Productivity and Community Composition of Low Biomass/High Silica Precipitation Hot Springs: A Possible Window to Earth’s Early Biosphere?
Abstract
:1. Introduction
2. Methods
2.1. Geochemistry
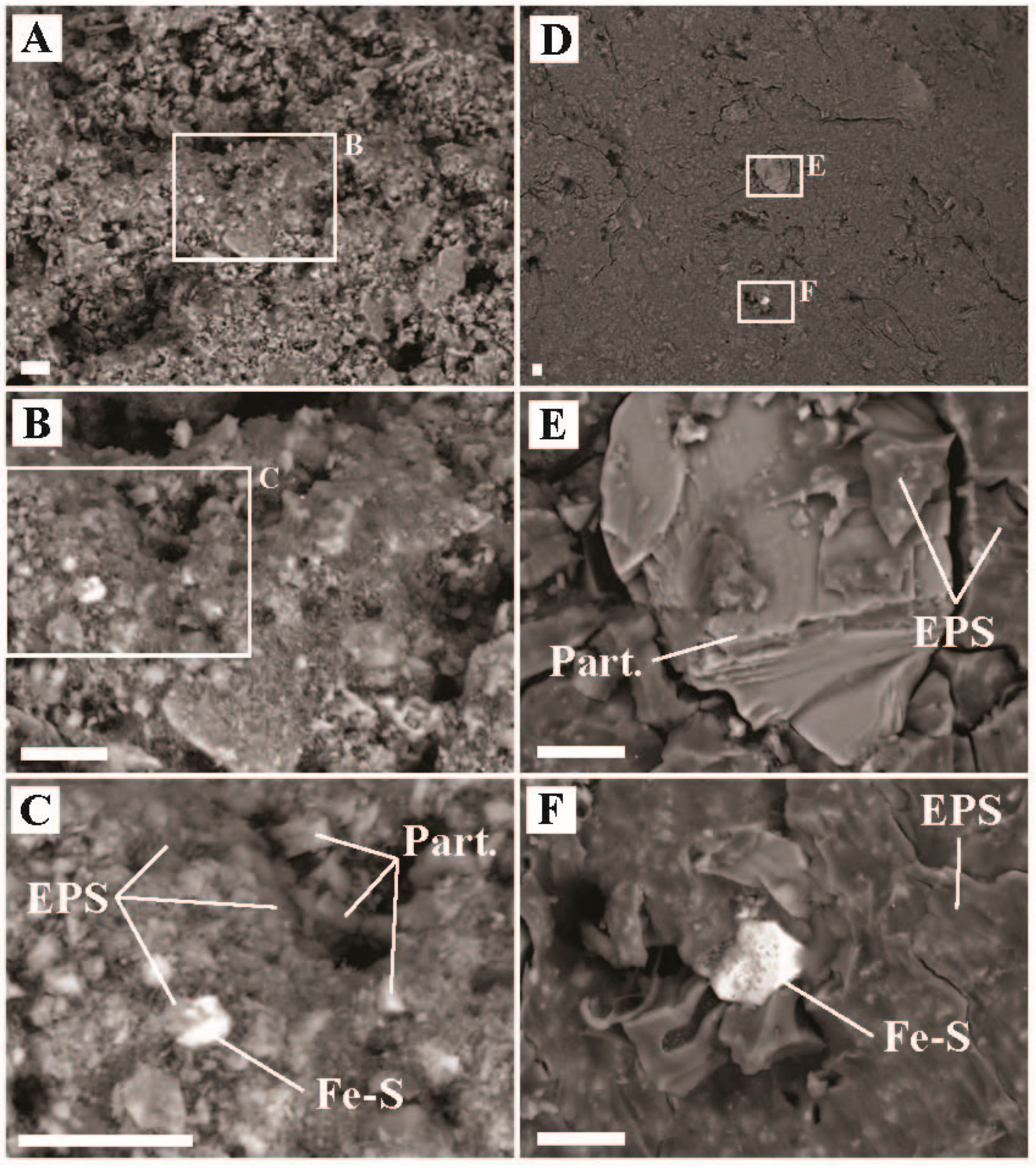
2.2. Imaging and Elemental Quantification via SEM-EDX
2.3. Photoassimilation of CO2 and Analysis of Solid Samples for 13C and 15N
2.4. DNA Extraction and Quantification
2.5. Sequence Analysis
3. Results
3.1. Site Descriptions
3.2. Rabbit Creek Area, MGB
3.3. Imperial Geyser Basin, LGB
3.4. Sylvan Spring Area, GGB
3.5. Geochemistry
3.6. Inorganic Carbon Uptake and Carbon and Nitrogen Stable Isotopes
3.7. Microbial Community Composition
4. Discussion
4.1. Acidic Site (‘Heartbeat Pool’)—Low Biomass, Low pH
4.2. Alkaline Sites (Boulder Geyser and ‘Rose Terrace Pool’)
4.3. Circum-Neutral Sites (Dante’s Inferno, ‘Avocado Spring’, ‘The Dryer’)
4.4. Chemoautotrophy
4.5. Photoautotrophy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Djokic, T.; Van Kranendonk, M.J.; Campbell, K.A.; Walter, M.R.; Ward, C.R. Earliest signs of life on land preserved in ca. 3.5 Ga hot spring deposits. Nat. Commun. 2017, 8, 15263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djokic, T.; Van Krandendonk, M.J.; Campbell, K.A.; Havig, J.R.; Walter, M.R.; Guido, D.M. A reconstructed subaerial hot spring field in the 3.5 billion-year-old Dresser Formation, North Pole Dome, Pilbara Craton, Western Australia. Astrobiology 2019. in review. [Google Scholar]
- Squyres, S.W.; Arvidson, R.E.; Ruff, S.; Gellert, R.; Morris, R.V.; Ming, D.W.; Crumpler, L.; Farmer, J.D.; Marais, D.J.D.; Yen, A.; et al. Detection of Silica-Rich Deposits on Mars. Science 2008, 320, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, R.E.; Bell, J.F., III; Bellutta, P.; Cabrol, N.A.; Catalano, J.G.; Cohen, J.; Crumpler, L.S.; Des Marais, D.J.; Estlin, T.A.; Farrand, W.H.; et al. Spirit Mars Rover Mission: Overview and selected results from the northern Home Plate Winter Haven to the side of Scamander crater. J. Geophys. Res. Planet. 2010, 115, E7. [Google Scholar] [CrossRef]
- Rice, M.S.; Bell, J.F., III; Cloutis, E.A.; Wang, A.; Ruff, S.W.; Craig, M.A.; Bailey, D.T.; Johnson, J.R.; de Souza, P.A., Jr.; Farrand, W.H. Silica-rich deposits and hydrated minerals at Gusev Crater, Mars: Vis-NIR spectral characterization and regional mapping. Icarus 2010, 205, 375–395. [Google Scholar] [CrossRef]
- Skok, J.R.; Mustard, J.F.; Ehlmann, B.L.; Milliken, R.E.; Murchie, S.L. Silica deposits in the Nili Patera caldera on the Syrtis Major volcanic complex on Mars. Nat. Geosci. 2010, 3, 838–841. [Google Scholar] [CrossRef] [Green Version]
- Schwenzer, S.P.; Abramov, O.; Allen, C.; Bridges, J.; Clifford, S.; Filiberto, J.; Kring, D.; Lasue, J.; McGovern, P.; Newsom, H.; et al. Gale Crater: Formation and post-impact hydrous environments. Planet. Space Sci. 2012, 70, 84–95. [Google Scholar] [CrossRef]
- Wray, J.J. Gale crater: The Mars Science Laboratory/Curiosity rover landing site. Int. J. Astrobiol. 2013, 12, 25–38. [Google Scholar] [CrossRef]
- Ruff, S.W.; Farmer, J.D. Silica deposits on Mars with features resembling hot spring biosignatures at El Tatio in Chile. Nat. Commun. 2016, 7, 13554. [Google Scholar] [CrossRef]
- Crowe, S.A.; Døssing, L.N.; Beukes, N.J.; Bau, M.; Kruger, S.J.; Frei, R.; Canfield, D.E. Atmospheric oxygenation three billion years ago. Nature 2013, 501, 535–538. [Google Scholar] [CrossRef]
- Planavsky, N.J.; Asael, D.; Hofmann, A.; Reinhard, C.T.; LaLonde, S.V.; Knudsen, A.; Wang, X.; Ossa, F.O.; Pecoits, E.; Smith, A.J.B.; et al. Evidence for oxygenic photosynthesis half a billion years before the Great Oxidation Event. Nat. Geosci. 2014, 7, 283–286. [Google Scholar] [CrossRef] [Green Version]
- Blankenship, R.E.; Sadekar, S.; Raymond, J. The evolutionary transition from anoxygenic to oxygenic photosynthesis. In Evolution of Primary Producers in the Sea; Academic Press: Cambridge, MA, USA, 2007; pp. 21–35. [Google Scholar]
- Havig, J.R.; Hamilton, T.L.; Bachan, A.; Kump, L.R. Sulfur and carbon isotopic evidence for metabolic pathway evolution and a four-stepped Earth system progression across the Archean and Paleoproterozoic. Earth Sci. Rev. 2017, 174, 1–21. [Google Scholar] [CrossRef]
- Follmann, H.; Brownson, C. Darwin’s warm little pond revisited: From molecules to the origin of life. Naturwissenschaften 2009, 96, 1265–1292. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W.; Georgiou, C.D. Hydrothermal Conditions and the Origin of Cellular Life. Astrobiology 2015, 15, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.G.; Yu, S.S.; Mamajanov, I.; Grover, M.A.; Krishnamurthy, R.; Fernández, F.M.; Hud, N.V. Ester-Mediated Amide Bond Formation Driven by Wet-Dry Cycles: A Possible Path to Polypeptides on the Prebiotic Earth. Angew. Chem. Int. Ed. 2015, 127, 10009–10013. [Google Scholar] [CrossRef]
- Van Kranendonk, M.J.; Deamer, D.W.; Djokic, T. Life springs. Sci. Am. 2017, 317, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.K.D.; Pudritz, R.E.; Semenov, D.A.; Henning, T.K. Origin of the RNA world: The fate of nucleobases in warm little ponds. Proc. Natl. Acad. Sci. USA 2017, 114, 11327–11332. [Google Scholar] [CrossRef] [Green Version]
- Milshteyn, D.; Damer, B.; Havig, J.; Deamer, D. Amphiphilic Compounds Assemble into Membranous Vesicles in Hydrothermal Hot Spring Water but Not in Seawater. Life 2018, 8, 11. [Google Scholar] [CrossRef]
- Ranjan, S.; Todd, Z.R.; Rimmer, P.B.; Sasselov, D.D.; Babbin, A.R. Nitrogen Oxide Concentrations in Natural Waters on Early Earth. Geochem. Geophys. Geosyst. 2019, 20, 2021–2039. [Google Scholar] [CrossRef] [Green Version]
- Abramov, O.; Kring, D.A. Impact-induced hydrothermal activity on early Mars. J. Geophys. Res. Space Phys. 2005, 110, E12. [Google Scholar] [CrossRef]
- Pirajno, F. Hydrothermal Processes Associated with Meteorite Impacts. In Hydrothermal Processes and Mineral Syst; Springer: Dordrecht, The Netherlands, 2009; pp. 1097–1130. [Google Scholar]
- Osinski, G.R.; Tornabene, L.L.; Banerjee, N.R.; Cockell, C.S.; Flemming, R.; Izawa, M.R.; McCutcheon, J.; Parnell, J.; Preston, L.J.; Pickersgill, A.E.; et al. Impact-generated hydrothermal systems on Earth and Mars. Icarus 2013, 224, 347–363. [Google Scholar] [CrossRef]
- Havig, J.R. Geochemistry of Hydrothermal Biofilms: Composition of Biofilms in a Siliceous Sinter-Deposition Hot Spring; Arizona State University: Tempe, AZ, USA, 2009. [Google Scholar]
- Havig, J.R.; Meyer-Dombard, D.R.; Raymond, J.; Shock, E.L. Merging Isotopes and Metagenomics: Coupling Biofilm C and N Isotopes and Metagenomics in a Siliceous Sinter-Depositing Hot Spring. J. Geophys. Res. Biogeosci. 2011, 116, G01005. [Google Scholar] [CrossRef]
- Schuler, C.G.; Havig, J.R.; Hamilton, T.L. Hot spring microbial community composition, morphology, and carbon fixation: implications for interpreting the ancient rock record. Front. Earth Sci., Biogeosci. 2017, 5, 97. [Google Scholar] [CrossRef]
- Havig, J.R.; Hamilton, T.L. Hypolithic photosynthesis in hydrothermal areas and implications for cryptic oxygen oases on Archean continental surfaces. Front. Earth Sci. Biogeosci. 2019. [Google Scholar] [CrossRef]
- Havig, J.R.; Kuether, J.; Gangidine, A.; Schroeder, S.; Hamilton, T.L. Hot Spring Microbial Community Elemental Composition: Hot Spring and Soil Inputs, and the Transition from Biocumulus to Siliceous Sinter. Astrobiology 2019. in review. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; A Walters, W.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- White, D.E.; Muffler, L.J.P.; Truesdell, A.H. Vapor-dominated hydrothermal systems compared with hot-water systems. Econ. Geol. 1971, 66, 75–97. [Google Scholar] [CrossRef]
- Truesdell, A.H.; Fournier, R.O. Conditions in the Deeper Parts of the Hot Spring Systems of Yellowstone National Park, Wyoming; US Geological Survey: Reston, VA, USA, 1976.
- White, D.E.; Hutchinson, R.A.; Keith, T.E. The Geology and Remarkable Thermal Activity of Norris Geyser Basin, Yellowstone National Park, Wyoming; US Geological Survey: Reston, VA, USA, 1988.
- Fournier, R.O. Geochemistry and Dynamics of the Yellowstone National Park Hydrothermal System. Annu. Rev. Earth Planet. Sci. 1989, 17, 13–53. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; McCleskey, R.B.; Ball, J.W. Sulfur geochemistry of hydrothermal waters in Yellowstone National Park: IV Acid–sulfate waters. Appl. Geochem. 2009, 24, 191–207. [Google Scholar] [CrossRef]
- Hurwitz, S.; Lowenstern, J.B. Dynamics of the Yellowstone hydrothermal system. Rev. Geophys. 2014, 52, 375–411. [Google Scholar] [CrossRef]
- Hamilton, T.L.; Lange, R.K.; Boyd, E.S.; Peters, J.W. Biological nitrogen fixation in acidic high-temperature geothermal springs in Yellowstone National Park, Wyoming. Environ. Microbiol. 2011, 13, 2204–2215. [Google Scholar] [CrossRef]
- Holloway, J.M.; Nordstrom, D.K.; Bohlke, J.K.; McCleskey, R.B.; Ball, J.W.; Mc Cleskey, R. Ammonium in thermal waters of Yellowstone National Park: Processes affecting speciation and isotope fractionation. Geochim. Cosmochim. Acta 2011, 75, 4611–4636. [Google Scholar] [CrossRef]
- Huber, G.; Spinnler, C.; Gambacorta, A.; Stetter, K.O. Metallosphaera sedula gen, and sp. nov. Represents a New Genus of Aerobic, Metal-Mobilizing, Thermoacidophilic Archaebacteria. Syst. Appl. Microbiol. 1989, 12, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Segerer, A.H.; Trincone, A.; Gahrtz, M.; Stetter, K.O. Stygiolobus azoricus gen. nov., sp. nov. Represents a Novel Genus of Anaerobic, Extremely Thermoacidophilic Archaebacteria of the Order Sulfolobales. Int. J. Syst. Evol. Microbiol. 1991, 41, 495–501. [Google Scholar] [CrossRef] [Green Version]
- She, Q.; Singh, R.K.; Confalonieri, F.; Zivanovic, Y.; Allard, G.; Awayez, M.J.; Chan-Weiher, C.C.-Y.; Clausen, I.G.; Curtis, B.A.; De Moors, A.; et al. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc. Natl. Acad. Sci. USA 2001, 98, 7835–7840. [Google Scholar] [CrossRef]
- Suzuki, T.; Iwasaki, T.; Uzawa, T.; Hara, K.; Nemoto, N.; Kon, T.; Ueki, T.; Yamagishi, A.; Oshima, T. Sulfolobus tokodaii sp. nov. (f. Sulfolobus sp. strain 7), a new member of the genus Sulfolobus isolated from Beppu Hot Springs, Japan. Extremophiles 2002, 6, 39–44. [Google Scholar] [CrossRef]
- Küsel, K.; Dorsch, T.; Acker, G.; Stackebrandt, E. Microbial Reduction of Fe(III) in Acidic Sediments: Isolation of Acidiphilium cryptum JF-5 Capable of Coupling the Reduction of Fe(III) to the Oxidation of Glucose. Appl. Environ. Microbiol. 1999, 65, 3633–3640. [Google Scholar]
- Rohwerder, T.; Janosch, C.; Sand, W. Elemental Sulfur Oxidation in Acidiphilium spp. In Advanced Materials Research; Trans Tech Publications: Zurich, Switzerland, 2007; p. 583. [Google Scholar]
- Jones, D.S.; Lapakko, K.A.; Wenz, Z.J.; Olson, M.C.; Roepke, E.W.; Sadowsky, M.J.; Novak, P.J.; Bailey, J.V. Novel Microbial Assemblages Dominate Weathered Sulfide-Bearing Rock from Copper-Nickel Deposits in the Duluth Complex, Minnesota, USA. Appl. Environ. Microbiol. 2017, 83, e00909-17. [Google Scholar] [CrossRef] [Green Version]
- Fischer, F.; Zillig, W.; Stetter, K.O.; Schreiber, G. Chemolithoautotrophic metabolism of anaerobic extremely thermophilic archaebacteria. Nature 1983, 301, 511–513. [Google Scholar] [CrossRef]
- Itoh, T.; Suzuki, K.-I.; Nakase, T. Vulcanisaeta distributa gen. nov., sp. nov., and Vulcanisaeta souniana sp. nov., novel hyperthermophilic, rod-shaped crenarchaeotes isolated from hot springs in Japan. Int. J. Syst. Evol. Microbiol. 2002, 52, 1097–1104. [Google Scholar]
- Reysenbach, A.L.; Hamamura, N.; Podar, M.; Griffiths, E.; Ferreira, S.; Hochstein, R.; Heidelberg, J.; Johnson, J.; Mead, D.; Pohorille, A.; et al. Complete and Draft Genome Sequences of Six Members of the Aquificales. J. Bacteriol. 2009, 191, 1992–1993. [Google Scholar] [CrossRef] [Green Version]
- Kashefi, K.; Holmes, D.E.; Reysenbach, A.-L.; Lovley, D.R. Use of Fe(III) as an Electron Acceptor to Recover Previously Uncultured Hyperthermophiles: Isolation and Characterization of Geothermobacterium ferrireducens gen. nov., sp. nov. Appl. Environ. Microbiol. 2002, 68, 1735–1742. [Google Scholar] [CrossRef]
- Zillig, W.; Gierl, A.; Schreiber, G.; Wunderl, S.; Janekovic, D.; Stetter, K.; Klenk, H. The Archaebacterium Thermofilum pendens Represents, a Novel Genus of the Thermophilic, Anaerobic Sulfur Respiring Thermoproteales. Syst. Appl. Microbiol. 1983, 4, 79–87. [Google Scholar] [CrossRef]
- Huber, R.; Kristjansson, J.K.; Stetter, K.O. Pyrobaculum gen. nov., a new genus of neutrophilic, rod-shaped archaebacteria from continental solfataras growing optimally at 100 C. Arch. Microbiol. 1987, 149, 95–101. [Google Scholar] [CrossRef]
- Hensel, R.; Matussek, K.; Michalke, K.; Tacke, L.; Tindall, B.; Kohlhoff, M.; Siebers, B.; Dielenschneider, J. Sulfophobococcus zilligii gen. nov., spec. nov. a Novel Hyperthermophilic Archaeum Isolated from Hot Alkaline Springs of Iceland. Syst. Appl. Microbiol. 1997, 20, 102–110. [Google Scholar] [CrossRef]
- Huber, R.; Dyba, D.; Huber, H.; Burggraf, S.; Rachel, R. Sulfur-inhibited Thermosphaera aggregans sp. nov., a new genus of hyperthermophilic archaea isolated after its prediction from environmentally derived 16S rRNA sequences. Int. J. Syst. Bacteriol. 1998, 48, 31–38. [Google Scholar] [CrossRef]
- Huber, H.; Stetter, K.O. Desulfurococcales. In The Prokaryotes; Springer: New York, NY, USA, 2006; pp. 52–68. [Google Scholar]
- Anderson, I.; Rodriguez, J.; Susanti, D.; Porat, I.; Reich, C.; Ulrich, L.E.; Elkins, J.G.; Mavromatis, K.; Lykidis, A.; Kim, E.; et al. Genome Sequence of Thermofilum pendens Reveals an Exceptional Loss of Biosynthetic Pathways without Genome Reduction. J. Bacteriol. 2008, 190, 2957–2965. [Google Scholar] [CrossRef]
- Arsène-Ploetze, F.; Koechler, S.; Marchal, M.; Coppée, J.Y.; Chandler, M.; Bonnefoy, V.; Brochier-Armanet, C.; Barakat, M.; Barbe, V.; Battaglia-Brunet, F.; et al. Structure, Function, and Evolution of the Thiomonas spp. Genome. PLoS Genet. 2010, 6, e1000859. [Google Scholar] [CrossRef]
- Aguiar, P. Sulfurihydrogenibium azorense, sp. nov., a thermophilic hydrogen-oxidizing microaerophile from terrestrial hot springs in the Azores. Int. J. Syst. Evol. Microbiol. 2004, 54, 33–39. [Google Scholar] [CrossRef]
- Nakagawa, S.; Shtaih, Z.; Banta, A.; Beveridge, T.J.; Sako, Y.; Reysenbach, A.-L. Sulfurihydrogenibium yellowstonense sp. nov., an extremely thermophilic, facultatively heterotrophic, sulfur-oxidizing bacterium from Yellowstone National Park, and emended descriptions of the genus Sulfurihydrogenibium, Sulfurihydrogenibium subterraneum and Sulfurihydrogenibium azorense. Int. J. Syst. Evol. Microbiol. 2005, 55, 2263–2268. [Google Scholar]
- O’Neill, A.H.; Liu, Y.; Ferrera, I.; Beveridge, T.J.; Reysenbach, A.-L. Sulfurihydrogenibium rodmanii sp. nov., a sulfur-oxidizing chemolithoautotroph from the Uzon Caldera, Kamchatka Peninsula, Russia, and emended description of the genus Sulfurihydrogenibium. Int. J. Syst. Evol. Microbiol. 2008, 58, 1147–1152. [Google Scholar] [CrossRef]
- Prokofeva, M.I.; Miroshnichenko, M.L.; Kostrikina, N.A.; Chernyh, N.A.; Kuznetsov, B.B.; Tourova, T.P.; Bonch-Osmolovskaya, E.A. Acidilobus aceticus gen. nov., sp. nov., a novel anaerobic thermoacidophilic archaeon from continental hot vents in Kamchatka. Int. J. Syst. Evol. Microbiol. 2000, 50, 2001–2008. [Google Scholar] [CrossRef]
- Meyer-Dombard, D.R.; Shock, E.L.; Amend, J.P. Archaeal and bacterial communities in geochemically diverse hot springs of Yellowstone National Park, USA. Geobiology 2005, 3, 211–227. [Google Scholar] [CrossRef]
- Wood, A.P.; Kelly, D.P. Physiological Characteristics of a New Thermophilic Obligately Chemolithotrophic Thiobacillus Species, Thiobacillus tepidarius. Int. J. Syst. Evol. Microbiol. 1985, 35, 434–437. [Google Scholar] [CrossRef]
- Wood, A.P.; Kelly, D.P. Isolation and physiological characterisation of Thiobacillus thyasiris sp. nov., a novel marine facultative autotroph and the putative symbiont of Thyasira flexuosa. Arch. Microbiol. 1989, 152, 160–166. [Google Scholar] [CrossRef]
- Shooner, F.; Bousquet, J.; Tyagi, R.D. Isolation, Phenotypic Characterization, and Phylogenetic Position of a Novel, Facultatively Autotrophic, Moderately Thermophilic Bacterium, Thiobacillus thermosulfatus sp. nov. Int. J. Syst. Evol. Microbiol. 1996, 46, 409–415. [Google Scholar] [CrossRef]
- Weiner, R.M.; Melick, M.; O’Neill, K.; Quintero, E. Hyphomonas adhaerens sp. nov., Hyphomonas johnsonii sp. nov. and Hyphomonas rosenbergii sp. nov., marine budding and prosthecate bacteria. Int. J. Syst. Evol. Microbiol. 2000, 50, 459–469. [Google Scholar] [CrossRef]
- Nedashkovskaya, O.I.; Vancanneyt, M.; Van Trappen, S.; Vandemeulebroecke, K.; Lysenko, A.M.; Falsen, E.; Frolova, G.M.; Mikhailov, V.V.; Rohde, M.; Swings, J. Description of Algoriphagus aquimarinus sp. nov., Algoriphagus chordae sp. nov. and Algoriphagus winogradskyi sp. nov., from sea water and algae, transfer of Hongiella halophila Yi and Chun 2004 to the genus Algoriphagus as Algoriphagus halophilus comb. nov. and emended descriptions of the genera Algoriphagus Bowman et al. 2003 and Hongiella Yi and Chun 2004. Int. J. Syst. Evol. Microbiol. 2004, 54, 1757–1764. [Google Scholar]
- Xia, S.; Shi, Y.; Fu, Y.; Ma, X. DGGE analysis of 16S rDNA of ammonia-oxidizing bacteria in chemical–biological flocculation and chemical coagulation systems. Appl. Microbiol. Biotechnol. 2005, 69, 99–105. [Google Scholar] [CrossRef]
- Kellermann, C.; Griebler, C. Thiobacillus thiophilus sp. nov., a chemolithoautotrophic, thiosulfate-oxidizing bacterium isolated from contaminated aquifer sediments. Int. J. Syst. Evol. Microbiol. 2009, 59, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Lei, X.; Lai, Q.; Li, Y.; Zhang, B.; Zhang, J.; Zhang, H.; Yang, L.; Zheng, W.; Tian, Y.; et al. Phaeodactylibacter xiamenensis gen. nov., sp. nov., a member of the family Saprospiraceae isolated from the marine alga Phaeodactylum tricornutum. Int. J. Syst. Evol. Microbiol. 2014, 64, 3496–3502. [Google Scholar] [CrossRef]
- Zhen-Li, Z.; Xin-Qi, Z.; Nan, W.; Wen-Wu, Z.; Xu-Fen, Z.; Yi, C.; Min, W. Amphiplicatus metriothermophilus gen. nov., sp. nov., a thermotolerant alphaproteobacterium isolated from a hot spring. Int. J. Syst. Evol. Microbiol. 2014, 64, 2805–2811. [Google Scholar] [CrossRef]
- Lei, X.; Chen, Z.; Chen, Y.; Zheng, T.; Zhu, H.; Zhang, J.; Lai, Q.; Zheng, W.; Wang, G.; Liao, P.; et al. Phaeodactylibacter luteus sp. nov., isolated from the oleaginous microalga Picochlorum sp. Int. J. Syst. Evol. Microbiol. 2015, 65, 2666–2670. [Google Scholar] [CrossRef]
- Speth, D.R.; Zandt, M.H.I.; Guerrero-Cruz, S.; Dutilh, B.E.; Jetten, M.S.M. Genome-based microbial ecology of anammox granules in a full-scale wastewater treatment system. Nat. Commun. 2016, 7, 11172. [Google Scholar] [CrossRef]
- Stieglmeier, M.; Klingl, A.; Alves, R.J.E.; Rittmann, S.K.; Melcher, M.; Leisch, N.; Schleper, C. Nitrososphaera viennensis gen. nov., sp. nov., an aerobic and mesophilic, ammonia-oxidizing archaeon from soil and a member of the archaeal phylum Thaumarchaeota. Int. J. Syst. Evol. Microbiol. 2014, 64, 2738–2752. [Google Scholar] [CrossRef]
- Jung, M.Y.; Kim, J.G.; Sinninghe Damsté, J.S.; Rijpstra, W.I.C.; Madsen, E.L.; Kim, S.J.; Hong, H.; Si, O.J.; Kerou, M.; Schleper, C.; et al. A hydrophobic ammonia-oxidizing archaeon of the Nitrosocosmicus clade isolated from coal tar-contaminated sediment. Envi. Microbiol. Rep. 2016, 8, 983–992. [Google Scholar] [CrossRef]
- Lehtovirta-Morley, L.E.; Ross, J.; Hink, L.; Weber, E.B.; Gubry-Rangin, C.; Thion, C.; Prosser, J.I.; Nicol, G.W. Isolation of ‘Candidatus Nitrosocosmicus franklandus’, a novel ureolytic soil archaeal ammonia oxidiser with tolerance to high ammonia concentration. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef]
- Takami, H.; Noguchi, H.; Takaki, Y.; Uchiyama, I.; Toyoda, A.; Nishi, S.; Chee, G.J.; Arai, W.; Nunoura, T.; Itoh, T.; et al. A Deeply Branching Thermophilic Bacterium with an Ancient Acetyl-CoA Pathway Dominates a Subsurface Ecosystem. PLoS ONE 2012, 7, e30559. [Google Scholar] [CrossRef]
- Takami, H.; Arai, W.; Takemoto, K.; Uchiyama, I.; Taniguchi, T. Functional Classification of Uncultured “Candidatus Caldiarchaeum subterraneum” Using the Maple System. PLoS ONE 2015, 10, e0132994. [Google Scholar] [CrossRef]
- Luther, G.W.; Findlay, A.J.; Macdonald, D.J.; Owings, S.M.; Hanson, T.E.; Beinart, R.A.; Girguis, P.R. Thermodynamics and Kinetics of Sulfide Oxidation by Oxygen: A Look at Inorganically Controlled Reactions and Biologically Mediated Processes in the Environment. Front. Microbiol. 2011, 2, 62. [Google Scholar] [CrossRef] [Green Version]









| Sample Site | ‘Heartbeat Pool’ | Dante’s Inferno | ‘Avocado Spring’ | ‘The Dryer’ | Boulder Geyser OF | ‘Rose Terrace Pool’ | |
|---|---|---|---|---|---|---|---|
| Hydrothermal Area | RCA, MGB | SSA, GGB | SSA | GGB | SSA, GGB | IGB, LGB | RCA, MGB |
| Date Sampled | 06/06/18 | 07/24/16 | 07/24/16 | 06/05/18 | 07/24/16 | 06/29/17 | 06/06/18 |
| YNP Inventory IDa | (none) | Dante’s Inferno | GSSGNN025 | GSSGNN037 | LRNN781 | (none) | |
| UTM | 4929806 | 4949526 | 4949715 | 4949604 | 4933949 | 4929847 | |
| 12 T | 0515019 | 0518360 | 0518576 | 0518442 | 0512398 | 0515040 | |
| T (°C) | 79.2 | 79.4 | 69.5 | 65.6 | 40.5 | 84.9 | 74.8 |
| pH | 3.01 | 5.19 | 6.39 | 6.71 | 7.13 | 8.47 | 8.62 |
| Conductivity (µS/cm) | 505 | 2250 | 1925 | 3596 | 2310 | 3280 | 2799 |
| SiO2(aq) (mmol/L) | 4.34 | 2.91 | 2.55 | 4.32 | 2.19 | 2.86 | 4.83 |
| Cl− (mmol/L) | 0.24 ± 0.00 | 15.94 | 6.57 | 7.26 ± 0.04 | 15.95 | 7.90 ± 0.04 | 6.30 ± 0.01 |
| SO42− (mmol/L) | 0.66 ± 0.00 | 2.13 | 3.27 | 1.71 ± 0.00 | 2.15 | 0.17 ± 0.01 | 0.14 ± 0.00 |
| DIC (mmol/L) | 0.72 ± 0.06 | 1.42 | 5.27 | 10.1 | 0.18 | 2.41 | 39.11 ± 3.26 |
| DOC (µmol/L) | nd | 195 ± 0.2 | 118 ± 0.4 | nd | 97.9 ± 0.2 | 31.9 ± 9.9 | nd |
| Na+ (mmol/L) | 0.81 ± 0.01 | 17.59 | 16.66 | 16.36 ± 0.34 | 17.61 | 14.89 ± 3.98 | 11.06 ± 0.17 |
| K+ (mmol/L) | 0.17 ± 0.01 | 1.53 | 0.68 | 0.46 ± 0.03 | 0.95 | 0.30 ± 0.08 | 0.23 ± 0.01 |
| Mg2+ (µmol/L) | 7.90 ± 0.17 | 0.83 | 0.68 | 0.52 ± 0.32 | 3.57 | 0.31 ± 0.08 | 0.21 ± 0.17 |
| Ca2+ (µmol/L) | 37.27 ± 1.14 | 106.16 | 80.64 | 96.93 ± 1.31 | 128.98 | 28.55 ± 7.36 | 14.31 ± 0.72 |
| Sulfide (µmol/L) | 7.8 | 538.0 | 313.0 | 46.0 | 78.0 | 45.5 | 1.0 |
| Fe2+ (µmol/L) | bdl | 0.25 | 0.10 | bdl | bdl | 0.90 | bdl |
| P (µmol/L) | 2.40 ± 0.10 | nd | nd | 8.96 ± 0.05 | nd | 4.25 ± 0.08 | 6.57 ± 0.05 |
| Al (µmol/L) | 42.01 ± 0.08 | 12.59 | 5.56 | 4.42 ± 0.05 | 3.38 | 12.06 ± 0.14 | 11.64 ± 0.03 |
| Mn (nmol/L) | 675 ± 0.8 | 684 | 218 | 206 ± 1 | 475 | 30.1 ± 0.08 | 7.71 ± 0.14 |
| Fe(T) (nmol/L) | 507 ± 5 | 169 | 85 | 252 ± 16 | 381 | 384 ± 7 | 111 ± 2 |
| As (nmol/L) | 137 ± 16 | 33915 | 14338 | nd | 34269 | 15229 ± 170 | 16951 ± 94 |
| Biomass C (%) | 0.672 | 0.081 | 0.586 | 0.451 | 0.269 | 1.099 | 0.118 |
| Biomass N (%) | 0.049 | 0.016 | 0.060 | 0.061 | 0.034 | 0.056 | 0.010 |
| Sample Site | ‘Heartbeat Pool’ | Dante’s Inferno | ‘Avocado Spring’ | ‘The Dryer’ | Boulder Geyser OF | ‘Rose Terrace Pool’ | |
|---|---|---|---|---|---|---|---|
| Sample Description | Sediments | Sediments | Grey Mat (‘16) | Red Mat (‘18) | Silica Precipitate | Sediments | Sediments |
| T (°C) | 79.2 | 79.4 | 69.5 | 65.6 | 40.5 | 84.9 | 74.8 |
| pH | 3.01 | 5.19 | 6.39 | 6.71 | 7.13 | 8.47 | 8.62 |
| DIC δ13C (‰) | −8.44 ± 0.08 | −0.51 ± 0.12 | 0.74 ± 0.12 | −0.46 ± 0.08 | 3.06 ± 0.12 | −1.17 ± 0.08 | −1.65 ± 0.08 |
| DOC δ13C (‰) | nd | −22.16 ± 1.00 | −22.55 ± 0.05 | nd | −27.80 ± 0.09 | −24.47 ± 0.89 | nd |
| Biomass δ13C (‰) | −25.59 ± 0.06 | −14.69 ± 1.58 | −22.87 ± 0.73 | −15.83 ± 0.06 | −16.67 ± 0.96 | −24.33 ± 0.06 | −24.33 ± 0.06 |
| Biomass δ15N (‰) | −2.26 ± 0.12 | 0.24 ± 0.12 | 1.35 ± 0.12 | 4.08 ± 0.12 | −4.13 ± 0.12 | 1.93 ± 0.12 | 2.50 ± 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havig, J.R.; Hamilton, T.L. Productivity and Community Composition of Low Biomass/High Silica Precipitation Hot Springs: A Possible Window to Earth’s Early Biosphere? Life 2019, 9, 64. https://doi.org/10.3390/life9030064
Havig JR, Hamilton TL. Productivity and Community Composition of Low Biomass/High Silica Precipitation Hot Springs: A Possible Window to Earth’s Early Biosphere? Life. 2019; 9(3):64. https://doi.org/10.3390/life9030064
Chicago/Turabian StyleHavig, Jeff R., and Trinity L. Hamilton. 2019. "Productivity and Community Composition of Low Biomass/High Silica Precipitation Hot Springs: A Possible Window to Earth’s Early Biosphere?" Life 9, no. 3: 64. https://doi.org/10.3390/life9030064





