Abstract
Soluble organic compositions of extraterrestrial samples offer valuable insights into the prebiotic organic chemistry of the solar system. This review provides a summary of the techniques commonly used for analyzing amino acids, amines, monocarboxylic acids, aldehydes, and ketones in extraterrestrial samples. Here, we discuss possible effects of various experimental factors (e.g., extraction protocols, derivatization methods, and chromatographic techniques) in order to highlight potential influences on the results obtained from different methodologies. This detailed summary and assessment of current techniques is intended to serve as a basic guide for selecting methodologies for soluble organic analyses and to emphasize some key considerations for future method development.
1. Extraterrestrial Organic Matter
For most extraterrestrial samples, the organic content can be divided into two main components: (1) insoluble organic matter (IOM)―a macromolecular fraction that is insoluble in organic solvents [1]; and (2) soluble organic matter (SOM)―a solvent-soluble fraction that may contain a diverse range of prebiotic organic compounds, including amino acids, amines, carboxylic acids, aldehydes, ketones, alcohols, sugars, nucleobases, and aliphatic and aromatic hydrocarbons [2,3,4]. These biologically relevant molecules are synthesized by abiotic reactions during the early evolution of the pre-solar cloud and protoplanetary disk, and during subsequent photochemical-, energetic particle-, thermal and aqueous alteration-driven reactions in the solar system [5]. Extracting and analyzing soluble organic compounds from extraterrestrial samples and investigating the chemical processes involved in their synthesis brings us closer to understanding the origin of prebiotic organic molecules in the early solar system, and how these species may have contributed to the origin of life on Earth and possibly elsewhere.
2. Sample Types
2.1. Meteorites
The majority of soluble organic analyses of extraterrestrial materials are carried out on meteorites―rocky fragments of extraterrestrial bodies that have landed on the surface of a planetary body. Most meteorites originate from asteroids or comets, while a smaller number are derived from fragments of planets or moons that were ejected from their parent surfaces during large impact events. Based on their bulk compositions and textures, meteorites can be subdivided into two main categories: non-chondrites and chondrites [6]. The non-chondrites, which include the achondrites, stony–iron meteorites, and iron meteorites, have experienced partial or complete melting and planetary differentiation. In contrast, chondrites are derived from primitive, undifferentiated asteroids or comets, and their contents may represent some of the oldest materials in our solar system. They are generally characterized by the presence of chondrules―small, round olivine- and pyroxene-rich grains that formed as molten or partially-molten droplets during early solar system formation. Due to their primitive nature and often comparatively high (~3%) abundances of organic matter, chondrites are the most commonly studied meteorite samples for soluble organic analyses. Of the five mineralogic classes of chondrite meteorites (carbonaceous, ordinary, enstatite, R (Rumuruti-like), and K (Kakangari-like)), some of the most carbon- and soluble organic-rich meteorite groups belong to the carbonaceous chondrites (CI, CM, CR, CO, CB, CH, CV, CK and Cungrouped chondrites) and they constitute the bulk of the meteorite materials analyzed for soluble organics. The letters designating the categories of carbonaceous chondrites represent typical chondrites within each group (CI―Ivuna; CM―Mighei; CR―Renazzo; CO―Ornans; CB―Bencubbin; CV―Vigarano; CK―Karoonda), with the exception of the CH group which is assigned the letter ‘H’ in reference to its high metal and iron content. The Cungrouped category represents meteorite specimens with unique mineralogical and chemical properties that currently do not have enough known specimens to warrant the creation of a new class. Following the mineralogic class, a petrologic type is often assigned to indicate the relative degree of parent body alteration, with types 1–3 having experienced aqueous alteration at low temperature (1 = most aqueously altered; 3 = least aqueously altered) and types 4– 6 having experienced varying levels of thermal alteration (4 = least thermally altered; 6 = most thermally altered) [7].
The first published report of organic matter within a carbonaceous chondrite was a study of the Alais CI1 carbonaceous chondrite in 1834 [8]. The earliest investigations of the chemical composition of soluble organic matter in carbonaceous chondrites began with bulk carbon measurements of solvent extracts, and analyses of hydrocarbons and amino acids, with some uncertainties distinguishing between terrestrial and extraterrestrial origins (e.g., [9,10,11,12]). The fall of the Murchison CM2 carbonaceous chondrite in 1969 and, subsequently, the first measurements of non-protein amino acids in Murchison [13] led to greater scientific interest in meteoritic organics and significant improvements in analytical sensitivity. The database of known soluble organic compositions quickly expanded from only a handful of compounds to large datasets comprised of isomers and stereoisomers of multiple classes of soluble organics (e.g., [14,15,16,17,18]).
Obtaining Meteorite Samples for Analysis
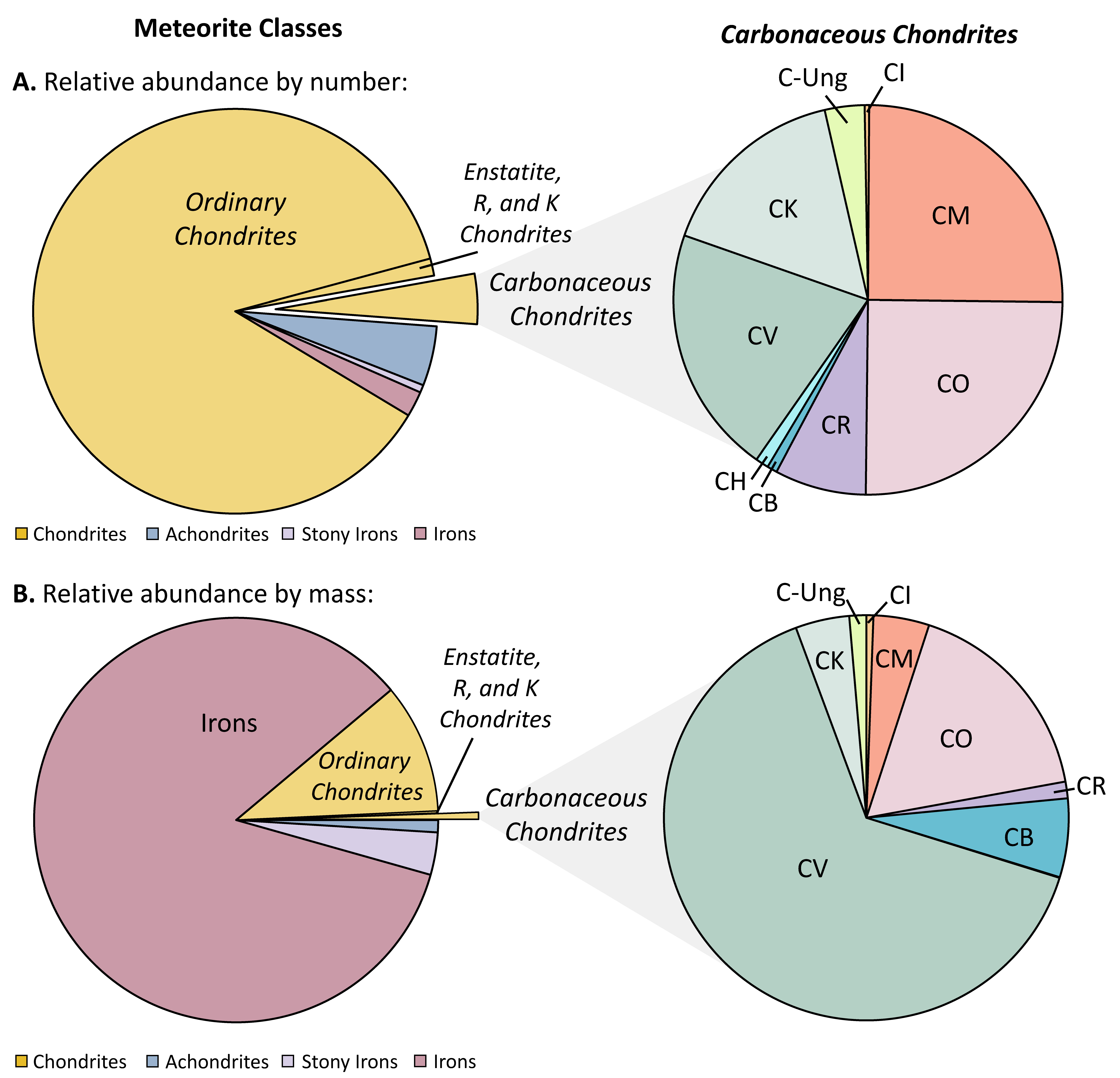
Meteorite falls occur all across the Earth’s surface. However, the vast majority of meteorites are collected in hot and cold desert environments (e.g., the Sahara and Antarctica, respectively) where dark meteorite fragments are easily distinguishable across vast regions of light-colored terrain with minimal surface features that would otherwise inhibit detection of these extraterrestrial specimens. Furthermore, cold-arid environments, such as Antarctica, provide an added benefit of exposing meteorites to minimal terrestrial alteration and contamination. Certain regions along the Transantarctic Mountains often harbor large numbers of meteorites because meteorites are naturally transported across the continent within vast sheets of flowing glacier ice and become concentrated along mountain ridges, which can act as natural barriers. Meteorites are annually collected by government-funded organizations (e.g., the Antarctic Search for Meteorites [19] (ANSMET) and the Japanese Antarctic Research Expedition [20] (JARE) programs), as well as by professional meteorite collectors and amateurs. Although there are currently > 60,000 meteorites available for study [21], the most organic-rich and scientifically valuable specimens for soluble organic analyses are relatively few in number. Chondrites comprise the largest proportion of meteorites known to date (~92%, based on number of meteorites) but the bulk of the group is comprised of relatively organic-poor samples (ordinary chondrites, which constitute ~87% of all meteorites, by number (Figure 1; [21]). Carbonaceous chondrites constitute only ~4% of all meteorites identified and represent ~0.5% of the total mass of meteorite material available. In terms of alteration history, the end-member groups also have the fewest number of samples. For instance, CI carbonaceous chondrites―some of the most highly aqueously altered and most primitive in terms of bulk elemental compositions―are represented by only nine known examples to date. Six of the CIs have a total available mass of only a few grams, and two of the CIs (Alais and Revelstoke) are not available for destructive analysis at all. For some carbonaceous chondrite groups (e.g., CIs and CVs), only one petrologic type has been observed to date. The rarity and generally low sample masses of meteorite samples that have experienced unique parent body histories makes these samples particularly desirable for soluble organic studies. For these reasons, obtaining a meteorite sample from an academic or government-owned collection often requires a formal sample request with valid justification for analyzing a specific sample mass of a particular meteorite specimen.
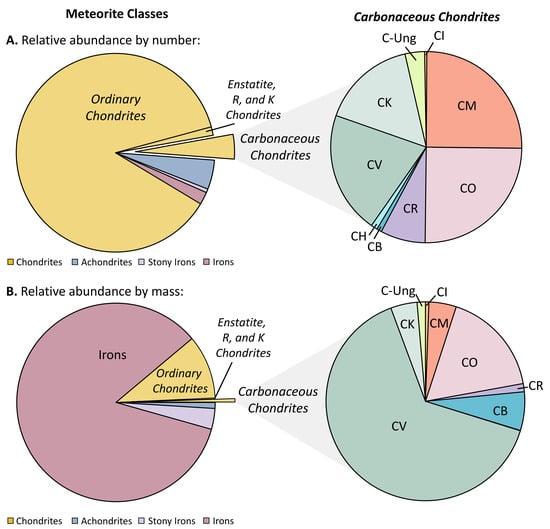
Figure 1.
Relative abundances of chondrites, achondrites, stony iron meteorites and iron meteorites, and of individual chondrite groups, based on (A) total numbers of meteorites (falls + finds) identified for each category and (B) total mass of meteorite material available from each category. Relative proportions are approximate values based on data obtained from the Meteoritical Bulletin Database [21] (March 2019).
2.2. Dust and Micrometeorites
Like meteorite samples, micrometeorites (MMs) and interplanetary dust particles (IDPs) can originate from asteroids, comets, or other planetary bodies; however, they are much smaller in size (MMs ~50–500 µm; IDPs ~10–40 µm), which presents a unique analytical challenge. Most organic analyses of these samples are focused on spatially resolved techniques without the ability to differentiate between soluble compounds beyond measurements of mass (e.g., microprobe two-step laser mass spectrometry (µL2MS) [22,23]), bulk isotopes (e.g., nanoscale secondary ion mass spectrometry (nanoSIMS) [24]), and functional groups (e.g., x-ray absorption near edge structure (XANES) spectroscopy and infrared spectroscopy [25]). In addition to these targeted analyses of individual MMs or IDPs, bulk solvent extractions of collections of MM grains have been performed for compound-specific measurements of amino acids using methodologies commonly applied to carbonaceous chondrite samples [24,26,27,28]. For amino acid analysis, disaggregation of MMs prior to the hot-water extraction step has been carried out by crushing and sonicating MM particles in ultrapure water [24,26]. Hot-water extractions have also been carried out on intact MMs directly, without weighing or crushing the particles, in order to mitigate potential contamination of the samples [27].
2.3. Spaceflight Mission Samples
Unlike most meteorites, extraterrestrial samples collected by robotic or crewed spacecraft are from a known location, they offer the opportunity to collect material from a desired sample source, and potentially provide a means by which to mitigate terrestrial contamination. However, these types of samples are generally more limited in abundance and more difficult to obtain than meteorites. Typically, a researcher must demonstrate proficiency in meteorite analyses and relevant analogs of the appropriate size and class in order to credibly propose to analyze samples collected through these missions. Such samples are carefully controlled by the government that curates them (e.g., https://curator.jsc.nasa.gov/lunar/index.cfm). Soluble organic analyses of sample return material often involve applying the same techniques as those used for analyzing carbonaceous chondrites. In fact, many of these chromatographic techniques were developed or refined to analyze the lunar samples returned by the NASA Apollo 11, 12, 14–17 missions [29,30,31,32,33,34,35]. Future analyses of samples returned from asteroid Ryugu by JAXA’s Hayabusa2 mission and from asteroid Bennu by NASA’s OSIRIS-REx mission [36] are expected to apply an array of methods. Small particles from the Itokawa asteroid obtained through the Hayabusa sample return mission have been analyzed similarly to IDPs and MMs, though their compositions are relatively depleted in organics [37,38]. Alternative collection techniques (e.g., aerogels and aluminum foils exposed to the Stardust comet Wild 2 coma [39,40,41]) have been used to examine bulk material, instead of individual particles, to make compound-specific measurements via chromatographic methods such as those described here. In contrast to sample return analyses, in situ soluble organic analyses of relatively distant planetary bodies require easily transportable instrumentation with minimal mass, power, and volume requirements. Technologies in development for these purposes include, for example, capillary electrophoresis and a new miniaturized variant of the method, microchip electrophoresis, for chiral analysis of amino acids [42].
3. Review Objectives
In the present literature review, we aim to provide a comprehensive guide for the analysis of soluble organic compounds in extraterrestrial materials, summarizing key modifications and new techniques introduced over the past >50 years of research. Methodologies developed for terrestrial sample analyses (e.g., for pharmaceutical or environmental purposes) are not always applicable to extraterrestrial studies. The organic compositions of astromaterials are often unique to extraterrestrial environments and, due to limited sample quantities and low abundances of organics, techniques with relatively high sensitivity are required. This review focuses specifically on methodologies targeting five classes of meteoritic soluble organics that are readily extractable in water or polar solvents and that are structurally related by synthetic pathways potentially occurring inside the asteroid parent body: amino acids, amines, monocarboxylic acids, aldehydes, and ketones (Scheme 1).
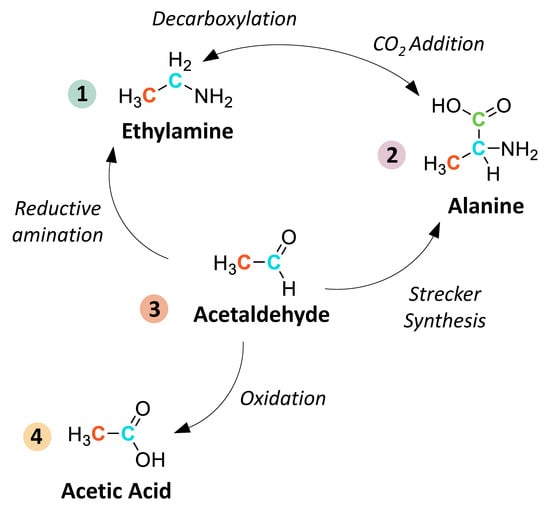
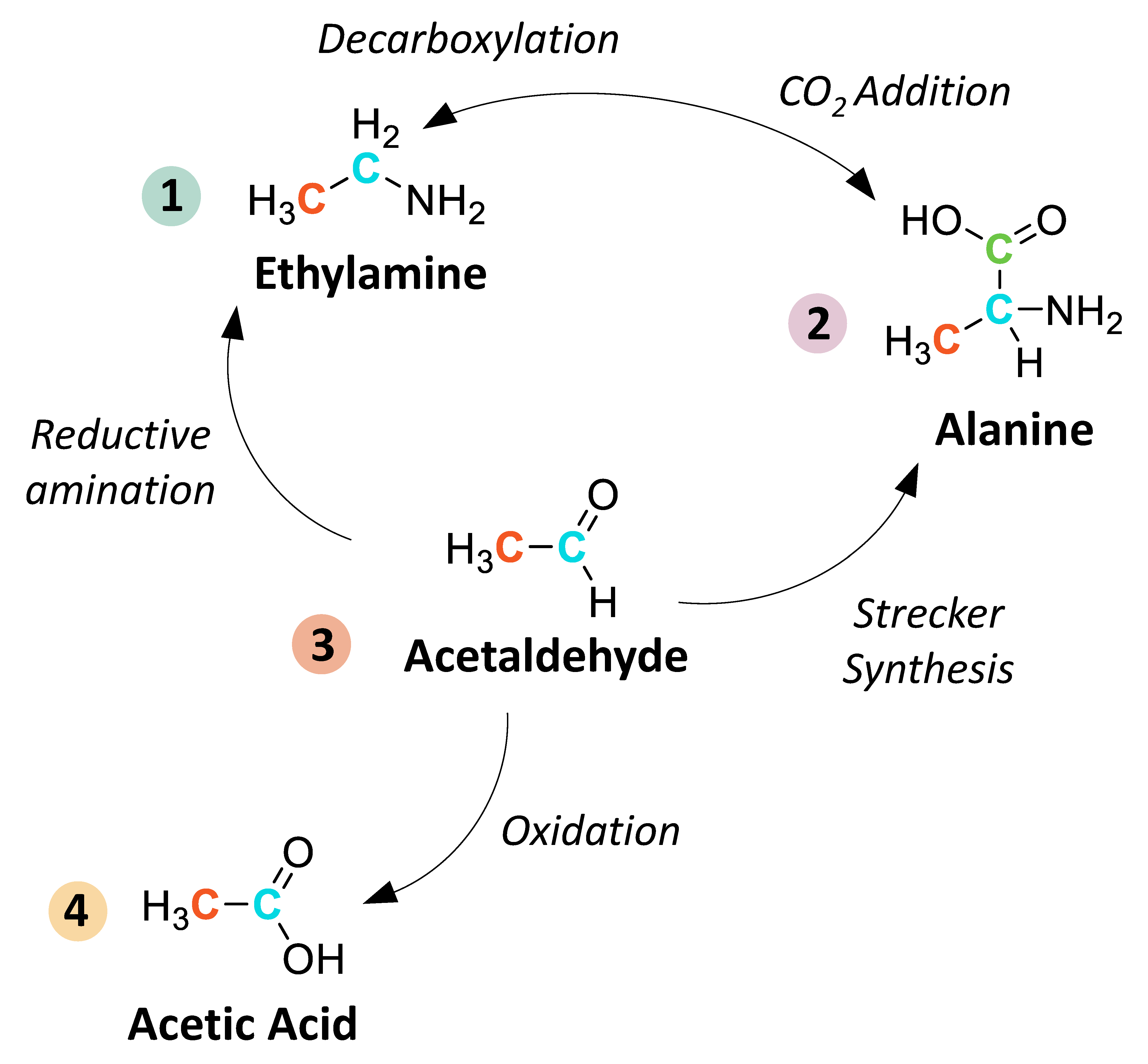
Scheme 1.
A simplified schematic of potential synthetic relationships between extraterrestrial water-soluble (“free”) amines(1), amino acids(2), aldehydes/ketones(3), and monocarboxylic acids(4). These reactions include amine synthesis via reductive amination of aldehydes/ketones [43] and decarboxylation/deamination of amino acids, carboxylic acid synthesis via oxidation of aldehydes [44], and amino acid synthesis via Strecker reactions and CO2 addition to amines [45]. Other potential mechanisms for amino acid synthesis not illustrated here include, for example, reductive amination of keto acids [46], β-amino acid synthesis via Michael addition of ammonia to α,β-unsaturated nitriles [17], and γ- and δ-amino acid synthesis via decarboxylation of α-amino dicarboxylic acids [47]. Bound amino acids that are released from samples via acid hydrolysis may be derived from larger organic polymers or from transition metal complexes. Detailed descriptions of these reaction mechanisms have been described elsewhere (see [5] and references therein).
A second objective of this review is to highlight observed variability between meteorite studies and discuss the potential impact of meteorite sample heterogeneity and the effects of differing analytical techniques (e.g., solvent extraction methods, derivatization methods, and chromatographic separation techniques). The ultimate goal of this discussion is to raise awareness of potential limitations to be considered during method development and new targeted analyses, in order to help mitigate ambiguity among collaborative cross-comparisons. With these considerations in mind, we provide a summary and assessment of the current state of knowledge regarding meteoritic soluble organics and discuss potential avenues for improvements during future analyses, including exploring new analytical techniques and targeting new classes of organic compounds.
4. Analysis of Extraterrestrial Organic Compounds: Method Overview
4.1. Mitigation and Monitoring of Sample Contamination
Special care must be taken prior to and during organic analyses of any extraterrestrial sample. Meteorite specimens that have been exposed to minimal terrestrial contamination and have known curatorial histories are preferable for soluble organic analyses and, when available, terrestrial environmental samples collected from the fall site should be analyzed in parallel with meteorite samples in order to assess whether there has been any terrestrial input [48,49,50,51,52,53]. As a common rule, any glassware or tools (e.g., forceps, mortar and pestle) used for sample storage, processing, and analyses are typically wrapped in aluminum foil and heated in air at or above 500 °C for several hours (usually > 6 h) prior to use. This combustion process drives off organics that could be introduced into the samples. Other measures that are commonly taken to mitigate and monitor terrestrial contamination and cross-contamination in extraterrestrial samples include the following: (1) storing and processing samples in clean laboratory conditions (e.g., cleanroom facilities, low-temperature laboratory facilities [54], high efficiency air (HEPA) filtered laminar flow hoods, and/or gaseous nitrogen or argon gloveboxes [54,55]; (2) bead-blasting reusable ceramic mortars and pestles before baking them to remove residual sample powder; (3) removing the fusion crust outer surfaces of meteorite chips before powdering samples; (4) substituting plastic tools with polytetrafluoroethylene (PTFE) materials (e.g., pipette bulbs, storage containers), and using PTFE-lined lids and caps for glass containers; (5) wearing powder-free nitrile gloves during sample handling and analyses (not latex or nylon, as these materials may degrade to form amino acids); (6) using only high purity (>99%) solvents and ultrapure water (triple-distilled, or Milli-Q® (18.2 MΩ cm, <5 ppb total organic carbon)) for carrying out sample extractions and preparing solutions; (7) analyzing the cleanliness of chemical reagents prior to using them for sample processing and analysis, to measure background levels of organics present in derivatization and purification reagents, as well as in the analytical instrumentation; and (8) analyzing blanks in parallel with samples, including solvent blanks (i.e., solvent only, no meteorite sample powder) and organic-free mineral analog blanks (usually baked serpentine mineral or glass powder, sand, or meteorite powder, extracted and carried through the method). Some of these techniques (e.g., cleanroom protocols) are designed to reduce particulate contamination, without special attention to soluble organic compounds. The challenges associated with soluble organic analyses are different from those typically experienced in a cleanroom environment [56]; for example, the presence of inorganic dust is of minimal consequence when executing soluble organic analyses, but the presence of organic gases (e.g., HCN, HCHO) that are not removed by filtration steps can often pose a problem. Conversely, aluminum-wrapped glassware cleaned by heating in a ceramic muffle furnace, which is ideal for soluble organic analysis, can introduce contaminants for bulk inorganic analyses. Further details regarding specialized curation and contamination control protocols can be found in the literature (e.g., [54,56]).
Certain molecular structures and isotopic signatures may be used to assess the potential influence of terrestrial contamination. Monitoring the presence of these terrestrial signatures in addition to performing the types of procedural blank analyses described above is valuable for identifying contaminants introduced prior to sample analyses. Biological and terrestrial signatures include, for example, the presence of large l-enantiomeric excesses (“non-racemic” mixtures) of proteinogenic amino acids (e.g., serine, threonine, isoleucine, and glutamine), d-enantiomeric excesses of isovaline potentially derived from fungal peptides [57], and the presence of polymers and plasticizers (e.g., nylons, polyethylene glycol, and phthalates) and silicon-based grease. In contrast, the presence of isotopically heavy organic compounds (δ13C > 0‰ and δD >> 0‰, which are characteristic of extraterrestrial low-temperature gas-phase reactions [58,59,60]) can be indicative of extraterrestrial origins.
4.2. Typical Sample Extraction Protocols
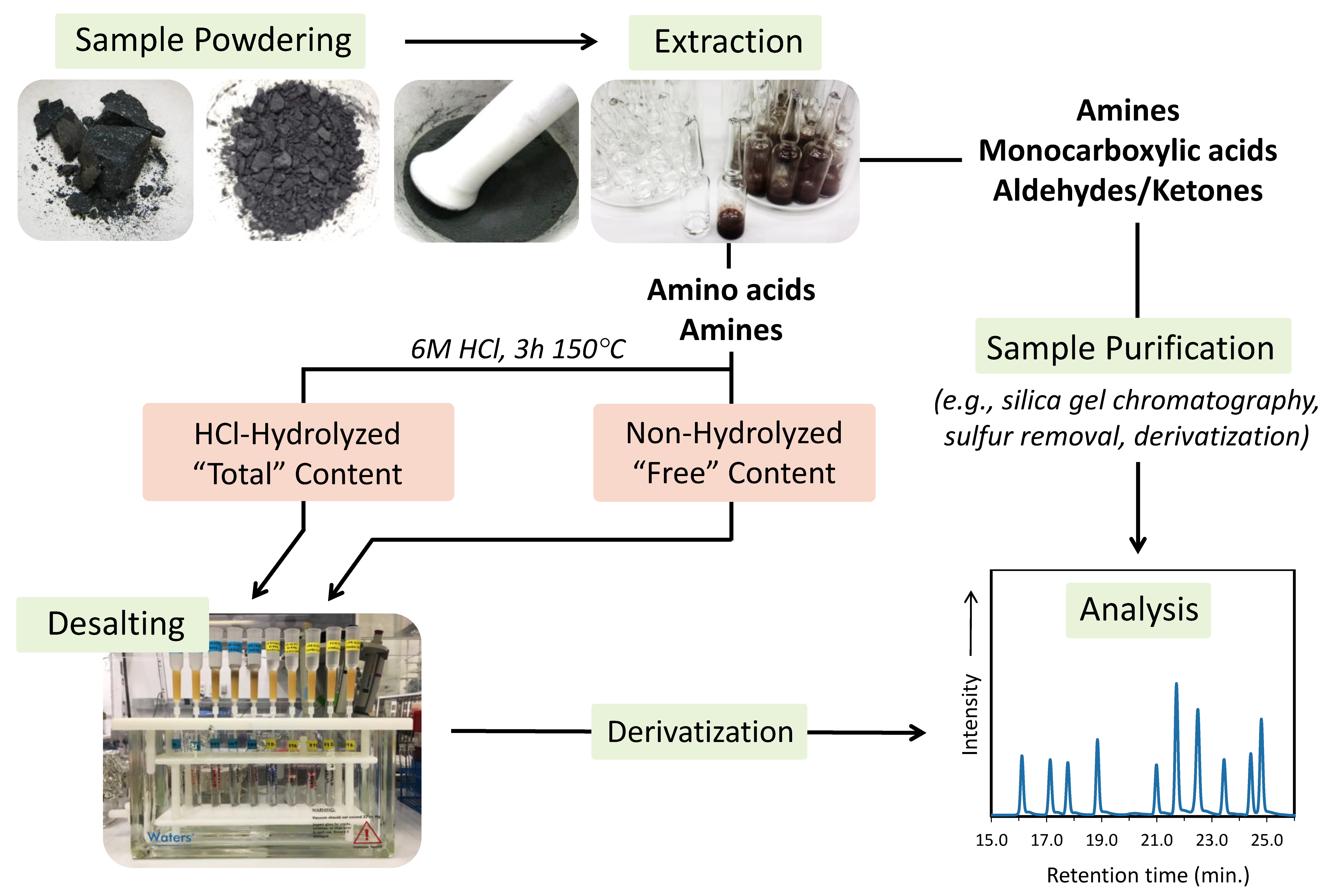
The general procedure for extracting and analyzing soluble organic compounds from extraterrestrial samples is illustrated in Figure 2. Typically, a solid sample is powdered using a ceramic or corundum mortar and pestle. An agate mortar and pestle can also be used; however, as agate cannot be heated to high temperatures for cleaning, extra care must be taken to sufficiently remove organic contaminants using ultrapure water and solvents. The fine powder is then weighed and transferred to a receptacle (e.g., glass tube, ampule, or round-bottom flask) for the solvent extraction step. Polar organic compounds such as amino acids, amines, monocarboxylic acids, aldehydes, and ketones, are typically extracted in ultrapure water for varying periods of up to 24 h, commonly at 100 or 110 °C (Table 1, Table 2, Table 3 and Table 4). These hot-water extractions can be carried out using a reflux set-up or as small aliquots (1–2 mL water for 0.5–1 g of powder). A number of studies have carried out meteorite extractions of polar organic compounds in organic solvents, such as KOH/CH3OH mixtures or 1 N NaOH for the extraction of carboxylic acids [61,62,63,64,65], and dichloromethane (DCM) for the extraction of aldehydes and ketones [66]. Relatively non-polar organic species (e.g., hydrocarbons and heterocycles) are typically extracted using either CH3OH, DCM, DCM/CH3OH mixtures, or benzene/CH3OH mixtures [13,67,68,69,70,71,72,73,74]. In this review, we focus on those compounds that are readily accessible through aqueous extractions.
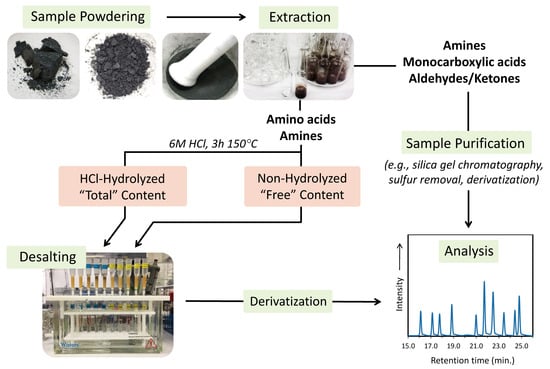
Figure 2.
Simplified schematic of a typical protocol for extracting and analyzing amino acids, amines, monocarboxylic acids, aldehydes, and ketones in extraterrestrial materials.

Table 1.
Analyses of meteoritic amino acids.

Table 2.
Compound-specific analyses of meteoritic amines.

Table 3.
Compound-specific analyses of meteoritic monocarboxylic acids.

Table 4.
Compound-specific analyses of meteoritic aldehydes and ketones.
4.3. Processing and Purifying the Extract
4.3.1. Separating and Concentrating the Supernatant
Following the extraction step, the solution containing the extracted organics is commonly centrifuged and/or filtered to separate the supernatant from the solid sample residue, as solids suspended in solution can interfere with subsequent derivatization or purification reactions. Rinsing the residual powder with additional solvent and repeating the centrifugation/filtering step helps to maximize the recovery of extracted compounds. Methods for concentrating the supernatant include using centrifugal vacuum concentrators, rotary evaporators, and cryodessicators, or drying with nitrogen gas. For some analyses, volatile compounds (e.g., monocarboxylic acids, amines) that may otherwise be lost from the extract during solvent evaporation are retained in the sample by adjusting the pH to convert the analytes to organic salts [65,70,75,76,77,78,79,80].
4.3.2. Acid Hydrolysis
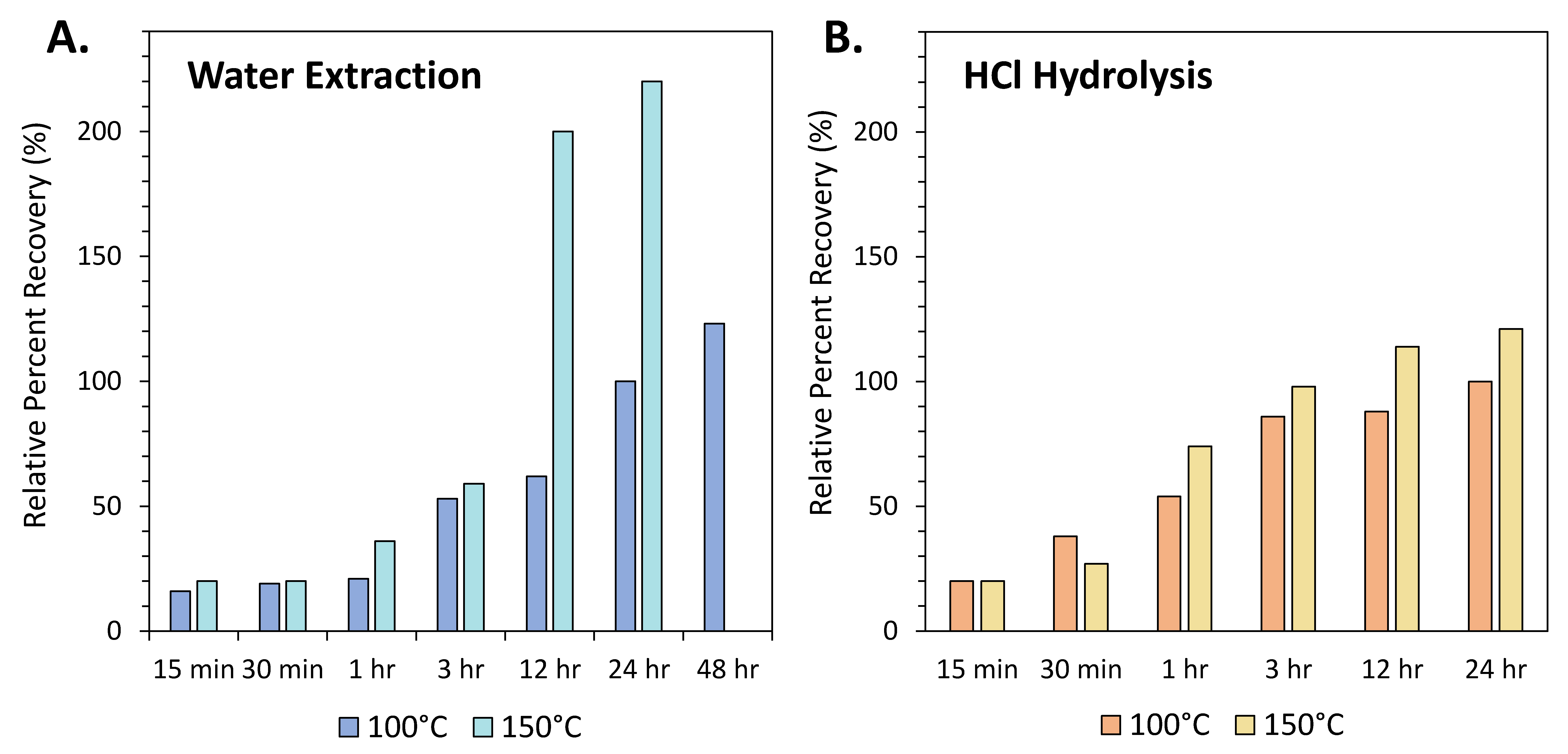
A meteorite extract may be carried through an acid hydrolysis step to hydrolyze and release organic molecules that may be bound as larger polymers or complexed with transition metal species within the meteorite [81]. This process may also produce amino acids from precursor molecules, including amino esters, amides, amino nitriles and lactams [47]. Acid hydrolysis is most commonly carried out for amino acid analysis (Table 1, Figure 2), but has also been used for the analysis of meteoritic amines (e.g., [15,82]). The acid-hydrolyzed fraction of a meteorite extract often exhibits a distinct composition and, in many cases, comprises the bulk of the amino acid content (e.g., [50,83,84]). The earliest hydrolysis experiments involved adding acid directly to the dried water extract residue [85,86,87]. This procedure was later modified to minimize the potential for contamination during direct liquid hydrolysis by optimizing a vapor-hydrolysis technique [88]. The duration of the hydrolysis step was also reduced to 3 hours in order to minimize the potential for amino acid racemization (Figure 3; [49]). Carrying out acid hydrolysis at higher temperatures and for longer durations has been shown to lead to significant racemization of chiral amino acids [89].
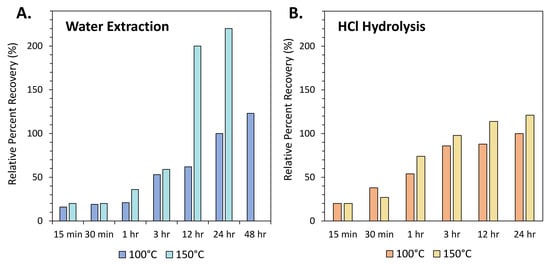
Figure 3.
Average percent recoveries (%) of amino acids extracted from Nile Delta deep-sea sediment (see Glavin et al., 1999 [49] for sample source information), relative to the standard amino acid extraction protocol (100 °C water extraction for 24 h, followed by 6 M HCl hydrolysis at 100 °C for 24 h). (A) Effects of varying temperature (100 °C and 150 °C) and duration (15 min–48 h) for the hot-water extraction. Increasing the duration and the temperature of the water extraction results in a continuous increase in amino acid recovery. (B) Effects of varying temperature (100 °C and 150 °C) and duration (15 min–24 h) for 6 M HCl hydrolysis. In most cases, increased temperature and duration results in higher amino acid recoveries; however, the duration of the hydrolysis step is generally kept at 3 h to avoid potential amino acid racemization [89].
4.3.3. Purification Methods
Sample purification steps are commonly carried out to remove impurities and isolate soluble organic compounds of interest for chromatographic analysis. Common purification techniques include treating the meteorite extract with activated silica gels (e.g., [65,82]), performing liquid-liquid extractions to isolate specific target molecules (e.g., [61,63]) and removing co-extracted elemental sulfur from solution using copper powder or granules (e.g., [66,70]). These purification steps may be carried out before or after derivatization, depending on the extraction procedure, target analytes, and derivatization chemistry. For amino acid analysis, passing the aqueous extract through an ion exchange chromatography column (“desalting”, Figure 2) prior to derivatization helps remove inorganic salts that may otherwise interfere with subsequent derivatization reactions and/or damage gas chromatography (GC) or liquid chromatography (LC) columns. Given the importance of desalting for the analyses of meteoritic amino acids, a detailed description of this purification effort, what types of ion exchange approaches are typically used, and how to minimize contamination when executing the desalting step, are discussed here.
Ion exchange chromatography provides separation of molecules based on charge by utilizing resins that are typically composed of beads of polymers, with electrolytes covalently bound to the resin beads ([90], and references therein). One of the ions of the electrolyte is fixed (“stationary”) to the resin beads, while the counterion is of the opposite charge and is capable of being exchanged (i.e., is “mobile”). During sample elution through the chromatography column, compounds that are neutral, or possess an identical charge to the stationary ion, are eluted through the column. In contrast, sample molecules with a charge opposite to the stationary ion, compete with the mobile ions for binding sites on the resin beads. Sample ions that have a greater affinity for the stationary ions are capable of displacing mobile ions to become bound to the resin beads. Bound sample ions can then be selectively eluted from the chromatography column using an eluant with an appropriate pH and ionic strength, such that the eluent contains either (A) an ion with a greater affinity for the stationary ions than do the sample ions, or (B) an ion with a selectivity for the stationary ions that is equal to, or less than, that of the sample ions, but is present at a high concentration.
For analyses of amino acids in meteorites, cation exchange chromatography has proven beneficial for removing inorganic salts and other interfering species, while concomitantly enabling the elution of amino acids in a solution that does not complicate their subsequent analyses [91]. Cation exchange chromatography resins that are typically used when preparing meteorites for amino acid analyses include AG 50W-X8, 100–200 mesh, hydrogen form (Bio-Rad Laboratories, Hercules, CA, USA [50]), and AG 50W-X4, 200–400 mesh, hydrogen form (Bio-Rad Laboratories, Hercules, CA, USA [92]). These resins have a SO3− stationary ion fixed to the beads, and an H+ mobile ion bound to the stationary ion. When a meteorite extract containing amino acids is loaded onto the chromatography column, the R-NH3+ ions of the amino acids will outcompete the H+ mobile ions for binding sites, and thus displace the mobile ions to become bound to the resin beads. Afterward, the amino acids can be selectively eluted from the resin typically using 2 M NH4OH.
Prior to use, cation exchange chromatography columns are typically rinsed to minimize potential sources of contamination ([93], and references therein). First, the columns are rinsed with 6 bed volumes of ultrapure water (until the eluting solution has a neutral pH). Second, a base wash is executed to remove the H+ mobile ions originally present on the resin using 4 bed volumes of 2 M NaOH (until the eluting solution has a basic pH). Third, the chromatography columns are rinsed with 12 bed volumes of ultrapure water (until the eluting solution has a neutral pH). Fourth, the resin is recharged with fresh H+ mobile ions using 2 bed volumes of 1.5 M double distilled hydrochloric acid (ddHCl) (until the eluting solution has an acidic pH). Finally, the chromatography columns undergo a final water rinse using 12 bed volumes of water (until the eluting solution has a neutral pH).
There are certain important considerations to bear in mind when preparing and using ion exchange chromatography columns, including: (1) Do not allow resins to dry, which may allow cracks to form, and (2) The water rinses performed after the NaOH wash and the ddHCl recharging steps are important to execute in order to return the columns to a neutral pH environment without exposing the columns to a pH shock that may otherwise crack the resins.
4.4. Chromatographic Separation and Detection
The separation and detection of meteoritic soluble organics is most commonly performed using gas chromatography-mass spectrometry (GC-MS), or high-performance liquid chromatography coupled to a UV fluorescence detector (HPLC-FD), and/or a mass spectrometer (e.g., time-of-flight mass spectrometer (ToF-MS)). Both GC and LC separation techniques offer different analytical advantages, and certain meteoritic organic compounds have been detected using only one method and not the other. GC is generally used for analyzing relatively volatile, low molecular weight, and thermally stable compounds, while LC techniques are more appropriate for analyzing non-volatile and thermally unstable analytes. The chromatographic separation technique best suited for a particular study is also dependent on the concentrations of the analytes in the sample and the level of instrumental sensitivity needed. Typically, LC methods using a fluorescent detector are one to a few orders of magnitude more sensitive than GC methods. On the other hand, if stable isotopic measurements are needed and sufficient sample mass is available, then GC protocols are followed. Nevertheless, LC and GC methods can be mutually beneficial; a small fraction of a sample can be analyzed using LC as an initial assessment of concentrations to provide feedback for further LC or GC analyses.
Detection methods vary depending on the chromatographic separation technique used. For GC analysis, parent molecules are typically ionized via electron impact ionization, and characteristic fragmentation patterns can be compared to mass spectral libraries for compound identification. LC techniques are more amenable to parallel detection methods (e.g., optical/fluorescence detection and mass spectrometry). Positive electrospray ionization is the most common ionization method for LC due to high sensitivity. Unlike electron impact ionization, the electrospray method results in minimal mass fragmentation. Other fragmentation methods within the ion optics of the instrument are possible to gain structural information, and different mass analyzers (e.g., quadrupole, time-of-flight, ion trap, etc.) provide different resolutions and scan speeds. A more thorough review of mass spectrometry is outside the scope of this manuscript.
While some compound classes can be separated chromatographically as underivatized samples (e.g., GC analysis of monocarboxylic acids via direct solvent injection or solid-phase micro-extraction (SPME)), most meteoritic analyses involve an intermediate chemical derivatization step to convert the target analytes to more GC- or LC-amenable derivatives. Table 1, Table 2, Table 3 and Table 4 summarize the range of chromatographic methods that have historically been used for analyses of meteoritic amino acids, amines, monocarboxylic acids, aldehydes, and ketones. In the following sections, we discuss the various methodologies used, with an emphasis on the relatively recent techniques. While amino acid analyses have been successfully carried out using a wide range of methods, the number of techniques used for analyses of other compound classes is relatively limited.
4.4.1. Amino Acids
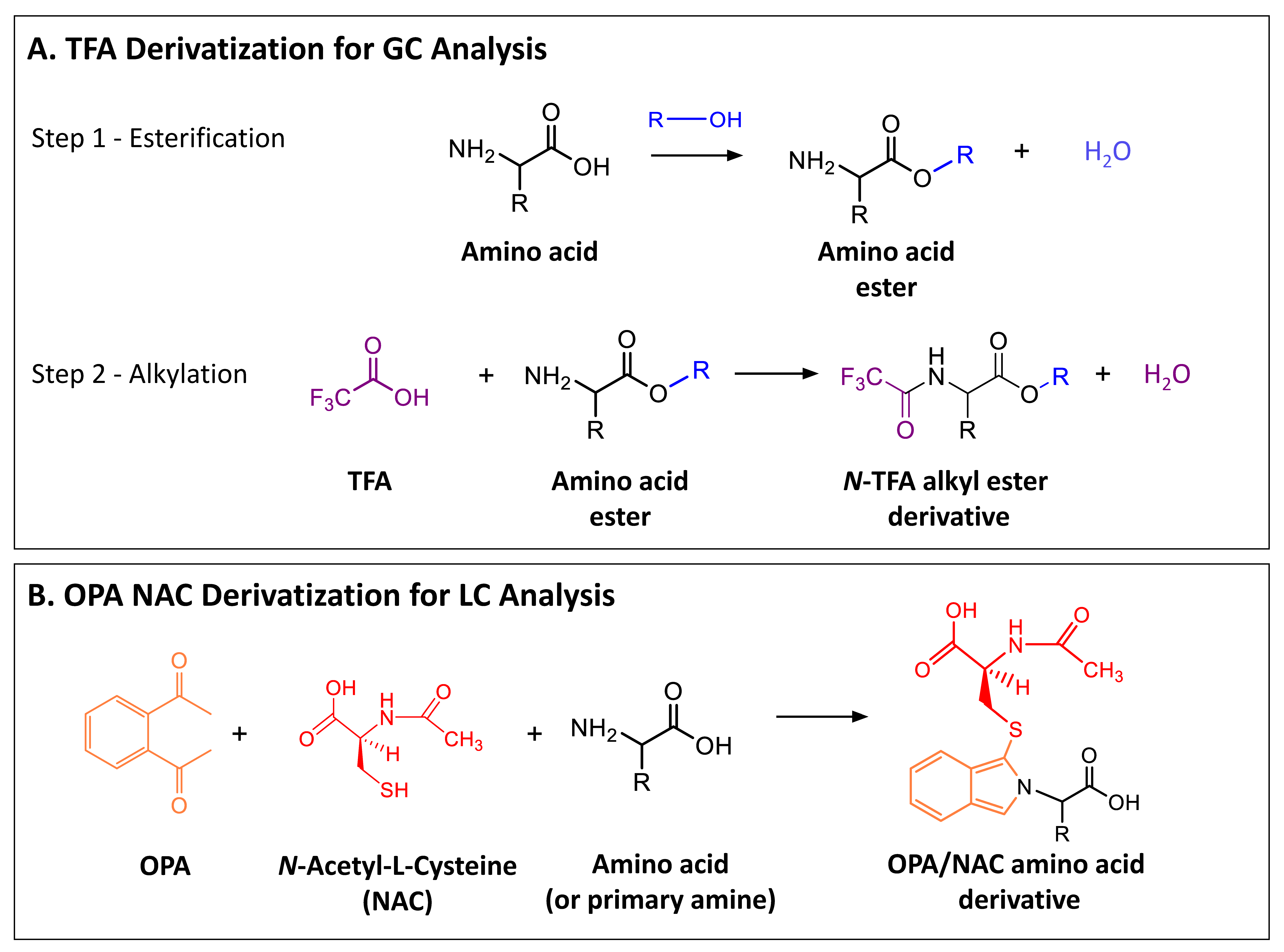
The large majority of reports of aliphatic amino acids in extraterrestrial samples can be grouped into two main categories based on methodologies for derivatization and analysis: (1) analyses using trifluoroacetic acid (TFA) or trifluoroacetic anhydride (TFAA) derivatization for GC analysis (Scheme 2, panel A); and (2) analyses using o-phthalaldehyde-N-acetyl-l-cysteine (OPA/NAC) derivatization for LC (Scheme 2, panel B). The TFA/TFAA derivatization method involves esterification by reaction with an alcohol, followed by alkylation of the amino acid ester (Scheme 2, panel A). Two enantiomers of a chiral amino acid can be separated if a chiral alcohol is used for the esterification step (e.g., (S)-(+)-2-pentanol [86]) and/or if a chiral GC-column is used for the separation of derivatives. For OPA/NAC, the reaction between chiral amino acids and the chiral thiol N-acetyl-l-cysteine produces two diastereomers (representing the two amino acid enantiomers) (Scheme 2, panel B) which can be separated using non-chiral reversed phase LC columns. Other methodologies used for chiral separation of meteoritic amino acids include the use of enantioselective chiral columns for GC analysis of N-ethoxycarbonyl ethyl ester (ECEE) and N-ethoxycarbonyl heptafluorobutyl ester (ECHFBE) derivatives [94,95], GC analysis of amino acids as n-pentafluoropropionic anhydride (PFP) alkyl ester diastereomers [96,97,98], and the use of a chiral zwitterionic surfactant for micellar electrokinetic chromatography of Pacific Blue succinimidyl ester (PBSE) derivatives [99]. Further details regarding both enantiomeric and stable isotopic analyses of meteoritic amino acids are reviewed in the literature (e.g., [91,100,101,102,103,104]).
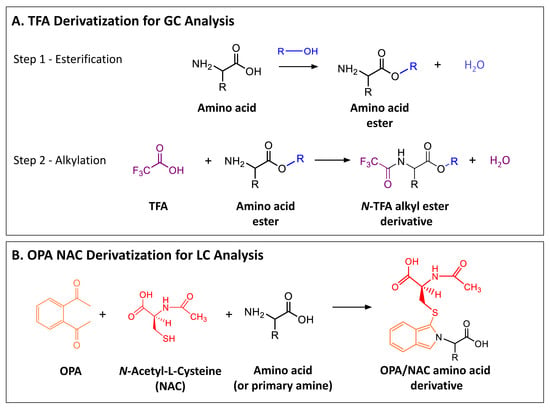
Scheme 2.
Schematic of the (A) trifluoroacetic acid (TFA) derivatization reaction and (B) o-phthalaldehyde-N-acetyl-l-cysteine (OPA/NAC) derivatization reaction commonly used for analyses of meteoritic amino acids.
The TFA/TFAA derivatization method for GC analysis has proven to be particularly advantageous in allowing a wide range of C6 and C7 amino acid TFA derivatives to be separated and detected [105,106]. For OPA/NAC derivatization, the introduction of smaller column resins and higher pressures (ultra high precision liquid chromatography (UHPLC)) and the use of UV fluorescence detection coupled with ToF or other high resolution mass spectrometry [50] was uniquely advantageous in allowing individual amino acids to be simultaneously identified based on their characteristic chromatographic retention times, their fluorescence absorbance patterns, and their accurate masses. This analytical approach provided greater accuracy for compound identification compared to chromatography or mass spectrometry approaches, alone. Despite these advantages, there are some known limitations associated with compound selectivity and/or chromatographic co-elutions. For instance, the basic proteinogenic amino acids (arginine, histidine, and lysine) are not detectable using either TFA/TFAA, or OPA/NAC derivatization approaches [107,108,109,110]. Therefore, these techniques are insufficient for determining if basic amino acids are present in chondrite extracts. Other derivatization methods (e.g., “Accq∙TAG” [56,111,112]) can detect these species but cannot discriminate enantiomers. Likewise, some unknown meteoritic organics are potentially masked by chromatographic co-elutions in conventional one-dimensional chromatography approaches. For example, glutamic acid and pyroglutamic acid are known to coelute with one another when implementing one-dimensional GC, but can be resolved when using novel two-dimensional GC×GC techniques [95].
4.4.2. Amines
The earliest report of individual meteoritic amines [15] compared measurements from three analytical techniques: GC-MS analysis of both free underivatized amines and 2,4-dinitrophenyl (DNP) amine derivatives, and ion exchange chromatographic analysis of amine OPA derivatives. Low molecular weight aliphatic monoamines were detected; however, unambiguous identifications were not possible for several of the compounds due to incomplete chromatographic separation. As shown in Table 2, subsequent analyses of meteoritic amines were performed with improved GC techniques that incorporated N-pentafluoropropionic anhydride (PFPA) derivatization [113] and trifluoroacetic anhydride (TFA) derivatization techniques [76,114,115,116,117]. A derivatization method using an enantiopure reagent ((S)-(–)-N-(trifluoroacetyl) pyrrolidine-2-carbonyl chloride; TPC) was later introduced to allow for both enantiomeric and compound-specific stable isotopic analyses (δ13C) of meteoritic amines [82].
4.4.3. Monocarboxylic Acids
Meteoritic monocarboxylic acids are most often analyzed via GC-MS as underivatized compounds, either through direct solvent injection [74,76,115,117,118] or using SPME [70,75,77,78,80,119,120] (Table 3). Avoiding sample derivatization decreases sample processing time, minimizes sample transfer steps which may otherwise result in partial loss of relatively volatile acids, and reduces the risk of introducing contamination. Using the SPME method, target analytes can be extracted from a filtered aqueous meteorite extract onto a solid coated fiber, which is then injected directly into the GC injection port. This method is particularly beneficial in eliminating the need for a liquid-liquid extraction step (e.g., water–CH2Cl2; [61,63]) which could potentially result in a loss of volatile, water-miscible, low molecular weight acids. Despite these advantages, however, complete chromatographic separation of some chiral monocarboxylic acids is more readily achieved using an intermediate derivatization step. For chiral analysis of monocarboxylic acids, novel derivatization methods have been developed and applied (e.g., (S)-1-phenylethyl and (S)-(–)-2-methylbutanol ester derivatives [65,79]; Table 3) to minimize co-elutions that would otherwise hinder accurate chiral quantification and simultaneous compound-specific δ13C analysis, respectively.
4.4.4. Aldehydes and Ketones
Individual aldehydes and ketones were first identified in meteorite samples via GC-MS as underivatized compounds, and by colorimetric analysis as 3-methyl-2-benzothiazolone hydrazine hydrochloride (MBTH) derivatives [14]. The Environmental Protection Agency (EPA) method #556, which uses 2,3,4,5,6-pentafluorobenzyl hydroxylamine (PFBHA) as the derivatization reagent for aldehyde and ketone analysis, was later applied to the analysis of these carbonyl species in several carbonaceous chondrites [74,114,115,119,121] (Table 4). The PFBHA derivatization method results in the formation of two derivatives ((E)- and (Z)-isomers) for all aldehydes and ketones that have asymmetric chemical structures. For most low molecular weight aldehydes and ketones, the generation of two PFBHA-derivatives does not pose a problem for chromatographic separation and quantification. However, any resulting partial co-elutions between the isomer peaks may complicate measurements, and the conversion of a single aldehyde or ketone to produce two separate derivatives could potentially result in isotopic fractionations. As such, a novel derivatization method ((S,S)-(–)1,4-dimethoxy-2,3-butanediol (DMB) derivatization [66] has recently been developed for performing accurate δ13C analysis without the aforementioned potential influences of the PFBHA method (Table 4).
5. Identifying Limitations of Cross-Comparisons between Studies
Studies of meteoritic organics rely heavily on cross-comparisons between studies, largely due to sample availability limitations. As a whole, this process of comparing and contrasting values between reports is practical and informative. However, as the field begins to more thoroughly investigate small variations in reported values―for instance, comparing relative abundance measurements of individual compounds in chondrites to infer relative degrees of parent body alteration―it becomes increasingly important to understand how several factors (e.g., meteorite sample heterogeneity and differences in laboratory procedures) can influence the measurements. The main objectives of the following sections are to highlight potential influences on reported values, emphasizing the need for detailed method development, method comparisons, and careful reporting of meteorite specimen information. The intent of this discussion is not to discredit data comparisons across different studies but to caution that there are several potential sources of variability to consider for future research.
5.1. Meteorite Sample Heterogeneity
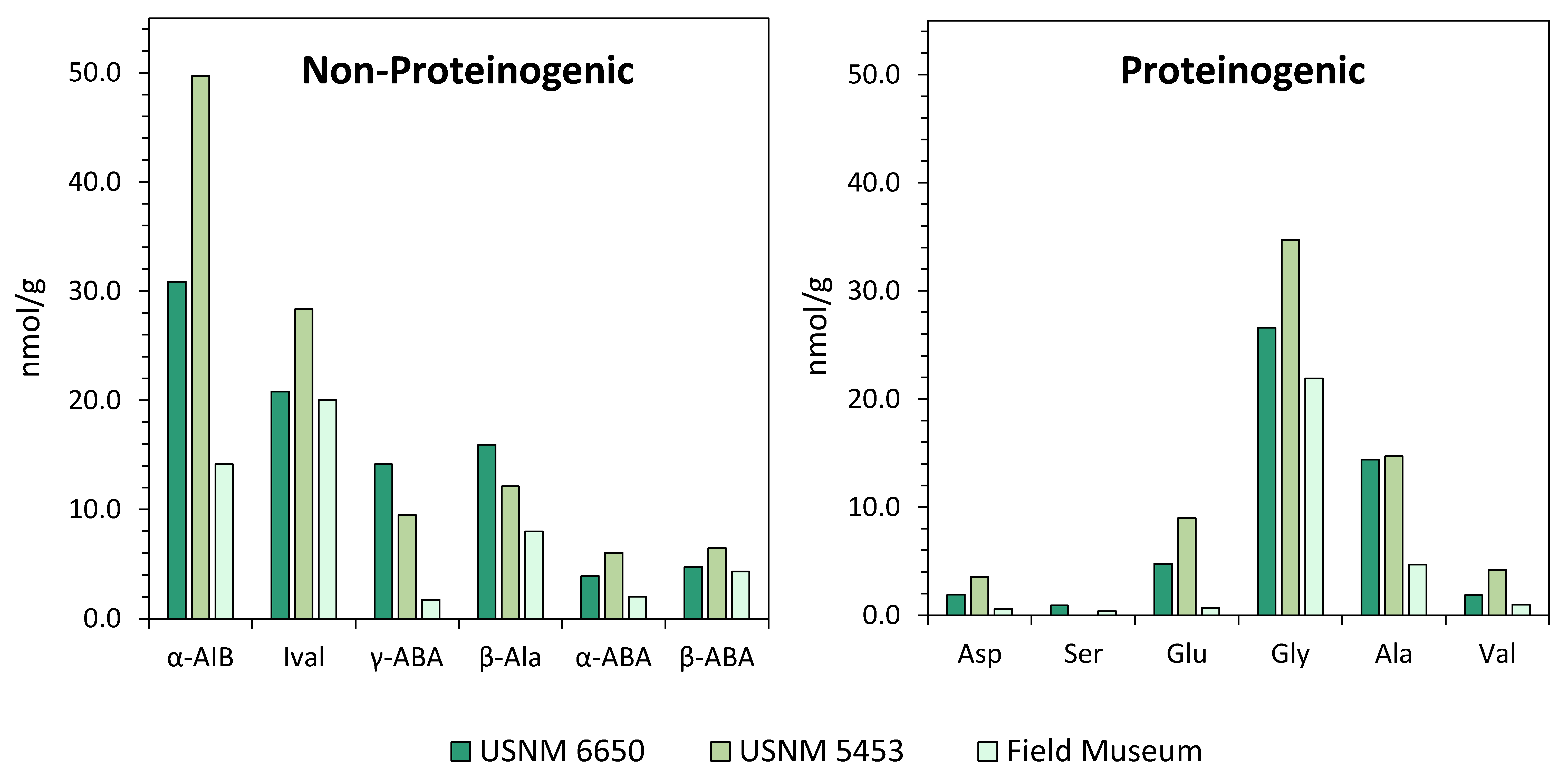
For soluble organic analyses, the Murchison meteorite is one of the most widely studied carbonaceous chondrites in the literature, due to the large quantity of sample material available for analysis, its relatively high abundance of soluble organic compounds, and the reasonably pristine nature of the meteorite. Like most meteorites, Murchison is generally assumed to exhibit a low compositional heterogeneity; however, there have been reports of significant discrepancies in soluble organic compositions across different Murchison specimens [105,122]. These variations potentially reflect heterogeneity within the asteroid parent body, contributions from terrestrial sources, and/or loss of organics through sample weathering. Like many fallen meteorites, the Murchison fragments landed across many kilometers within a strewn field [123] and consequently, likely experienced differing levels of fracturing and weathering [124]. This potential variability between meteorite specimens should be taken into consideration for cross-comparisons between different analyses. Figure 4 illustrates a specific case study of observed compositional heterogeneity for Murchison meteorite specimens. The dataset is split into non-proteinogenic and proteinogenic amino acid distributions to illustrate intrinsic sample heterogeneity versus variations potentially induced by terrestrial inputs. Alpha-aminoisobutyric acid (α-AIB) shows the largest variation among the non-proteinogenic amino acids, as has been demonstrated previously [105]. The larger variability seen for some non-proteinogenic amino acids, such as α-AIB and isovaline, may be explained by their greater steric hindrance, which can inhibit reaction efficiencies with derivatization reagents, potentially leading to more quantitation errors.
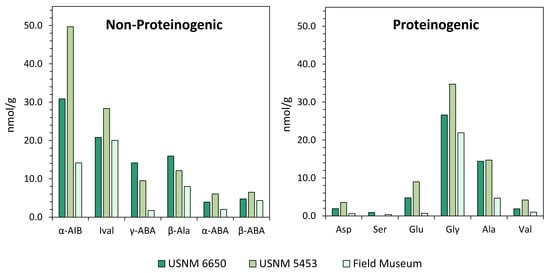
Figure 4.
Compositional heterogeneity among Murchison meteorite specimens. Abundances (nmol/g) of non-proteinogenic and proteinogenic amino acids in three Murchison specimens (United States National Museum (USNM) 6650 [50]; USNM 5453 [122]; specimen from the Field Museum of Natural History, Chicago [125]) analyzed using the same methodology (o-phthalaldehyde-N-acetyl-l-cysteine (OPA/NAC) derivatization and high-performance liquid chromatography coupled to a UV fluorescence detector (HPLC-FD) and liquid chromatography (LC)- time-of-flight mass spectrometer (ToF-MS)) within the same laboratory facility. Abundances reported in ng/g [50,122] were converted to nmol/g values for cross-comparison. α-AIB: α-aminoisobutyric acid; Ival: isovaline; γ-ABA: γ-amino-n-butyric acid; β-Ala: β-alanine; α-ABA: α-amino-n-butyric acid; β-ABA: β-amino-n-butyric acid; Asp: aspartic acid; Ser: serine; Glu: glutamic acid; Gly: glycine; Ala: alanine; Val: valine.
Improved analytical sensitivity over the years has allowed sample sizes for amino acid analyses to be reduced from several grams in the 1960s to milligram and microgram quantities for present-day analyses (e.g., [126,127]). As a result, over time, meteoritic amino acid analyses have become more targeted and potentially less representative of the bulk average composition. Several recent investigations have aimed to assess potential small-scale heterogeneity of organic matter in meteorites and have identified organic compounds distributed within the fine-grained mineral matrix [127,128,129] possibly in association with altered mineral grains [130]. As such, differing sample sizes may be an important consideration for comparisons between earlier datasets and more recent analyses. In addition, the rarity and generally limited sample availability of carbonaceous chondrites restrict the number of analyses that can be carried out for each individual meteorite specimen. More often than not, a manuscript will report and discuss the soluble organic content of only one or a few meteorite specimens with comparisons to datasets reported by other studies. Across research groups, different methodologies (e.g., different extraction procedures or derivatization reactions) can produce small variations in reported values (Section 5.2), which may complicate comparisons across studies.
5.2. Influence of Varying Methodologies
5.2.1. Influence of Extraction and Sample Processing
The types and abundances of soluble organic compounds extracted from a meteorite sample depend on: (1) the solvent type, (2) the extraction temperature, and (3) the duration of the extraction process. In order to optimize an extraction protocol for meteorite samples, each of the above factors should be tested on a suite of meteorite powders and procedural blanks, and monitored for total yields, reproducibility, potential reaction by-products when derivatization steps are used, and isotopic fractionation (if stable isotopes are measured). In practice, meteorite powders are not commonly accessible for method development. Industrial sediments [131] and other organic-rich geologic samples [49,132] have been used in some studies as alternative mineral matrices; however, the lack of a suitable meteorite analog on Earth for these purposes makes method development and optimization more challenging. The influence of individual factors (solvent, temperature, and time) have not been fully assessed for most soluble organic compound classes. For amino acid studies, the extraction conditions have remained consistent over time (aqueous extraction at ~100–110 °C for 18–24 h; Table 1). Likewise, extractions of meteoritic amines are generally carried out in water at 100–110 °C for 24 h (Table 2). However, extraction methods for other polar soluble organics such as monocarboxylic acids, aldehydes, and ketones, have been more variable and demonstrate the potential impact of differing protocols (Table 3 and Table 4). For instance, different yields of aliphatic aldehydes and ketones recently obtained by separate analyses of the same Murchison meteorite specimen may be partially explained by differing extraction procedures (DCM (100 °C for 24 h) versus aqueous extraction (100 °C for 24 h) [66,121]).
Successive solvent extractions of meteorite powders have been shown to yield larger abundances of soluble organics [114,115,133]. Simply increasing the temperature and duration of the extraction step has been shown to produce a similar effect (Figure 3). This continuous release of soluble organic matter during successive, longer, and warmer extractions indicates that the compounds may be bound to mineral substrates (e.g., chemisorption with phyllosilicate minerals) or as larger organic polymers, or synthesized in solution during the solvent extraction step. Post-extraction sample processing can also influence the yield of soluble organics. For instance, acid-vapor hydrolysis for amino acid analyses can have a major influence on abundances, in many cases doubling the yield (e.g., [50,83,84]). Increasing the temperature and duration of acid-hydrolysis results in higher amino acid recoveries (Figure 3), but can also lead to significant amino acid racemization. Recognizing this distinction between meteoritic soluble organics that are readily solvent-extractable versus organic byproducts of prolonged solvent extractions/sample processing steps is important to consider for our assessment of the organic inventory of asteroids in our solar system. The more readily extractable organics may be more representative of the types of organics involved in low-temperature aqueous alteration-driven reactions within parent body asteroids. Keeping the duration and temperature of extractions and hydrolyses consistent across studies is also important for maintaining consistency among study comparisons.
5.2.2. Influence of Derivatization and Chromatographic Techniques
Several different derivatization and chromatographic techniques have been tested for meteoritic amino acid analyses (Table 1), though potential influences of all techniques have not been fully assessed. A method comparison study of a single Murchison specimen [124] demonstrated that the two most commonly applied amino acid methods (TFA derivatization for GC analysis vs. OPA/NAC derivatization for LC analysis) produced some slight differences in yields, with the GC analysis yielding ~50% higher abundance of α-AIB, and the LC analysis yielding ~3 times higher abundance of β-alanine. This type of method comparison is critical for validating cross-comparisons between studies. Some similarly variable results have been observed for other classes of soluble organic compounds in meteorites using differing chromatographic techniques (e.g., GC vs. LC analysis of nucleobases [69,71]). For the relatively volatile soluble organics discussed in this review (amines, monocarboxylic acids, aldehydes, and ketones), very few methodologies have been tested and the effects of each technique are poorly understood. Recent findings have shown that small changes to the derivatization protocol may have a significant impact on the types and abundances of meteoritic organics detected. For example, analyses of monocarboxylic acids as (S)-1-phenylethyl esters have yielded a substantially higher total abundance of monocarboxylic acids compared to other techniques [65], and analyses of monocarboxylic acids as (S)-2-methylbutyl ester derivatives have yielded a particularly high relative abundance of acetic acid (91% of the total monocarboxylic acid content) compared to other studies (13–28% of the total) [79] (Table 3). Similarly, differing techniques used for aldehyde and ketone analyses have been shown to produce notably distinct results. DCM extraction and DMB derivatization yielded a relatively higher abundance of formaldehyde, relatively 13C-enriched isotopic values for acetaldehyde and propionaldehyde and relatively 13C-depleted isotopic compositions for acetone and butyraldehyde, compared to measurements obtained via aqueous extraction and derivatization with PFBHA [66,114,121]. Thus, it may be advisable that a common methodology be used for analysis in order to allow direct comparisons of organics across meteorites, and direct comparisons of the same meteorite obtained through different sources.
6. Conclusions
This review compiles and summarizes the range of analytical techniques applied to studies of extraterrestrial amino acids and structurally related amines, monocarboxylic acids, aldehydes, and ketones, to help facilitate data comparisons between studies, and to provide guidance for method selections for future sample analyses. Potential sources of variability among datasets are discussed, including compositional heterogeneity of meteorite samples, and the influences of differing extraction and purification protocols, derivatization methods, and chromatographic separation techniques. With these factors in mind, some potential considerations to implement for future analyses may include: (1) maintaining consistency in methodologies applied across studies to allow for more informative cross-comparisons of datasets; (2) carrying out direct comparisons of methodologies during method development experiments in order to gain a better understanding of the various effects of different analytical techniques; and (3) reporting detailed meteorite sample information for each soluble organic study to track compositional variability within single meteorite specimens.
Funding
This research was supported in part by a NASA Postdoctoral Research Program fellowship at NASA Goddard Space Flight Center (administered by Universities Space Research Association under contract with NASA) to D.N.S., NASA’s Planetary Science Division Internal Scientist Funding Program through the Fundamental Laboratory Research (FLaRe) work package to J.E.E., the NASA Astrobiology Institute through funding awarded to the Goddard Center for Astrobiology under proposal 13-13NAI7-0032, and a grant from the Simons Foundation (SCOL award 302497 to J.P.D.
Acknowledgments
The authors would like to thank three anonymous reviewers for their careful review of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alexander, C.M.O.D.; Fogel, M.; Yabuta, H.; Cody, G.D. The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim. Cosmochim. Acta 2007, 71, 4380–4403. [Google Scholar] [CrossRef]
- Botta, O.; Bada, J.L. Extraterrestrial organic compounds in meteorites. Surv. Geophys. 2002, 23, 411–467. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cooper, G.W.; Flynn, G.J. The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In Meteorites and the Early Solar System II; Lauretta, D.S., Ed.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 625–651. [Google Scholar]
- Sephton, M.A. Organic geochemistry of meteorites. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: New York, NY, USA, 2014; Volume 12, pp. 1–31. [Google Scholar]
- Glavin, D.P.; Alexander, C.M.O.; Aponte, J.C.; Dworkin, J.P.; Elsila, J.E.; Yabuta, H. The origin and evolution of organic matter in carbonaceous chondrites and links to their parent bodies. In Primitive Meteorites and Asteroids; Abreu, N.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–271. [Google Scholar]
- Krot, A.N.; Keil, K.; Scott, E.R.D.; Goodrich, C.A.; Weisberg, M.K. Classification of meteorites and their genetic relationships. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: New York, NY, USA, 2014; Volume 1, pp. 1–63. [Google Scholar]
- Weisberg, M.K.; McCoy, T.J.; Krot, A.N. Systematics and evaluation of meteorite classification. In Meteorites and the Early Solar System II, 2nd ed.; Lauretta, D.S., McSween, H.Y., Jr., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 19–52. [Google Scholar]
- Berzelius, J. Ueber Meteorsteine IV. Meteorstein von Alais. Ann. Phys. Chem. 1834, 33, 113–148. [Google Scholar] [CrossRef]
- Nagy, B.; Meinschein, W.G.; Hennessy, D.J. Mass spectroscopic analysis of the Orgueil meteorite: Evidence for biogenic hydrocarbons. Ann. N. Y. Acad. Sci. 1961, 93, 27–35. [Google Scholar] [CrossRef]
- Mueller, G. The properties and theory of genesis of the carbonaceous complex within the Cold Bokevelt meteorite. Geochim. Cosmochim. Acta 1953, 4, 1–10. [Google Scholar] [CrossRef]
- Briggs, M.H. Organic constituents of meteorites. Nature 1961, 191, 1137–1140. [Google Scholar] [CrossRef]
- Meinschein, W.G. Benzene Extracts of the Orgueil Meteorite. Nature 1963, 197, 833–836. [Google Scholar] [CrossRef]
- Kvenvolden, K.; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C.; Kaplan, I.R.; Moore, C. Evidence for extraterrestrial amino acids and hydrocarbons in the Murchison meteorite. Nature 1970, 228, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Jungclaus, G.A.; Yuen, G.U.; Moore, C.B.; Lawless, J.G. Evidence for the presence of low molecular weight alcohols and carbonyl compounds in the Murchison meteorite. Meteoritics 1976, 11, 231–237. [Google Scholar] [CrossRef]
- Jungclaus, G.; Cronin, J.R.; Moore, C.B.; Yuen, G.U. Aliphatic amines in the Murchison meteorite. Nature 1976, 261, 126–128. [Google Scholar] [CrossRef]
- Cronin, J.R.; Gandy, W.E.; Pizzarello, S. Amino-Acids of the Murchison Meteorite: I. Six Carbon Acyclic Primary Alpha-Amino Alkanoic Acids. J. Mol. Evol. 1981, 17, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfreund, P.; Glavin, D.P.; Botta, O.; Cooper, G.; Bada, J.L. Extraterrestrial amino acids in Orgueil and Ivuna: Tracing the parent body of Cl type carbonaceous chondrites. Proc. Natl. Acad. Sci. USA 2001, 98, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Huang, Y.; Fuller, M. The carbon isotopic distribution of Murchison amino acids. Geochim. Cosmochim. Acta 2004, 68, 4963–4969. [Google Scholar] [CrossRef]
- Righter, K.; Corrigan, C.; McCoy, T.; Harvey, R. 35 Seasons of U.S. Antarctic Meteorites (1976–2010): A Pictorial Guide to the Collection; American Geophysical Union: Washington, DC, USA; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Kojima, H. The history of Japanese Antarctic meteorites. Geol. Soc. Lond. Spec. Publ. 2006, 256, 291–303. [Google Scholar] [CrossRef]
- Meteoritical Bulletin Database. Available online: https://www.lpi.usra.edu/meteor/ (accessed on 30 March 2019).
- Clemett, S.J.; Maechling, C.R.; Zare, R.N.; Swan, P.D.; Walker, R.M. Identification of complex aromatic molecules in individual interplanetary dust particles. Science 1993, 262, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Clemett, S.J.; Chillier, X.D.F.; Gillette, J.S.; Zare, R.N.; Maurette, M.; Engrand, C.; Kurat, G. Observation of indigenous polycyclic aromatic hydrocarbons in ‘giant’ carbonaceous Antarctic micrometeorites. Orig. Life Evol. Biosph. 1998, 28, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, G.; Pizzarello, S.; Taylor, S.; Brownlee, D. Concentration and variability of the AIB amino acid in polar micrometeorites: Implications for the exogenous delivery of amino acids to the primitive Earth. Meteorit. Planet. Sci. 2004, 39, 1849–1858. [Google Scholar] [CrossRef]
- Flynn, G.J.; Keller, L.P.; Feser, M.; Wirick, S.; Jacobsen, C. The origin of organic matter in the solar system: Evidence from the interplanetary dust particles. Geochim. Cosmochim. Acta 2003, 67, 4791–4806. [Google Scholar] [CrossRef]
- Brinton, K.L.F.; Engrand, C.; Glavin, D.P.; Bada, J.L.; Maurette, M. A search for extraterrestrial amino acids in carbonaceous Antarctic micrometeorites. Orig. Life Evol. Biosph. 1998, 28, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Matrajt, G.; Bada, J.L. Re-examination of amino acids in Antarctic micrometeorites. Adv. Space Res. 2004, 33, 106–113. [Google Scholar] [CrossRef]
- Parker, E.T.; Engrand, C.; Dworkin, J.P.; Glavin, D.P. Amino acids in Antarctic micrometeorites. In Proceedings of the American Geophyical Union Fall Meeting, Washington, DC, USA, 10–14 December 2018. [Google Scholar]
- Fox, S.W.; Harada, K.; Hare, P.E. Accumulated analyses of amino acid precursors in returned lunar samples. Proc. Fourth Lunar Sci. Conf. Geochim. Cosmochim. Acta 1973, 2 (Suppl. 4), 2241–2248. [Google Scholar]
- Gehrke, C.W.; Zumwalt, R.W.; Kuo, K.; Rash, J.J.; Aue, W.A.; Stalling, D.L.; Kvenvolden, K.A.; Ponnamperuma, C. Research for amino-acids in lunar samples. Space Life Sci. 1972, 3, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Hare, P.E.; Windsor, C.R.; Fox, S.W. Evidence for compounds hydrolyzable to amino acids in aqueous extracts of Apollo 11 and Apollo 12 lunar fines. Science 1971, 173, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Modzeleski, V.E.; Modzeleski, J.E.; Mohammed, M.A.; Nagy, L.A.; Nagy, B.; Mcewan, W.S.; Urey, H.C.; Hamilton, P.B. Carbon-compounds in pyrolysates and amino-acids in extracts of Apollo-14 lunar samples. Nat. Phys. Sci. 1973, 242, 50–52. [Google Scholar] [CrossRef]
- Nagy, B.; Drew, C.M.; Hamilton, P.B.; Modzeles, V.E.; Murphy, M.E.; Scott, W.M.; Urey, H.C.; Young, M. Organic compounds in lunar samples. Pyrolysis products, hydrocarbons, amino acids. Science 1970, 167, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Modzeleski, J.E.; Modzeleski, V.E.; Mohammad, M.A.J.; Nagy, L.A.; Scott, W.M.; Drew, C.M.; Thomas, J.E.; Ward, R.; Hamilton, P.B.; et al. Carbon compounds in Apollo 12 lunar samples. Nature 1971, 232, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.E.; Harada, K.; Fox, S.W. Analyses for amino acids in lunar fines. Proc. Apollo 11 Lunar Sci. Conf. Geochim. Et Cosmochim. Acta 1970, 2 (Suppl. 4), 1799–1803. [Google Scholar]
- Lauretta, D.S.; Balram-Knutson, S.S.; Beshore, E.; Boynton, W.V.; d’Aubigny, C.D.; DellaGiustina, D.N.; Enos, H.L.; Gholish, D.R.; Hergenrother, C.W.; Howell, E.S.; et al. OSIRIS-REx: Sample Return from Asteroid (101955) Bennu. Space Sci. Rev. 2017, 212, 925–984. [Google Scholar] [CrossRef]
- Naraoka, H.; Aoki, D.; Fukushima, K.; Uesugi, M.; Ito, M.; Kitajima, F.; Mita, H.; Yabuta, H.; Takano, Y.; Yada, T.; et al. ToF-SIMS analysis of carbonaceous particles in the sample catcher of the Hayabusa spacecraft. Earth Planets Space 2015, 67, 67. [Google Scholar] [CrossRef]
- Naraoka, H.; Mita, H.; Hamase, K.; Mita, M.; Yabuta, H.; Saito, K.; Fukushima, K.; Kitajima, F.; Sandford, S.A.; Nakamura, T.; et al. Preliminary organic compound analysis of microparticles returned from Asteroid 25143 Itokawa by the Hayabusa mission. Geochem. J. 2012, 46, 61–72. [Google Scholar] [CrossRef]
- Sandford, S.A.; Aleon, J.; Alexander, C.M.O.D.; Araki, T.; Bajt, S.; Baratta, G.A.; Borg, J.; Bradley, J.P.; Brownlee, D.E.; Brucato, J.R.; et al. Organics Captured from Comet 81P/Wild 2 by the Stardust Spacecraft. Science 2006, 314, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Dworkin, J.P.; Sandford, S.A. Detection of cometary amines in samples returned by Stardust. Meteorit. Planet. Sci. 2008, 43, 399–413. [Google Scholar] [CrossRef]
- Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Cometary glycine detected in samples returned by Stardust. Meteorit. Planet. Sci. 2009, 44, 1323–1330. [Google Scholar] [CrossRef]
- Creamer, J.S.; Mora, M.F.; Willis, P.A. Enhanced resolution of chiral amino acids with capillary electrophoresis for biosignature detection in extraterrestrial samples. Anal. Chem. 2016, 89, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Burk, M.J.; Martinez, J.E.; Feaster, J.E.; Cosford, N. Catalytic asymmetric reductive amination of ketones via highly enantioselective hydrogenation of the C-N double bond. Tetrahedron 1994, 50, 4399–4428. [Google Scholar] [CrossRef]
- Corey, E.J.; Gilman, N.W.; Ganem, B.E. New methods for the oxidation of aldehydes to carboxylic acids and esters. J. Am. Chem. Soc. 1968, 90, 5616–5617. [Google Scholar] [CrossRef]
- Peltzer, E.T.; Bada, J.L.; Schlesinger, G.; Miller, S.L. The chemical conditions on the parent body of the Murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Adv. Space Res. 1984, 4, 69–74. [Google Scholar] [CrossRef]
- Huber, C.; Wächtershäuser, G. Primordial reductive amination revisited. Tetrahedron Lett. 2003, 44, 1695–1697. [Google Scholar] [CrossRef]
- Cooper, G.W.; Cronin, J.R. Linear and cyclic aliphatic carboxamides of the Murchison meteorite: Hydrolyzable derivatives of amino acids and other carboxylic acids. Geochim. Cosmochim. Acta 1995, 59, 1003–1015. [Google Scholar] [CrossRef]
- Bada, J.L.; Glavin, D.P.; McDonald, G.D.; Becker, L. A search for endogenous amino acids in martian meteorite ALH84001. Science 1998, 279, 362–365. [Google Scholar] [CrossRef]
- Glavin, D.P.; Bada, J.L.; Brinton, K.L.F.; McDonald, G.D. Amino acids in the Martian meteorite Nakhla. Proc. Natl. Acad. Sci. USA 1999, 96, 8835–8838. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Dworkin, J.P.; Aubrey, A.; Botta, O.; Doty, J.H., III; Martins, Z.; Bada, J.L. Amino acid analyses of Antarctic CM2 meteorites using liquid chromatography-time of flight-mass spectrometry. Meteorit. Planet. Sci. 2006, 41, 889–902. [Google Scholar] [CrossRef]
- Burton, A.S.; Glavin, D.P.; Callahan, M.P.; Dworkin, J.P.; Jenniskens, P.; Shaddad, M.H. Heterogeneous distributions of amino acids provide evidence of multiple sources within the Almahata Sitta parent body, asteroid 2008 TC3. Meteorit. Planet. Sci. 2011, 46, 1703–1712. [Google Scholar] [CrossRef]
- Burton, A.S.; Glavin, D.P.; Elsila, J.E.; Dworkin, J.P.; Jenniskens, P.; Yin, Q.Z. The amino acid composition of the Sutter’s Mill CM2 carbonaceous chondrite. Meteorit. Planet. Sci. 2014, 49, 2074–2086. [Google Scholar] [CrossRef]
- Mcdonald, G.D.; Bada, J.L. A Search for Endogenous Amino-Acids in the Martian Meteorite Eeta79001. Geochim. Cosmochim. Acta 1995, 59, 1179–1184. [Google Scholar] [CrossRef]
- Herd, C.D.K.; Hilts, R.W.; Skelhorne, A.W.; Simkus, D.N. Cold curation of pristine astromaterials: Insights from the Tagish Lake meteorite. Meteorit. Planet. Sci. 2016, 51, 499–519. [Google Scholar] [CrossRef]
- Calaway, M.J.; Allen, C.C.; Allton, J.H. Organic Contamination Baseline Study in NASA Johnson Space Center Astromaterials Curation Laboratories; NASA Center for AeroSpace Information: Hanover, MD, USA, 2014.
- Dworkin, J.P.; Adelman, L.A.; Ajluni, T.; Andronikov, A.V.; Aponte, J.C.; Bartels, A.E.; Beshore, E.; Bierhaus, E.B.; Brucato, J.R.; Bryan, B.H.; et al. OSIRIS-REx Contamination Control Strategy and Implementation. Space Sci. Rev. 2017, 214, 19. [Google Scholar] [CrossRef] [PubMed]
- Elsila, J.E.; Callahan, M.P.; Glavin, D.P.; Dworkin, J.P.; Brueckner, H. Distribution and stable isotopic composition of amino acids from fungal peptaibiotics: Assessing the potential for meteoritic contamination. Astrobiology 2011, 11, 123–133. [Google Scholar] [CrossRef]
- Penzias, A.A. Nuclear processing and isotopes in the galaxy. Science 1980, 208, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Robert, F.; Epstein, S. The concentration and isotopic composition of hydrogen, carbon, and nitrogen in carbonaceous meteorites. Geochim. Cosmochim. Acta 1982, 46, 81–95. [Google Scholar] [CrossRef]
- Sandford, S.A.; Bernstein, M.P.; Dworkin, J.P. Assessment of the interstellar processes leading to deuterium enrichment in meteoritic organics. Meteorit. Planet. Sci. 2001, 36, 1117–1133. [Google Scholar] [CrossRef]
- Yuen, G.U.; Kvenvolden, K.A. Monocarboxylic acids in Murray and Murchison carbonaceous meteorites. Nature 1973, 246, 301–302. [Google Scholar] [CrossRef]
- Lawless, J.G.; Yuen, G.U. Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 1979, 282, 396–398. [Google Scholar] [CrossRef]
- Shimoyama, A.; Naraoka, H.; Yamamoto, H.; Harada, K. Carboxylic acids in the Yamato-791198 carbonaceous chondrites from Antarctica. Chem. Lett. 1986, 15, 1561–1564. [Google Scholar] [CrossRef]
- Naraoka, H.; Shimoyama, A.; Harada, K. Molecular distribution of monocarboxylic acids in Asuka carbonaceous chondrites from Antarctica. Orig. Life Evol. Biosph. 1999, 29, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Aponte, J.C.; Tarozo, R.; Alexandre, M.R.; Alexander, C.M.O.D.; Charnley, S.B.; Hallmann, C.; Summons, R.E.; Huang, Y. Chirality of meteoritic free and IOM-derived monocarboxylic acids and implications for prebiotic organic synthesis. Geochim. Cosmochim. Acta 2014, 131, 1–12. [Google Scholar] [CrossRef]
- Aponte, J.C.; Whitaker, D.; Powner, M.W.; Elsila, J.E.; Dworkin, J.P. Analyses of aliphatic aldehydes and ketones in carbonaceous chondrites. Acs Earth Space Chem. 2019, 3, 463–472. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Aliphatic hydrocarbons of the Murchison meteorite. Geochim. Cosmochim. Acta 1990, 54, 2859–2868. [Google Scholar] [CrossRef]
- Krishnamurthy, R.V.; Epstein, S.; Cronin, J.R.; Pizzarello, S.; Yuen, G.U. Isotopic and molecular analyses of hydrocarbons and monocarboxylic acids of the Murchison meteorite. Geochim. Cosmochim. Acta 1992, 56, 4045–4058. [Google Scholar] [CrossRef]
- Callahan, M.P.; Smith, K.E.; Cleaves, H.J.; Ruzicka, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc. Natl. Acad. Sci. USA 2011, 108, 13995–13998. [Google Scholar] [CrossRef]
- Hilts, R.W.; Herd, C.D.K.; Simkus, D.N.; Slater, G.F. Soluble organic compounds in the Tagish Lake meteorite. Meteorit. Planet. Sci. 2014, 49, 526–549. [Google Scholar] [CrossRef]
- Martins, Z.; Botta, O.; Fogel, M.L.; Sephton, M.A.; Glavin, D.P.; Watson, J.S.; Dworkin, J.P.; Schwartz, A.W.; Ehrenfreund, P. Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet. Sci. Lett. 2008, 270, 130–136. [Google Scholar] [CrossRef]
- Martins, Z.; Modica, P.; Zanda, B.; d’Hendecourt, L.L.S. The amino acid and hydrocarbon contents of the Paris meteorite: Insights into the most primitive CM chondrite. Meteorit. Planet. Sci. 2015, 50, 926–943. [Google Scholar] [CrossRef]
- Huang, Y.; Aponte, J.C.; Zhao, J.; Tarozo, R.; Hallmann, C. Hydrogen and carbon isotopic ratios of polycyclic aromatic compounds in two CM2 carbonaceous chondrites and implications for prebiotic organic synthesis. Earth Planet. Sci. Lett. 2015, 426, 101–108. [Google Scholar] [CrossRef]
- Pizzarello, S.; Schrader, D.L.; Monroe, A.A.; Lauretta, D.S. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 11949–11954. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Wang, Y.; Alexandre, M.R.; Lee, T.; Rose-Petruck, C.; Fuller, M.; Pizzarello, S. Molecular and compound-specific isotopic characterization of monocarboxylic acids in carbonaceous meteorites. Geochim. Cosmochim. Acta 2005, 69, 1073–1084. [Google Scholar] [CrossRef]
- Pizzarello, S.; Huang, Y.; Alexandre, M.R. Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proc. Natl. Acad. Sci. USA 2008, 105, 3700–3704. [Google Scholar] [CrossRef] [PubMed]
- Herd, C.D.K.; Blinova, A.; Simkus, D.N.; Huang, Y.S.; Tarozo, R.; Alexander, C.M.O.; Gyngard, F.; Nittler, L.R.; Cody, G.D.; Fogel, M.L.; et al. Origin and evolution of prebiotic organic matter as inferred from the Tagish Lake meteorite. Science 2011, 332, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Aponte, J.C.; Alexandre, M.R.; Wang, Y.; Brearley, A.J.; Alexander, C.M.O.D.; Huang, Y. Effects of secondary alteration on the composition of free and IOM-derived monocarboxylic acids in carbonaceous chondrites. Geochim. Cosmochim. Acta 2011, 75, 2309–2323. [Google Scholar] [CrossRef]
- Aponte, J.C.; Woodward, H.K.; Abreu, N.M.; Elsila, J.E.; Dworkin, J.P. Molecular distribution, 13C-isotope, and enantiomeric compositions of carbonaceous chondrite monocarboxylic acids. Meteorit. Planet. Sci. 2019, 54, 415–430. [Google Scholar] [CrossRef]
- Dillon, J.T.; Tarozo, R.; Yin, Q.; Huang, Y. Analysis of carboxylic acid compounds in the Sutter’s Mill meteorite. In Proceedings of the 44th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 18–22 March 2013. [Google Scholar]
- Cronin, J.R. Acid-labile amino acid precursors in the Murchison meteorite I: Chromatographic fractionation. Orig. Life 1976, 7, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Aponte, J.C.; Dworkin, J.P.; Elsila, J.E. Assessing the origins of aliphatic amines in the Murchison meteorite from their compound-specific carbon isotopic ratios and enantiomeric composition. Geochim. Cosmochim. Acta 2014, 141, 331–345. [Google Scholar] [CrossRef]
- Glavin, D.P.; Elsila, J.E.; Burton, A.S.; Callahan, M.P.; Dworkin, J.P.; Hilts, R.W.; Herd, C.D.K. Unusual nonterrestrial L-proteinogenic amino acid excesses in the Tagish Lake meteorite. Meteorit. Planet. Sci. 2012, 47, 1347–1364. [Google Scholar] [CrossRef]
- Burton, A.S.; Elsila, J.E.; Hein, J.E.; Glavin, D.P.; Dworkin, J.P. Extraterrestrial amino acids identified in metal-rich CH and CB carbonaceous chondrites from Antarctica. Meteorit. Planet. Sci. 2013, 48, 390–402. [Google Scholar] [CrossRef]
- Cronin, J.R.; Moore, C.B. Amino acid analyses of the Murchison, Murray, and Allende carbonaceous chondrites. Science 1971, 172, 1327–1329. [Google Scholar] [CrossRef] [PubMed]
- Pollock, G.E.; Cheng, C.N.; Cronin, S.E.; Kvenvolden, K.A. Stereoisomers of isovaline in the Murchison meteorite. Geochim. Cosmochim. Acta 1975, 39, 1571–1573. [Google Scholar] [CrossRef]
- Peltzer, E.T.; Bada, J.L. Alpha-hydroxycarboxylic acids in the Murchison meteorite. Nature 1978, 272, 443–444. [Google Scholar] [CrossRef]
- Glavin, D.P.; Bada, J. Isolation of amino acids from natural samples using sublimation. Anal. Chem. 1998, 70, 3119–3122. [Google Scholar] [CrossRef]
- Nakaparksin, S.; Gilav, E.; Oro, J. Study of the racemization of some neutral a-amino acids in acid solution using gas chromatographic techniques. Anal. Biochem. 1970, 33, 374–382. [Google Scholar] [CrossRef]
- Jungbauer, A.; Hahn, R. Ion-Exchange Chromatography. In Methods in Enzymology Guide to Protein Purification, 2nd ed.; Burgess, R.R., Deutscher, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; Volume 463, pp. 349–371. [Google Scholar]
- Burton, A.S.; Stern, J.C.; Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 2012, 41, 5459–5472. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cronin, J.R. Non-racemic amino acids in the Murray and Murchison meteorites. Geochim. Cosmochim. Acta 2000, 64, 329–338. [Google Scholar] [CrossRef]
- Amelung, W.; Zhang, X. Determination of amino acid enantiomers in soils. Soil Biol. Biochem. 2001, 33, 553–562. [Google Scholar] [CrossRef]
- Meierhenrich, U.J.; Muñoz Caro, G.M.; Bredehöft, J.H.; Jessberger, E.K.; Thiemann, W.H.-P. Identification of diamino acids in the Murchison meteorite. Proc. Natl. Acad. Sci. USA 2004, 101, 9182–9186. [Google Scholar] [CrossRef] [PubMed]
- Myrgorodska, I.; Meinert, C.; Martins, Z.; d’Hendecourt, L.L.; Meierhenrich, U.J. Quantitative enantioseparation of amino acids by comprehensive two-dimensional gas chromatography applied to non-terrestrial samples. J. Chromatogr. A 2016, 1433, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.H.; Nagy, B. Distribution and enantiomeric composition of amino acids in the Murchison meteorite. Nature 1982, 296, 837–840. [Google Scholar] [CrossRef]
- Engel, M.H.; Macko, S.A.; Silfer, J.A. Carbon isotope composition of individual amino acids in the Murchison meteorite. Nature 1990, 348, 47–49. [Google Scholar] [CrossRef]
- Engel, M.H.; Macko, S.A. Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature 1997, 389, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Chiesl, T.N.; Chu, W.K.; Stockton, A.M.; Amashukeli, X.; Grunthaner, F.; Mathies, R.A. Enhanced Amine and Amino Acid Analysis Using Pacific Blue and the Mars Organic Analyzer Microchip Capillary Electrophoresis System. Anal. Chem. 2009, 81, 2537–2544. [Google Scholar] [CrossRef]
- Myrgorodska, I.; Javelle, T.; Meinert, C.; Meierhenrich, U.J. Enantioselective gas chromatography in search of the origin of biomolecular asymmetry in outer space. Isr. J. Chem. 2016, 56, 1016–1026. [Google Scholar] [CrossRef]
- Pizzarello, S. Identifying chiral molecules and their enantiomeric excesses in extraterrestrial samples: An experimental journey. Isr. J. Chem. 2016, 56, 1027–1035. [Google Scholar] [CrossRef]
- Burton, A.S.; Berger, E.L. Insights into abiotically-generated amino acid enantiomeric excesses found in meteorites. Life 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Sephton, M.A.; Gilmour, I. Compound-specific isotope analysis of the organic constituents in carbonaceous chondrites. Mass Spectrom. Rev. 2001, 20, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Eiler, J.M.; Cesar, J.; Chimiak, L.; Dallas, B.; Grice, K.; Griep-Raming, J.; Juchelka, D.; Kitchen, N.; Lloyd, M.; Makarov, A.; et al. Analysis of molecular isotopic structures at high precision and accuracy by Orbitrap mass spectrometry. Int. J. Mass Spectrom. 2017, 422, 126–142. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Amino acids in meteorites. Adv. Space Res. 1983, 3, 5–18. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Amino-Acids of the Murchison Meteorite. III. Seven Carbon Acyclic Primary Alpha-Amino Alkanoic Acids. Geochim. Cosmochim. Acta 1986, 50, 2419–2427. [Google Scholar] [CrossRef]
- Lamkin, W.M.; Gehrke, C.W. Quantitative gas chromatography of amino acids—Preparation of n-butyl N-trifluoroacetyl esters. Anal. Chem. 1965, 37, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Pereira, W.E.; Summons, R.E.; Rindfleisch, T.C.; Duffield, A.M.; Zeitman, B.; Lawless, J.G. Stable isotope mass fragmentography: Identification and hydrogen-deuterium exchange studies of eight Murchison meteorite amino acids. Geochim. Cosmochim. Acta 1975, 39, 163–172. [Google Scholar] [CrossRef]
- Mengerink, Y.; Kutlan, D.; Toth, F.; Csampai, A.; Molnar-Perl, I. Advances in the evaluation of the stability and characteristics of the amino acid and amine derivatives obtained with the o-phthaldialdehyde/3-mercaptopropionic acid and o-phthaldialdehyde/N-acetyl-l-cysteine reagents—High-performance liquid chromatography-mass spectrometry study. J. Chromatogr. A 2002, 949, 99–124. [Google Scholar] [CrossRef]
- Nuevo, M.; Auger, G.; Blanot, D.; d’Hendecourt, L. A detailed study of the amino acids produced from the vacuum UV irradiation of interstellar ice analogs. Orig. Life Evol. Biosph. 2008, 38, 37–56. [Google Scholar] [CrossRef]
- Salazar, C.; Armenta, J.M.; Cortes, D.F.; Shulaev, V. Combination of an AccQ·Tag-Ultra Performance Liquid Chromatographic Method with Tandem Mass Spectrometry for the Analysis of Amino Acids. In Amino Acid Analysis—Methods and Protocols; Alterman, M., Hunziker, P., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 828. [Google Scholar]
- Boogers, I.; Plugge, W.; Stokkermans, Y.Q.; Duchateau, A.L.L. Ultra-performance liquid chromatographic analysis of amino acids in protein hydrolysates using an automated pre-column derivatisation method. J. Chromatogr. A 2008, 1189, 406–409. [Google Scholar] [CrossRef]
- Pizzarello, S.; Feng, X.; Epstein, S.; Cronin, J.R. Isotopic analyses of nitrogenous compounds from the Murchison meteorite: Ammonia, amines, amino acids, and polar hydrocarbons. Geochim. Cosmochim. Acta 1994, 58, 5579–5587. [Google Scholar] [CrossRef]
- Pizzarello, S.; Holmes, W. Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim. Cosmochim. Acta 2009, 73, 2150–2162. [Google Scholar] [CrossRef]
- Monroe, A.A.; Pizzarello, S. The soluble organic compounds of the Bells meteorite: Not a unique or unusual composition. Geochim. Cosmochim. Acta 2011, 75, 7585–7595. [Google Scholar] [CrossRef]
- Pizzarello, S.; Yarnes, C.T. Enantiomeric excesses of chiral amines in ammonia-rich carbonaceous meteorites. Earth Planet. Sci. Lett. 2016, 443, 176–184. [Google Scholar] [CrossRef]
- Pizzarello, S.; Yarnes, C.T. The soluble organic compounds of the Mukundpura meteorite: A new CM chondrite fall. Planet Space Sci. 2018, 164, 127–131. [Google Scholar] [CrossRef]
- Yuen, G.; Blair, N.; Marais, D.J.D.; Chang, S. Carbon isotope composition of low molecular weight hydrocarbons and monocarboxylic acids from Murchison meteorite. Nature 1984, 307, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Simkus, D.N.; Aponte, J.C.; Elsila, J.E.; Hilts, R.W.; McLain, H.L.; Herd, C.D.K. New insights into the heterogeneity of the Tagish Lake meteorite: Soluble organic compositions of variously altered specimens. Meteorit. Planet. Sci. 2019. [Google Scholar] [CrossRef]
- Huang, Y.; Alexandre, M.R.; Wang, Y. Structure and isotopic ratios of aliphatic side chains in the insoluble organic matter of the Murchison carbonaceous chondrite. Earth Planet. Sci. Lett. 2007, 259, 517–525. [Google Scholar] [CrossRef]
- Simkus, D.N.; Aponte, J.C.; Hilts, R.W.; Elsila, J.E.; Herd, C.D.K. Compound-specific carbon isotope compositions of aldehydes and ketones in the Murchison meteorite. Meteorit. Planet. Sci. 2019, 54, 142–156. [Google Scholar] [CrossRef]
- Glavin, D.P.; Callahan, M.P.; Dworkin, J.P.; Elsila, J.E. The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 2011, 45, 1948–1972. [Google Scholar] [CrossRef]
- Seargent, D.A.J. The Murchison meteorite—Circumstances of its fall. Meteoritics 1990, 25, 341–342. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Glavin, D.P.; Bada, J. Extraterrestrial amino acids in the Murchison meteorite: Re-evaluation after thirty years. In Perspectives in Amino Acid and Protein Geochemistry; Goodfriend, G.A., Collins, M.J., Fogel, M.L., Macko, S.A., Wehmiller, J.F., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 7–14. [Google Scholar]
- Friedrich, J.M.; McLain, H.L.; Dworkin, J.P.; Glavin, D.P.; Towbin, W.H.; Hill, M.; Ebel, D.S. Effect of polychromatic X-ray microtomography imaging on the amino acid content of the Murchison CM chondrite. Meteorit. Planet. Sci. 2019, 54, 220–228. [Google Scholar] [CrossRef]
- Callahan, M.P.; Martin, M.G.; Burton, A.S.; Glavin, D.P.; Dworkin, J.P. Amino acid analysis in micrograms of meteorite sample by nanoliquid chromatography-high-resolution mass spectrometry. J. Chromatogr. A 2014, 1332, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.S.; Berger, E.L.; Locke, D.R.; Lewis, E.K. Correlated amino acid and mineralogical analyses of milligram and submilligram samples of carbonaceous chondrite Lonewolf Nunataks 94101. In Proceedings of the 49th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 19–23 March 2018. [Google Scholar]
- Simkus, D.N.; Goreva, Y.S.; McCoy, T.J.; Herd, C.D.K. TOF-SIMS analysis of prebiotic organic compounds in the Murchison meteorite. In Proceedings of the 46th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 16–20 March 2015. [Google Scholar]
- Hashiguchi, M.; Naraoka, H. High-mass resolution molecular imaging of organic compounds on the surface of Murchison meteorite. Meteorit. Planet. Sci. 2019, 54, 452–468. [Google Scholar] [CrossRef]
- Yesiltas, M.; Kebukawa, Y. Associations of organic matter with minerals in Tagish Lake meteorite via high spatial resolution synchrotron-based FTIR microspectroscopy. Meteorit. Planet. Sci. 2016, 51, 584–595. [Google Scholar] [CrossRef]
- Mita, H.; Shimoyama, A. Alpha-aminoisobutyric acid and isovaline in Tokyo Bay sediments. Geochim. Cosmochim. Acta 1998, 62, 47–50. [Google Scholar] [CrossRef]
- Glavin, D.P.; Brinkerhoff, W.; Dworkin, J.P.; Eigenbrode, J.; Franz, H.; Mahaffy, P.; Stern, J.; Allamandola, L.; Blake, D.; Sandford, S.A.; et al. Astrobiology Sample Analysis Program (ASAP) for advanced life detection instrumentation development and calibration. Astrobiology 2008, 8, 297. [Google Scholar]
- Peterson, E.; Horz, F.; Chang, S. Modification of amino acids at shock pressures of 3.5 to 32 GPa. Geochim. Cosmochim. Acta 1997, 61, 3937–3950. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Lawless, J.G.; Ponnamperuma, C. Nonprotein amino acids in Murchison meteorite. Proc. Natl. Acad. Sci. USA 1971, 68, 486–490. [Google Scholar] [CrossRef]
- Oró, J.; Gibert, J.; Lichtenstein, H.; Wikstrom, S.; Flory, D.A. Amino-acids, aliphatic and aromatic hydrocarbons in the Murchison meteorite. Nature 1971, 230, 105–106. [Google Scholar] [CrossRef]
- Oro, J.; Nakaparksin, S.; Lichtenstein, H.; Gilav, E. Configuration of amino-acids in carbonaceous chondrites and a Pre-Cambrian chert. Nature 1971, 230, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Lawless, J.G.; Kvenvolden, K.A.; Peterson, E.; Ponnamperuma, C.; Moore, C. Amino acids indigenous to Murray meteorite. Science 1971, 173, 626–627. [Google Scholar] [CrossRef] [PubMed]
- Lawless, J.G.; Kvenvolden, K.A.; Peterson, E.; Ponnamperuma, C.; Jarosewich, E. Physical sciences: Evidence for amino-acids of extraterrestrial origin in the Orgueil meteorite. Nature 1972, 236, 66–67. [Google Scholar] [CrossRef]
- Lawless, J.G.; Peterson, E. Amino-acids in carbonaceous chondrites. Orig. Life Evol. Biosph. 1975, 6, 3–8. [Google Scholar] [CrossRef]
- Kotra, R.K.; Shimoyama, A.; Ponnamperuma, C.; Hare, P.E. Amino acids in a carbonaceous chondrite from Antarctica. J. Mol. Evol. 1979, 13, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, A.; Ponnamperuma, C.; Yanai, K. Amino acids in the Yamato-74662 meteorite, an Antarctic carbonaceous chondrite. Proc. Fourth Symp. Antarct. Meteor. 1979, 15, 196–205. [Google Scholar]
- Shimoyama, A.; Harada, K.; Yanai, K. Amino acids from the Yamato-791198 carbonaceous chondrite from Antarctica. Chem. Lett. 1985, 14, 1183–1186. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S.; Yuen, G.U. Amino-Acids of the Murchison Meteorite: II. Five Carbon Acyclic Primary Beta-Amino, Gamma-Amino and Delta-Amino Alkanoic Acids. Geochim. Cosmochim. Acta 1985, 49, 2259–2265. [Google Scholar] [CrossRef]
- Shimoyama, A.; Harada, K. Amino acid depleted carbonaceous chondrites (C2) from Antarctica. Geochem. J. 1984, 18, 281–286. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–955. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cooper, G.W. Molecular and chiral analyses of some protein amino acid derivatives in the Murchison and Murray meteorites. Meteorit. Planet. Sci. 2001, 36, 897–909. [Google Scholar] [CrossRef]
- Shimoyama, A.; Ogasawara, R. Dipeptides and diketopiperazines in the Yamato-791198 and Murchison carbonaceous chondrites. Orig. Life Evol. Biosph. 2002, 32, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Zolensky, M.; Turk, K.A. Nonracemic isovaline in the Murchison meteorite: Chiral distribution and mineral association. Geochim. Cosmochim. Acta 2003, 67, 1589–1595. [Google Scholar] [CrossRef]
- Pizzarello, S.; Huang, Y.S. The deuterium enrichment of individual amino acids in carbonaceous meteorites: A case for the presolar distribution of biomolecule precursors. Geochim. Cosmochim. Acta 2005, 69, 5163. [Google Scholar] [CrossRef]
- Chan, H.S.; Martins, Z.; Sephton, M.A. Amino acid analyses of type 3 chondrites Colony, Ornans, Chainpur, and Bishunpur. Meteorit. Planet. Sci. 2012, 47, 1502–1516. [Google Scholar] [CrossRef]
- Koga, T.; Naraoka, H. A new family of extraterrestrial amino acids in the Murchison meteorite. Sci. Rep. 2017, 7, 636. [Google Scholar] [CrossRef]
- Zenobi, R.; Philippoz, J.-M.; Zare, R.N.; Wing, M.R.; Bada, J.L.; Marti, K. Organic compounds in the Forest Vale, H4 ordinary chondrite. Geochim. Cosmochim. Acta 1992, 56, 2899–2905. [Google Scholar] [CrossRef]
- Bruckner, H.; Haasmann, S.; Langer, M.; Westhauser, T.; Wittner, R.; Godel, H. Liquid chromatographic determination of d- and l-amino acids by derivatization with o-phthaldialdehyde and chiral thiols. J. Chromatogr. A 1994, 666, 259–273. [Google Scholar] [CrossRef]
- Glavin, D.P.; Aubrey, A.D.; Callahan, M.P.; Dworkin, J.P.; Elsila, J.E.; Parker, E.T.; Bada, J.L.; Jenniskens, P.; Shaddad, M.H. Extraterrestrial amino acids in the Almahata Sitta meteorite. Meteorit. Planet. Sci. 2010, 45, 1695–1709. [Google Scholar] [CrossRef]
- Hutt, L.D.; Glavin, D.P.; Bada, J.L.; Mathies, R.A. Microfabricated capillary electrophoresis amino acid chirality analyzer for extraterrestrial exploration. Anal. Chem. 1999, 71, 4000–4006. [Google Scholar] [CrossRef]
- Glavin, D.P.; Bada, J.L. Survival of amino acids in micrometeorites during atmospheric entry. Astrobiology 2001, 1, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Botta, O.; Glavin, D.P.; Kminek, G.; Bada, J.L. Relative amino acid concentrations as a signature for parent body processes of carbonaceous chondrites. Orig. Life Evol. Biosph. 2002, 32, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Botta, O.; Martins, Z.; Ehrenfreund, P. Amino acids in Antarctic CM1 meteorites and their relationship to other carbonaceous chondrites. Meteorit. Planet. Sci. 2007, 42, 81–92. [Google Scholar] [CrossRef]
- Botta, O.; Martins, Z.; Emmenegger, C.; Dworkin, J.P.; Glavin, D.P.; Harvey, R.P.; Zenobi, R.; Bada, J.; Ehrenfreund, P. Polycyclic aromatic hydrocarbons and amino acids in meteorites and ice samples from LaPaz icefield, Antarctica. Meteorit. Planet. Sci. 2008, 43, 1465–1480. [Google Scholar] [CrossRef]
- Kminek, G.; Botta, O.; Glavin, D.P.; Bada, J.L. Amino acids in the Tagish Lake meteorite. Meteorit. Planet. Sci. 2002, 37, 697–701. [Google Scholar] [CrossRef]
- Martins, Z.; Hofmann, B.A.; Greenwood, R.C.; Verchovsky, A.B.; Franchi, I.A.; Jull, A.J.T.; Botta, O.; Glavin, D.P.; Dworkin, J.P.; Ehrenfreund, P. Amino acid composition, petrology, geochemistry, 14C terrestrial age, and oxygen isotopes of the Shisr 033 CR chondrite. Meteorit. Planet. Sci. 2007, 42, 1581–1595. [Google Scholar] [CrossRef]
- Martins, Z.; Alexander, C.M.O.D.; Orzechowska, G.E.; Fogel, M.L.; Ehrenfreund, P. Indigenous amino acids in primitive CR meteorites. Meteorit. Planet. Sci. 2007, 42, 2125–2136. [Google Scholar] [CrossRef]
- Glavin, D.P.; Dworkin, J.P. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. USA 2009, 106, 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.S.; Elsila, J.E.; Callahan, M.P.; Martin, M.G.; Glavin, D.P.; Johnson, N.M.; Dworkin, J.P. A propensity for n-ω-amino acids in thermally altered Antarctic meteorites. Meteorit. Planet. Sci. 2012, 47, 374–386. [Google Scholar] [CrossRef]
- Burton, A.S.; Grunsfeld, S.; Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. The effects of parent-body hydrothermal heating on amino acid abundances in CI-like chondrites. Polar Sci. 2014, 8, 255–263. [Google Scholar] [CrossRef]
- Burton, A.S.; McLain, H.; Glavin, D.P.; Elsila, J.E.; Davidson, J.; Miller, K.E.; Andronikov, A.V.; Lauretta, D.; Dworkin, J.P. Amino acid analyses of R and CK chondrites. Meteorit. Planet. Sci. 2015, 50, 470–482. [Google Scholar] [CrossRef]
- Callahan, M.P.; Burton, A.S.; Elsila, J.E.; Baker, E.M.; Smith, K.E.; Glavin, D.P.; Dworkin, J.P. A search for amino acids and nucleobases in the Martian meteorite Roberts Massif 04262 using liquid chromatography-mass spectrometry. Meteorit. Planet. Sci. 2013, 48, 786–795. [Google Scholar] [CrossRef]
- Chan, Q.H.S.; Zolensky, M.E.; Kebukawa, Y.; Fries, M.; Ito, M.; Steele, A.; Rahman, Z.; Nakato, A.; Kilcoyne, A.L.D.; Suga, H.; et al. Organic matter in extraterrestrial water-bearing salt crystals. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Chan, Q.H.S.; Nakato, A.; Kebukawa, Y.; Zolensky, M.E.; Nakamura, T.; Maisano, J.A.; Colbert, M.W.; Martinez, J.E.; Kilcoyne, A.L.D.; Suga, H.; et al. Heating experiments of the Tagish Lake meteorite: Investigation of the effects of short-term heating on chondritic organics. Meteorit. Planet. Sci. 2019, 54, 104–125. [Google Scholar] [CrossRef]
- Cronin, J.R.; Moore, C.B. Amino acids of the Nogoya and Mokoia carbonaceous chondrites. Geochim. Cosmochim. Acta 1976, 40, 853–857. [Google Scholar] [CrossRef]
- Cronin, J.R. Acid-labile amino acid precursors in the Murchison meteorite II: A search for peptides and amino acyl amides. Orig. Life 1976, 7, 343–348. [Google Scholar] [CrossRef]
- Epstein, S.; Krishnamurthy, R.V.; Cronin, J.R.; Pizzarello, S.; Yuen, G.U. Unusual stable isotope ratios in amino acids and carboxylic acid extracts from the Murchison meteorite. Nature 1987, 326, 477–479. [Google Scholar] [CrossRef]
- Pizzarello, S.; Krishnamurthy, R.V.; Epstein, S.; Cronin, J.R. Isotopic analyses of amino acids from the Murchison meteorite. Geochim. Cosmochim. Acta 1991, 55, 905–910. [Google Scholar] [CrossRef]
- Lerner, N.R.; Cooper, G.W. Iminodicarboxylic acids in the Murchison meteorite: Evidence of Strecker reactions. Geochim. Cosmochim. Acta 2005, 69, 2901–2906. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S.; Moore, C.B. Amino-acids in an Antarctic carbonaceous chondrite. Science 1979, 206, 335–337. [Google Scholar] [CrossRef]
- Aponte, J.C.; Dworkin, J.P.; Elsila, J.E. Indigenous aliphatic amines in the aqueously altered Orgueil meteorite. Meteorit. Planet. Sci. 2015, 50, 1733–1749. [Google Scholar] [CrossRef]
- Aponte, J.C.; McLain, H.L.; Dworkin, J.P.; Elsila, J.E. Aliphatic amines in Antarctic CR2, CM2, and CM1/2 carbonaceous chondrites. Geochim. Cosmochim. Acta 2016, 189, 296–311. [Google Scholar] [CrossRef]
- Aponte, J.C.; Abreu, N.M.; Glavin, D.P.; Dworkin, J.P.; Elsila, J.E. Distribution of aliphatic amines in CO, CV, and CK carbonaceous chondrites and relation to mineralogy and processing history. Meteorit. Planet. Sci. 2017, 52, 2632–2646. [Google Scholar] [CrossRef]
- Aponte, J.C.; Abreu, N.M.; Keller, L.P.; Elsila, J.E.; Dworkin, J.P. Soluble amines in anomalous CR chondrite Miller Range (MIL) 090001. In Proceedings of the 49th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 19–23 March 2018. [Google Scholar]
- Shimoyama, A.; Naraoka, H.; Komiya, M.; Harada, K. Analyses of carboxylic acids and hydrocarbons in Antarctic carbonaceous chondrites, Yamato-74662 and Yamato-793321. Geochem. J. 1989, 23, 181–193. [Google Scholar] [CrossRef]
- Briscoe, J.F.; Moore, C.B. Determination of formic acid and acetic acid in chondritic meteorites. Meteoritics 1993, 28, 330. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).