Application of Noninvasive Vagal Nerve Stimulation to Stress-Related Psychiatric Disorders
Abstract
1. Introduction
2. Physiology of the Vagus Nerve
3. Neurobiology of Stress-Related Psychiatric Disorders
4. Neuromodulation for Stress-Related Psychiatric Disorders
5. Vagus Nerve and Neuroplasticity
6. Neural Circuits in Stress-Related Psychiatric Disorders and Vagal Nerve Stimulation
7. Noninvasive Vagal Nerve Stimulation: Safety and Reliability
8. Noninvasive Vagal Nerve Stimulation: Application to Stress-Related Psychiatric Disorders
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anda, R.F.; Felitti, V.J.; Walker, J.; Whitfield, C.; Bremner, J.D.; Perry, B.D.; Dube, S.R.; Giles, W.H. The enduring effects of childhood abuse and related experiences in childhood: A convergence of evidence from neurobiology and epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Magee, W.J. Childhood adversities and adult depression: Basic patterns of association in a US national survey. Psychol. Med. 1993, 23, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Thornton, L.M.; Gardner, C.O. Stressful life events and previous episodes in the etiology of major depression in women: An evaluation of the “kindling” hypothesis. Am. J. Psychiatry 2000, 157, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Weathers, F.W.; Bovin, M.J.; Lee, D.J.; Sloan, D.M.; Schnurr, P.P.; Kaloupek, D.G.; Keane, T.M.; Marx, B.P. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol. Assess. 2018, 30, 383–395. [Google Scholar] [CrossRef]
- Kessler, R.C.; McGonagle, K.A.; Zhao, S.; Nelson, C.B.; Hughes, M.; Eschleman, S.; Wittchen, H.-U.; Kendler, K. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: Results from the National Comorbidity Study. Arch. Gen. Psychiatry 1994, 51, 8–19. [Google Scholar] [CrossRef]
- Stewart, W.F.; Ricci, J.A.; Chee, E.; Hahn, S.R.; Morganstein, D. Cost of lost productive work time among US workers with depression. J. Am. Med. Assoc. 2003, 289, 3135–3144. [Google Scholar] [CrossRef]
- Pietrzak, R.H.; Goldstein, R.B.; Southwick, S.M.; Grant, B.F. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: Results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J. Anxiety Disord. 2011, 25, 456–465. [Google Scholar] [CrossRef]
- Eibner, C. The Invisible Wounds of War: Quantifying the Societal Costs of Psychological and Cognitive Injuries; RAND Corporation: Santa Monica, CA, USA, 2008. [Google Scholar]
- McCauley, J.; Kern, D.E.; Kolodner, K.; Dill, L.; Schroeder, A.F.; DeChant, H.K.; Ryden, J.; Derogatis, L.R.; Bass, E.G. Clinical characteristics of women with a history of childhood abuse: Unhealed wounds. J. Am. Med. Assoc. 1997, 277, 1362–1368. [Google Scholar] [CrossRef]
- MacMillan, H.L.; Fleming, J.E.; Trocme, N.; Boyle, M.H.; Wong, M.; Racine, Y.A.; Beardslee, W.R.; Offord, D.R. Prevalence of child physical and sexual abuse in the community: Results from the Ontario Health Supplement. J. Am. Med. Assoc. 1997, 278, 131–135. [Google Scholar] [CrossRef]
- Kessler, R.C.; Sonnega, A.; Bromet, E.; Hughes, M.; Nelson, C.B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1995, 52, 1048–1060. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D. (Ed.) Posttraumatic Stress Disorder: From Neurobiology to Treatment, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Blanchard, E.B.; Buckley, T.C.; Hickling, E.J.; Taylor, A.E. Posttraumatic stress disorder and comorbid major depression: Is the correlation an illusion? J. Anxiety Disord. 1998, 12, 1–37. [Google Scholar] [CrossRef]
- Franklin, C.L.; Zimmerman, M. Posttraumatic stress disorder and major depressive disorder: Investigating the role of overlapping symptoms in diagnostic comorbidity. J. Nerv. Ment. Dis. 2001, 189, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Flory, J.D.; Yehuda, R. Comorbidity between post-traumatic stress disorder and major depressive disorder: Alternative explanations and treatment considerations. Dialogues Clin. Neurosci. 2015, 17, 141–150. [Google Scholar]
- Nijdam, M.J.; Gersons, B.P.R.; Olff, M. The role of major depression in neurocognitive functioning in patients with posttraumatic stress disorder. Eur. J. Psychotraumatol. 2013, 4, 19979. [Google Scholar] [CrossRef]
- Shalev, A.Y.; Freedman, S.; Peri, T. Prospective study of post-traumatic stress disorder and depression following trauma. Am. J. Psychiatry 1988, 155, 630–637. [Google Scholar] [CrossRef]
- Rytwinski, N.K.; Scur, M.D.; Feeny, N.C.; Youngstrom, E.A. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: A meta-analysis. J. Trauma. Stress 2013, 26, 299–309. [Google Scholar] [CrossRef]
- Oquendo, M.; Brent, D.A.; Birmaher, B.; Greenhill, L.; Kolko, D.; Stanley, B.; Zelazny, J.; Burke, A.K.; Firinciogullari, S.; Ellis, S.P.; et al. Posttraumatic stress disorder comorbid with major depression: Factors mediating the association with suicidal behavior. Am. J. Psychiatry 2005, 162, 560–566. [Google Scholar] [CrossRef]
- Ramsawh, H.J.; Fullerton, C.S.; Mash, H.B.H.; Ng, T.H.H.; Kessler, R.C.; Stein, M.B.; Ursano, R.J. Risk for suicidal behaviors associated with PTSD, depression, and their comorbidity in the U.S. Army. J. Affect. Disord. 2014, 161, 116–122. [Google Scholar] [CrossRef]
- Ballenger, J.C.; Davidson, J.R.; Lecrubier, Y.; Nutt, D.J.; Foa, E.B.; Kessler, R.C.; McFarlane, A.C.; Shalev, A.Y. Consensus statement on posttraumatic stress disorder from the International Consensus Group on Depression and Anxiety. J. Clin. Psychiatry 2000, 61, 60–66. [Google Scholar]
- Foa, E.B.; Davidson, J.R.T.; Frances, A.; Culpepper, L.; Ross, R.; Ross, D. The expert consensus guideline series: Treatment of posttraumatic stress disorder. J. Clin. Psychiatry 1999, 60, 4–76. [Google Scholar]
- Schottenbauer, M.A.; Glass, C.R.; Arnkoff, D.B.; Tendick, V.; Gray, S.H. Nonresponse and dropout rates in outcome studies on PTSD: Review and methodological considerations. Psychiatry 2008, 71, 134–168. [Google Scholar] [CrossRef] [PubMed]
- Hembree, E.A.; Foa, E.B.; Dorfan, N.M.; Street, G.P.; Kowalski, J.; Tu, X. Do patients drop out prematurely from exposure therapy for PTSD? J. Trauma. Stress 2003, 16, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Ballenger, J.C.; Davidson, J.R.; Lecrubier, Y.; Nutt, D.J.; Marshall, R.D.; Nemeroff, C.B.; Shalev, A.Y.; Yehuda, R. Consensus statement update on posttraumatic stress disorder from the international consensus group on depression and anxiety. J. Clin. Psychiatry 2004, 65 (Suppl. 1), 55–62. [Google Scholar]
- Davis, L.; Hamner, M.; Bremner, J.D. Pharmacotherapy for PTSD: Effects on PTSD symptoms and the brain. In Posttraumatic Stress Disorder: From Neurobiology to Treatment; Bremner, J.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 389–412. [Google Scholar]
- Institute of Medicine of the National Academies. Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment; National Academies of Science, Engineering and Medicine, Health and Medicine Division: Washington, DC, USA, 2014. [Google Scholar]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1907. [Google Scholar] [CrossRef]
- Komegae, E.N.; Farmer, D.G.S.; Brooks, V.L.; McKinley, M.J.; McAllen, R.M.; Martelli, D. Vagal afferent activation suppresses systemic inflammation via the splanchnic anti-inflammatory pathway. Brain Behav. Immun. 2018, 73, 441–449. [Google Scholar] [CrossRef]
- Bremner, J.D.; Charney, D.S. Neural circuits in fear and anxiety. In Textbook of Anxiety Disorders, 2nd ed.; Stein, D.J., Hollander, E., Rothbaum, B.O., Eds.; American Psychiatric Publishing: Arlington, VA, USA, 2010; pp. 55–71. [Google Scholar]
- Charney, D.S.; Bremner, J.D. The neurobiology of anxiety disorders. In Neurobiology of Mental Illness; Charney, D.S., Nestler, E.J., Bunney, S.S., Eds.; Oxford University Press: Oxford, UK, 1999; pp. 494–517. [Google Scholar]
- Bremner, J.D.; Pearce, B. Neurotransmitter, neurohormonal, and neuropeptidal function in stress and PTSD. In Posttraumatic Stress Disorder: From Neurobiology to Treatment; Bremner, J.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 181–232. [Google Scholar]
- Campanella, C.; Bremner, J.D. Neuroimaging of PTSD. In Posttraumatic Stress Disorder: From Neurobiology to Treatment; Bremner, J.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 291–320. [Google Scholar]
- Yehuda, R. Post-traumatic stress disorder. N. Engl. J. Med. 2002, 346, 108–114. [Google Scholar] [CrossRef]
- Vermetten, E. Epilogue: Neuroendocrinology of PTSD. Prog. Brain Res. 2008, 167, 311–313. [Google Scholar] [CrossRef]
- De Kloet, C.S.; Vermetten, E.; Geuze, E.; Kavelaars, A.; Heijnen, C.J.; Westenberg, H.G. Assessment of HPA-axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. J. Psychiatr. Res. 2006, 40, 550–567. [Google Scholar] [CrossRef]
- Van Zuiden, M.; Kavelaars, A.; Geuze, E.; Olff, M.; Heijnen, C.J. Predicting PTSD: Pre-existing vulnerabilities in glucocorticoid-signaling and implications for preventive interventions. Brain Behav. Immun. 2013, 30, 12–21. [Google Scholar] [CrossRef]
- Yehuda, R.; Golier, J.A.; Yang, R.-K.; Tischler, L. Enhanced sensitivity to glucocorticoids in peripheral mononuclear leukocytes in posttraumatic stress disorder. Biol. Psychiatry 2004, 55, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Young, E.A.; Haskett, R.F.; Murphy-Weinberg, V.; Watson, S.J.; Akil, H. Loss of glucocorticoid fast feedback in depression. Arch. Gen. Psychiatry 1991, 48, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Teicher, M.H.; Trestman, R.L.; Levengood, R.A.; Siever, L.J. Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biol. Psychiatry 1996, 40, 79–88. [Google Scholar] [CrossRef]
- Carroll, B.J.; Curtis, G.C.; Davies, B.M.; Mendels, J.; Sugarman, A.A. Urinary free cortisol excretion in depression. Psychol. Med. 1976, 6, 43–50. [Google Scholar] [CrossRef]
- Hosoi, T.; Okuma, Y.; Nomura, Y. Electrical stimulation of afferent vagus nerve induces IL-1β expression in the brain and activates HPA axis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R141–R147. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F.; Goehler, L.E. Cytokine-to-brain communication: A review and analysis of alternative mechanisms. Life Sci. 1995, 57, 1011–1026. [Google Scholar] [CrossRef]
- Thrivikraman, K.V.; Zejnelovic, F.; Bonsall, R.W.; Owens, M.J. Neuroendocrine homeostasis after vagus nerve stimulation in rats. Psychoneuroendocrinology 2013, 38, 1067–1077. [Google Scholar] [CrossRef]
- Agorastos, A.; Boel, J.A.; Heppner, P.S.; Hager, T.; Moeller-Bertram, T.; Haji, U.; Motazedi, A.; Yanagi, M.A.; Baker, D.G.; Stiedl, O. Diminished vagal activity and blunted diurnal variation of heart rate dynamics in posttraumatic stress disorder. Stress 2013, 16, 300–310. [Google Scholar] [CrossRef]
- Delgado, P.L.; Moreno, F.A. Role of norepinephrine in depression. J. Clin. Psychiatry 2000, 61, S5–S12. [Google Scholar]
- Golden, R.N.; Markey, S.P.; Risby, E.D.; Rudorfer, M.V.; Cowdry, R.W.; Potter, W.Z. Antidepressants reduce whole-body norepinephrine turnover while enhancing 6-hydroxymelatonin output. Arch. Gen. Psychiatry 1988, 45, 150–154. [Google Scholar] [CrossRef]
- Lake, C.R.; Pickar, D.; Ziegler, M.G.; Lipper, S.; Slater, S.; Murphy, D.L. High plasma NE levels in patients with major affective disorder. Am. J. Psychiatry 1982, 139, 1315–1318. [Google Scholar] [PubMed]
- Veith, R.C.; Lewis, L.; Linares, O.A. Sympathetic nervous system activity in major depression: Basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch. Gen. Psychiatry 1994, 51, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D.; Krystal, J.H.; Southwick, S.M.; Charney, D.S. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse 1996, 23, 39–51. [Google Scholar] [CrossRef]
- Blanchard, E.B.; Kolb, L.C.; Prins, A.; Gates, S.; McCoy, G.C. Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with posttraumatic stress disorder. J. Nerv. Ment. Dis. 1991, 179, 371–373. [Google Scholar] [CrossRef]
- Geracioti, T.D.J.; Baker, D.G.; Ekhator, N.N.; West, S.A.; Hill, K.K.; Bruce, A.B.; Schmidt, D.; Rounds-Kugler, B.; Yehuda, R.; Keck, P.E.J.; et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am. J. Psychiatry 2001, 158, 1227–1230. [Google Scholar] [CrossRef]
- Mason, J.W.; Giller, E.L.; Kosten, T.R. Elevation of urinary norepinephrine/cortisol ratio in posttraumatic stress disorder. J. Nerv. Ment. Dis. 1988, 176, 498–502. [Google Scholar] [CrossRef]
- Zoladz, P.R.; Diamond, D.M. Current status on behavioral and biological markers of PTSD: A search for clarity in a conflicting literature. Neurosci. Biobehav. Rev. 2013, 37, 860–895. [Google Scholar] [CrossRef]
- Bremner, J.D.; Krystal, J.H.; Southwick, S.M.; Charney, D.S. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse 1996, 23, 28–38. [Google Scholar] [CrossRef]
- Southwick, S.M.; Krystal, J.H.; Bremner, J.D.; Morgan, C.A.; Nicolaou, A.; Nagy, L.M.; Johnson, D.R.; Heninger, G.R.; Charney, D.S. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch. Gen. Psychiatry 1997, 54, 749–758. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Pace, T.W.W.; Heim, C.M. A short review on the psychoneuroimmunology of posttraumatic stress disorder: From risk factors to medical comorbidities. Brain Behav. Immun. 2011, 25, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Marsland, A.L.; Walsh, C.; Lockwood, K.; John-Henderson, N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav. Immun. 2017, 64, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Hamer, M.; Chida, Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav. Immun. 2007, 21, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Sugama, S.; Conti, B. Interleukin-18 and stress. Brain Res. Rev. 2008, 58, 85–95. [Google Scholar] [CrossRef]
- Lima, B.B.; Hammadah, M.; Wilmot, K.; Pearce, B.D.; Shah, A.; Levantsevych, O.; Kaseer, B.; Obideen, M.; Gafeer, M.M.; Kim, J.H.; et al. Posttraumatic Stress Disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain Behav. Immun. 2019, 75, 26–33. [Google Scholar] [CrossRef]
- Pace, T.W.W.; Mletzko, T.C.; Alagbe, O.; Musselman, D.L.; Nemeroff, C.B.; Miller, A.H.; Heim, C.M. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 2006, 163, 1630–1633. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathphysiology of depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef]
- Bierhaus, A.; Wolf, J.; Andrassy, M.; Rohleder, N.; Humpert, P.M.; Petrov, D.; Ferstl, R.; von Eynatten, M.; Wendt, T.; Rudofsky, G.; et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA 2003, 100, 1920–1925. [Google Scholar] [CrossRef]
- Raison, C.L.; Miller, A.H. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Mol. Psychiatry 2013, 18, 15–37. [Google Scholar] [CrossRef]
- Passos, C.I.; Vasconcelos-Moreno, M.P.; Costa, L.G.; Kunz, M.; Brietzke, E.; Quevedo, J.; Salum, G.; Magalhães, P.V.; Kapczinski, F.; Kauer-Sant’Anna, M. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015, 2, 1002–1012. [Google Scholar] [CrossRef]
- Felger, J.C.; Li, L.; Marvar, P.J.; Woolwine, B.J.; Harrison, D.G.; Raison, C.L.; Miller, A.H. Tyrosine metabolism during interferon-α administration: Association with fatigue and CSF dopamine concentrations. Brain Behav. Immun. 2013, 31, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Kelley, K.W.; Lawson, M.A.; Woolwine, B.J.; Vogt, G.; Spivey, J.R.; Saito, K.; Miller, A.H. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: Relationship to CNS immune responses and depression. Mol. Psychiatry 2010, 15, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.L.; Price, L.H.; Miller, A.H.; Salomon, R.M.; Aghajanian, G.K.; Heninger, G.R.; Charney, D.S. Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Arch. Gen. Psychiatry 1994, 51, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.M. Kynurenines: From the perspective of major psychiatric disorders. FEBS J. 2012, 279, 1375–1385. [Google Scholar] [CrossRef]
- Duman, R.S.; Malberg, J.E.; Nakagawa, S. Regulation of adult neurogenesis by psychotropic drugs and stress. J. Pharmacol. Exp. Ther. 2001, 299, 401–407. [Google Scholar]
- Duman, R.S. Depression: A case of neuronal life and death? Biol. Psychiatry 2004, 56, 140–145. [Google Scholar] [CrossRef]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef]
- Nizri, E.; Brenner, T. Modulation of inflammatory pathways by the immune cholinergic system. Amino Acids 2013, 45, 73–85. [Google Scholar] [CrossRef]
- Griffin, G.D.; Charron, D.; Al-Daccak, R. Post-traumatic stress disorder: Revisiting adrenergics, glucocorticoids, immune system effects and homeostasis. Clin. Transl. Immunol. 2014, 3, e27. [Google Scholar] [CrossRef]
- Zhou, J.; Nagarkatti, P.; Zhong, Y.; Ginsberg, J.P.; Singh, N.P.; Zhang, J.; Nagarkatti, M. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS ONE 2014, 9, e94075. [Google Scholar] [CrossRef] [PubMed]
- Bremner, D.; Vermetten, E.; Kelley, M.E. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. J. Nerv. Ment. Dis. 2007, 195, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.N.; van der Kolk, B.; Burbridge, J.; Fisler, R.; Kradin, R. Phenotype of blood lymphocytes in PTSD suggests chronic immune activation. Psychosomatics 1999, 40, 222–225. [Google Scholar] [CrossRef]
- Altemus, M.; Cloitre, M.; Dhabhar, F.S. Enhanced cellular immune response in women with PTSD related to childhood abuse. Am. J. Psychiatry 2003, 160, 1705–1707. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Berg, P.A.; Klein, R. Method for the in vitro determination of an individual disposition towards Th1- or Th2-reactivity by the application of appropriate stimulatory antigens. Clin. Exp. Immunol. 2003, 134, 78–85. [Google Scholar] [CrossRef]
- Woods, A.B.; Page, G.G.; O’Campo, P.; Pugh, L.C.; Ford, D.; Campbell, J.C. The mediation effect of posttraumatic stress disorder symptoms on the relationship of intimate partner violence and IFN-gamma levels. Am. J. Community Psychol. 2005, 36, 159–175. [Google Scholar] [CrossRef]
- Lindqvist, D.; Wolkowitz, O.M.; Mellon, S.; Yehuda, R.; Flory, J.D.; Henn-Haase, C.; Bierer, L.M.; Abu-Amara, D.; Coy, M.; Neylan, T.C.; et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav. Immun. 2014, 42, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef]
- Bremner, J.D.; Gurel, N.Z.; Jiao, Y.; Wittbrodt, M.T.; Levantsevych, O.M.; Huang, M.; Jung, H.; Shandhi, M.H.; Beckwith, J.; Herring, I.; et al. Transcutaneous vagal nerve stimulation blocks stress-induced activation of interleukin-6 and interferon-γ in posttraumatic stress disorder: A double-blind, randomized, sham-controlled trial. Brain Behav. Immun. Health 2020, in press. [Google Scholar]
- Huston, J.M.; Gallowitsch-Puerta, M.; Ochani, M.; Ochani, K.; Yuan, R.; Rosas-Ballina, M.; Ashok, M.; Goldstein, R.S.; Chavan, S.; Pavlov, V.A. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med. 2007, 35, 2762–2768. [Google Scholar] [CrossRef]
- Wang, X.-W.; Karki, A.; Du, D.-Y.; Zhao, X.-J.; Xiang, X.-Y.; Lu, Z.-Q. Plasma levels of high mobility group box 1 increase in patients with posttraumatic stress disorder after severe blunt chest trauma: A prospective cohort study. J. Surg. Res. 2015, 193, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.L.; Cline, D.L. PACAP: Regulator of the stress response. In Stress: Physiology, Biochemistry, and Pathology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 279–291. [Google Scholar]
- Ressler, K.J.; Mercer, K.B.; Bradley, B.; Jovanovic, T.; Mahan, A.; Kerley, K.; Norrholm, S.D.; Kilaru, V.; Smith, A.K.; Myers, A.J.; et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 2011, 470, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, T.; Norrholm, S.D.; Davis, J.; Mercer, K.B.; Almli, L.; Nelson, A.; Cross, D.; Smith, A.; Ressler, K.J.; Bradley, B. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol. Psychiatry 2013, 18, 742–743. [Google Scholar] [CrossRef] [PubMed]
- Kamkwalala, A.; Norrholm, S.D.; Poole, J.M.; Brown, A.; Donley, S.; Duncan, E.; Bradley, B.; Ressler, K.J.; Jovanovic, T. Dark-enhanced startle responses and heart rate variability in a traumatized civilian sample: Putative sex-specific correlates of posttraumatic stress disorder. Psychosom. Med. 2012, 74, 153. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.A.; Grillon, C.; Lubin, H.; Southwick, S.M. Startle reflex abnormalities in women with sexual assault-related posttraumatic stress disorder. Am. J. Psychiatry 1997, 154, 1076–1080. [Google Scholar] [PubMed]
- Jovanovic, T.; Norrholm, S.D.; Blanding, N.Q.; Phifer, J.E.; Weiss, T.; Davis, M.; Duncan, E.; Bradley, B.; Ressler, K.J. Fear potentiation is associated with hypothalamic–pituitary–adrenal axis function in PTSD. Psychoneuroendocrinology 2010, 35, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Walker, D.L.; Lee, Y.S. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex: Possible relevance to PTSD. Ann. N. Y. Acad. Sci. 1997, 821, 305–331. [Google Scholar] [CrossRef]
- Starr, E.R.; Margiotta, J.F. PACAP modulates distinct neuronal components to induce cell-specific plasticity at central and autonomic synapses. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer: Berlin/Heidelberg, Germany, 2016; pp. 83–107. [Google Scholar]
- Cagampang, F.R.A.; Piggins, H.D.; Sheward, W.J.; Harmar, A.J.; Coen, C.W. Circadian changes in PACAP type 1 (PAC1) receptor mRNA in the rat suprachiasmatic and supraoptic nuclei. Brain Res. 1998, 813, 218–222. [Google Scholar] [CrossRef]
- Piggins, H.D.; Stamp, J.A.; Burns, J.; Rusak, B.; Semba, K. Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J. Comp. Neurol. 1996, 376, 278–294. [Google Scholar] [CrossRef]
- Adair, D.; Truong, D.; Esmaeilpour, Z.; Gebodh, N.; Borges, H.; Ho, L.; Bremner, J.D.; Badran, B.W.; Napadow, V.; Clark, V.P.; et al. Electrical stimulation of cranial nerves in cognition and disease. Brain Stimul. 2020, 13, 713–720. [Google Scholar] [CrossRef]
- Krames, E.; Peckham, P.H.; Rezai, A. Neuromodulation: Comprehensive Textbook of Principles, Technologies, and Therapies, 2nd ed.; Academic Press: London, UK, 2018. [Google Scholar]
- Brunoni, A.R.; Moffa, A.H.; Sampaio-Junior, B.; Borrione, L.; Moreno, M.L.; Fernandes, R.A.; Veronezi, B.P.; Nogueira, B.S.; Aparicio, L.V.M.; Razza, L.B.; et al. Trial of electrical Direct-Current Therapy versus escitalopram for depression. N. Engl. J. Med. 2017, 376, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Unal, G.; Brunoni, A.; Loo, C. What psychiatrists need to know about transcranial direct current stimulation. Psychiatr. Times 2017, 34, 1–3. [Google Scholar]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of transcranial Direct Current Stimulation: Evidence based update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Bikson, M.; Bulow, P.; Stiller, J.W.; Datta, A.; Battaglia, F.; Karnup, S.V.; Postolache, T.T. Transcranial direct current stimulation for major depression: A general system for quantifying transcranial electrotherapy dosage. Curr. Treat. Options Neurol. 2008, 10, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef]
- McCann, U.D.; Kimbrell, T.A.; Morgan, C.M.; Anderson, T.; Geraci, M.; Benson, B.E.; Wassermann, E.M.; Willis, M.W.; Post, R.M. Repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Arch. Gen. Psychiatry 1998, 55, 276–279. [Google Scholar] [CrossRef]
- Tortella, G.; Casati, R.; Aparicio, L.V.M.; Mantovani, A.; Senço, N.; D’Urso, G.; Brunelin, J.; Guarienti, F.; Lorencini Selingardi, P.M.; Muszkat, D.; et al. Transcranial direct current stimulation in psychiatric disorders. World J. Psychiatry 2015, 5, 88–102. [Google Scholar] [CrossRef]
- Schachter, S.C.; Saper, C.B. Vagus nerve stimulation. Epilepsia 1998, 39, 677–686. [Google Scholar] [CrossRef]
- Lisanby, S.H. Electroconvulsive therapy for depression. N. Engl. J. Med. 2007, 357, 1939–1945. [Google Scholar] [CrossRef]
- Tess, A.V.; Smetana, G.W. Medical evaluation of patients undergoing electroconvulsive therapy. N. Engl. J. Med. 2009, 360, 1437–1444. [Google Scholar] [CrossRef]
- Haq, A.U.; Sitzmann, A.F.; Goldman, M.L.; Maixner, D.F.; Mickey, B.J. Response of depression to electroconvulsive therapy: A meta-analysis of clinical predictors. J. Clin. Psychiatry 2015, 76, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.; Helm, S.; Toto, S.; Moschny, N.; Sperling, W.; Hillemacher, T.; Kahl, K.G.; Jakubovski, E.; Bleich, S.; Frieling, H.; et al. S100B, homocysteine, vitamin B12, folic acid, and procalcitonin serum levels in remitters to electroconvulsive therapy: A pilot study. Dis. Markers 2018. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.I.F.; Dougall, N.; Ross, M.; O’Carroll, R.E.; Riddle, W.; Ebmeier, K.P.; Goodwin, G.M. Short-term effects of electroconvulsive treatment on the uptake of [Tc-99m] exametazine into brain in major depression shown with single photon emission tomography. J. Affect. Disord. 1994, 30, 27–34. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; Hellström, K.; Waldton, C.; Augustinsson, L.E. Evaluation of refractory epilepsy treated with vagus nerve stimulation for up to 5 years. Neurology 1999, 52, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menachem, E.; Manon-Espaillat, R.; Ristanovic, R.; Wilder, B.J.; Stefan, H.; Mirza, W.; Tarver, W.B.; Wernicke, J.F. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia 1994, 35, 616–626. [Google Scholar] [CrossRef] [PubMed]
- George, R.; Salinsky, M.; Kuzniecky, R.; Rosenfeld, W.; Bergen, D.; Tarver, W.B.; Wernicke, J.F. Vagus nerve stimulation for treatment of partial seizures: 3. Long-term follow-up on the first 67 patients exiting a controlled study. Epilepsia 1994, 35, 637–643. [Google Scholar] [CrossRef]
- Handforth, A.; DeGiorgio, C.M.; Schachter, S.C.; Uthman, B.M.; Naritoku, D.K.; Tecoma, E.S.; Henry, T.R.; Collins, S.D.; Vaughn, B.V.; Gilmartin, R.C.; et al. Vagus nerve stimulation therapy for partial-onset seizures: A randomized active-control trial. Neurology 1998, 51, 48–55. [Google Scholar] [CrossRef]
- Salinsky, M.C.; Uthman, B.M.; Ristanovic, R.K.; Wernicke, J.F.; Tarver, W.B. Vagus nerve stimulation for the treatment of medically intractable seizures. Results of a 1-year open-extension trial. Arch. Neurol. 1999, 53, 1176–1180. [Google Scholar] [CrossRef]
- The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology 1995, 45, 224–230. [Google Scholar] [CrossRef]
- Berry, S.M.; Broglio, K.; Bunker, M.; Jayewardene, A.; Olin, B.; Rush, A.J. A patient-level meta-analysis of studies evaluating vagus nerve stimulation therapy for treatment-resistant depression. Med. Devices 2013, 6, 17–35. [Google Scholar]
- Dell-Osso, B.; Oldani, L.; Palazzo, M.C.; Balossi, I.; Ciabatti, M.; Altamura, A.C. Vagus nerve stimulation in treatment-resistant depression: Acute and follow-up results of an Italian case series. J. ECT 2013, 29, 41–44. [Google Scholar]
- George, M.S.; Rush, A.J.; Marangell, L.B.; Sackeim, H.A.; Brannan, S.K.; Davis, S.M.; Howland, R.; Kling, M.A.; Moreno, F.; Rittberg, B.; et al. A one-year comparison of Vagus Nerve Stimulation with treatment as usual for treatment-resistant depression. Biol. Psychiatry 2005, 58, 364–373. [Google Scholar] [CrossRef]
- George, M.S.; Rush, A.J.; Sackeim, H.A.; Marangell, L. Vagus Nerve Stimulation (VNS): Utility in neuropsychiatric disorders. Int. J. Neuropsychopharmacol. 2003, 6, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Marangell, L.B.; Rush, A.J.; George, M.S.; Sackeim, H.A.; Johnson, C.R.; Husain, M.M.; Nahas, Z.; Lisanby, S.H. Vagus Nerve Stimulation (VNS) for major depressive episodes: Longer-term outcome. Biol. Psychiatry 2002, 51, 280–287. [Google Scholar] [CrossRef]
- Rush, A.J.; George, M.S.; Sackeim, H.A.; Marangell, L.B.; Husain, M.; Giller, C.; Nahas, Z.; Haines, S.; Simson, R.K.; Goodman, R.; et al. Vagus Nerve Stimulation (VNS) for treatment-resistant depression: A multicenter study. Biol. Psychiatry 2000, 47, 276–286. [Google Scholar] [CrossRef]
- Rush, A.J.; Marangell, L.B.; Sackeim, H.A.; George, M.S.; Brannan, S.K.; Davis, S.M.; Howland, R.; Kling, M.A.; Rittberg, B.R.; Burke, W.J.; et al. Vagus Nerve Stimulation for treatment-resistant depression: A randomized, controlled acute phase trial. Biol. Psychiatry 2005, 58, 347–354. [Google Scholar] [CrossRef]
- Rush, A.J.; Sackeim, H.A.; Marangell, L.B.; George, M.S.; Brannan, S.K.; Davis, S.M.; Lavori, P.; Howland, R.; Kling, M.A.; Rittberg, B.; et al. Effects of 12 Months of Vagus Nerve Stimulation in treatment-resistant depression: A naturalistic study. Biol. Psychiatry 2005, 58, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Sackeim, H.A.; Brannan, S.K.; Rush, A.J.; George, M.S.; Marangell, L.B.; Allen, J. Durability of antidepressant response to vagus nerve stimulation (VNS). Int. J. Neuropsychopharmacol. 2007, 10, 817–826. [Google Scholar] [CrossRef]
- Sackeim, H.A.; Keilp, J.G.; Rush, A.J.; George, M.S.; Marangell, L.B.; Dormer, J.S.; Burt, T.; Lisanby, S.H.; Husain, M.; Collum, M.; et al. The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry Neuropsychol. Behav. Neurol. 2001, 14, 53–62. [Google Scholar]
- Sackeim, H.A.; Rush, A.J.; George, M.S.; Marangell, L.B.; Husain, M.M.; Nahas, Z.; Johnson, C.R.; Seidman, S.; Giller, C.; Haines, S.; et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: Efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 2001, 25, 713–728. [Google Scholar] [CrossRef]
- Johnson, R.L.; Wilson, C.G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 2018, 11, 203–211. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Sackeim, H.A.; Rush, A.J.; Marangell, L.B.; Nahas, Z.; Husain, M.M.; Lissanby, S.H.; Burt, T.; Goldman, J.; Ballenger, J.C. Vagus Nerve Stimulation: A new tool for brain research and therapy. Biol. Psychiatry 2000, 47, 287–295. [Google Scholar] [CrossRef]
- Aaronson, S.T.; Sears, P.; Ruvuna, F.; Bunker, M.; Conway, C.R.; Dougherty, D.D.; Reimherr, F.W.; Schwartz, T.L.; Zajecka, J.M. A five-year observational study of patients with treatment-resistant depression treated with VNS therapy or treatment-as-usual: Comparison of response, remission, and suicidality. Am. J. Psychiatry 2017, 174, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.S. Vagus Nerve Stimulation for Epilepsy. Medicine 2014. [Google Scholar] [CrossRef]
- Noble, I.J.; Gonzalez, I.J.; Meruva, V.B.; Callahan, K.A.; Belfort, B.D.; Ramanathan, K.R.; Meyers, E.; Kilgard, M.P.; Rennaker, R.L.; McIntyre, C.K. Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Transl. Psychiatry 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pena, D.F.; Childs, J.E.; Willett, S.; Vital, A.; McIntyre, C.K.; Kroener, S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front. Behav. Neurosci. 2014, 8, 1–8. [Google Scholar] [CrossRef]
- Schomer, A.C.; Nearing, B.D.; Schachter, S.C.; Verrier, R.L. Vagus nerve stimulation reduces cardiac electrical instability assessed by quantitative T-wave alternans analysis in patients with drug-resistant focal epilepsy. Epilepsia 2014, 55, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Groves, D.A.; Brown, V.J. Vagal nerve stimulation: A review of its applications and potential mechanisms that mediate its clinical effects. Neurosci. Biobehav. Rev. 2005, 29, 493–500. [Google Scholar] [CrossRef]
- Hays, S.A.; Rennaker, R.L.; Kilgard, M.P. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog. Brain Res. 2013, 207, 275–299. [Google Scholar]
- Polak, T.; Markulin, F.; Ehlis, A.-C.; Langer, J.B.M.; Ringel, T.M.; Fallgatter, A.J. Far field potentials from brain stem after transcutaneous vagus nerve stimulation: Optimization of stimulation and recording parameters. J. Neural Transm. 2009, 116, 1237–1242. [Google Scholar] [CrossRef]
- Player, M.J.; Taylor, J.L.; Weickert, C.S.; Alonzo, A.; Sachdev, P.S.; Martin, D.; Mitchell, P.B.; Loo, C.K. Increase in PAS-induced neuroplasticity after a treatment course of transcranial direct current stimulation for depression. J. Affect. Disord. 2014, 167, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Popovic, Z.B.; Bibevski, S.; Fakhry, I.; Sica, D.A.; Van Wagoner, D.R.; Mazgalev, T.N. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ. Heart Fail. 2009, 2, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Peña, D.F.; Engineer, N.D.; McIntyre, C.K. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol. Psychiatry 2013, 73, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.R.; Robertson, N.M.; Pruitt, D.T.; Gonzales, P.A.; Hays, S.A.; Rennaker, R.L.; Kilgard, M.P.; McIntyre, C.K. Vagus nerve stimulation reverses the extinction impairments in a model of PTSD with prolonged and repeated trauma. Stress 2019, 22, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Schacter, S.C. Vagus nerve stimulation: Mood and cognitive effects. Epilepsy Behav. 2004, 5, S56–S59. [Google Scholar] [CrossRef] [PubMed]
- McIntire, L.; McKinley, A.; Goodyear, C. Peripheral nerve stimulation to augment human analyst performeance. IEEE 2019. [Google Scholar] [CrossRef]
- Clark, K.B.; Krahl, S.E.; Smith, D.C.; Jensen, R.A. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol. Learn. Mem. 1995, 63, 213–216. [Google Scholar] [CrossRef]
- Clark, K.B.; Naritoku, D.K.; Smith, D.C.; Browning, R.A.; Jensen, R.A. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat. Neurosci. 1999, 2, 94–98. [Google Scholar] [CrossRef]
- Clark, K.B.; Smith, D.C.; Hassert, D.L.; Browning, R.A.; Naritoku, D.K.; Jensen, R.A. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol. Learn. Mem. 1998, 70, 364–373. [Google Scholar] [CrossRef]
- Flood, J.F.; Smith, G.E.; Morley, J.E. Modulation of memory processing by cholecystokinin: Dependence on the vagus nerve. Science 1987, 236, 832–834. [Google Scholar] [CrossRef]
- Ghacibeh, G.A.; Shenker, J.I.; Shenal, B.; Uthman, B.M.; Heilman, K.M. The influence of vagus nerve stimulation on memory. Cogn. Behav. Neurol. 2006, 19, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Ghacibeh, G.A.; Shenker, J.I.; Shenal, B.; Uthman, B.M.; Heilman, K.M. Effect of vagus nerve stimulation on creativity and cognitive flexibility. Epilepsy Behav. 2006, 8, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.I.L.; Riphagen, J.M.; Razat, C.M.; Wiese, S.; Sack, A.T. Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol. Aging 2015, 36, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Merrill, C.A.; Jonsson, M.A.; Minthon, L.; Ejnell, H.; Silander, H.C.; Blennow, K.; Karlsson, M.; Nordlund, A.; Rolstad, S.; Warkentin, S.; et al. Vagus nerve stimulation in patients with Alzheimer’s disease: Additional follow-up results of a pilot study through 1 year. J. Clin. Psychiatry 2006, 67, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Vonck, K.; Raedt, R.; Naulaerts, J.; De Vogelaere, F.; Thiery, E.; Van Roost, D.; Aldenkamp, B.; Miatton, M.; Boon, P. Vagus nerve stimulation. 25 years later! What do we know about the effects on cognition? Neurosci. Biobehav. Rev. 2014, 45, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Follesa, P.; Biggio, F.; Gorini, G.; Caria, S.; Talani, G.; Dazzi, L.; Puligheddu, M.; Marrosu, F.; Biggio, G. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007, 1179, 28–34. [Google Scholar] [CrossRef]
- Vida, G.; Pena, G.; Kanashiro, A.; Thompson-Bonilla, M.d.R.; Palange, D.; Deitch, E.A.; Ulloa, L. B2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 2011, 25, 4476–4485. [Google Scholar] [CrossRef]
- Bansal, V.; Ryu, S.Y.; Lopez, N.; Allexan, S.; Krzyzaniak, M.; Eliceiri, B.; Baird, A.; Coimbra, R. Vagal stimulation modulates inflammation through a ghrelin mediated mechanism in traumatic brain injury. Inflammation 2012, 35, 214–220. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Corsi-Zuelli, F.M.G.; Brognara, F.; Quirino, G.F.S.; Hiroki, C.H.; Fais, R.S.; Del-Ben, C.M.; Ulloa, L.; Salgado, H.C.; Kanashiro, A. Neuroimmune interactions in schizophrenia: Focus on vagus nerve stimulation and activation of the alpha-7 nicotinic acetylcholine receptor. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Cunningham, J.T.; Mifflin, S.W.; Gould, G.G.; Frazer, A. Induction of c-Fos and delta-FosB immunoreactivity in rat brain by vagal nerve stimulation. Neuropsychopharmacology 2008, 33, 1884–1895. [Google Scholar] [CrossRef] [PubMed]
- De Herdt, V.; Bogaert, S.; Bracke, K.R.; Raedt, R.; De Vos, M.; Vonck, K.; Boon, P. Effects of vagus nerve stimulation on pro- and anti-inflammatory cytokine induction in patients with refractory epilepsy. J. Neuroimmunol. 2009, 214, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Olshansky, B. Inflammatory cytokines and nitric oxide in heart failure and potential modulation by vagus nerve stimulation. Heart Fail. Rev. 2011, 16, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Majoie, H.J.M.; Rijkers, K.; Berfelo, M.W.; Hulsman, J.A.R.J.; Myint, A.; Schwarz, M.; Vles, J.S.H. Vagus nerve stimulation in refractory epilepsy: Effects on pro-and anti-inflammatory cytokines in peripheral blood. Neuroimmunomodulation 2011, 18, 52–56. [Google Scholar] [CrossRef]
- Elzinga, B.M.; Bremner, J.D. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J. Affect. Disord. 2002, 70, 1–17. [Google Scholar] [CrossRef]
- Chen, S.-P.; Ayd, I.; de Moraisa, A.L.; Qina, T.; Zhenga, Y.; Sadeghiana, H.; Okaa, F.; Simon, B.; Eikermann-Haertera, K.; Ayataa, C. Vagus nerve stimulation inhibits cortical spreading depression. Cephalagia 2015, 35, 219–221. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; Hamberger, A.; Hedner, T.; Hammond, E.J.; Uthman, B.M.; Slater, J.; Treig, T.; Stefan, H.; Ramsay, R.E.; Wernicke, J.F.; et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995, 20, 221–227. [Google Scholar] [CrossRef]
- Roosevelt, R.W.; Smith, D.C.; Clough, R.W.; Jensen, R.A.; Browning, R.A. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain 2006, 1119, 124–132. [Google Scholar] [CrossRef]
- Oshinsky, M.L.; Murphy, A.L.; Hekierski, H.; Cooper, M.; Simon, B.J. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain 2014, 155, 1042–2037. [Google Scholar] [CrossRef]
- Hays, S.A.; Khodaparast, N.; Hulsey, D.R.; Ruiz, A.; Sloan, A.M.; Rennaker, R.L.; Kilgard, M.P. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke 2014, 45, 3097–3100. [Google Scholar] [CrossRef]
- Engineer, C.T.; Engineer, N.D.; Riley, J.R.; Seale, J.D.; Kilgard, M.P. Pairing speech sounds with vagus nerve stimulation drives stimulus-specific cortical plasticity. Brain Stimul. 2015, 8, 637–644. [Google Scholar] [CrossRef]
- Engineer, N.D.; Riley, J.R.; Seale, J.D.; Vrana, W.A.; Shetake, J.A.; Sudanagunta, S.P.; Borland, M.S.; Kilgard, M.P. Reversing pathological neural activity using targeted plasticity. Nature 2011, 470, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Shim, H.-J.; Kwak, M.Y.; An, Y.-H.; Kim, D.H.; Kim, Y.J. Feasibility and safety of transcutaneous vagus nerve stimulation paired with notched music therapy for the treatment of chronic tinnitus. J. Audiol. Otol. 2015, 18, 159–167. [Google Scholar]
- Li, T.-T.; Wang, Z.-J.; Yang, S.-B.; Zhu, J.-H.; Zhang, S.-Z.; Cai, S.-J.; Ma, W.-H.; Zhang, D.-Q.; Mei, A.-G. Transcutaneous electrical stimulation at auricular acupoints innervated by auricular branch of vagus nerve pairing tone for tinnitus: Study protocol for a randomized controlled clinical trial. Trials 2015, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhao, F.-B.; Wang, J.; Lu, Y.F.; Tian, J.; Zhao, Y.; Gao, Y.; Hu, X.-J.; Liu, X.-Y.; Tan, J.; et al. Effects of vagus nerve stimulation on cognitive functioning in rats with cerebral ischemia reperfusion. J. Transl. Med. 2016, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.A. Enhancing rehabilitative therapies with vagus nerve stimulation. Neurotherapeutics 2016, 13, 382–394. [Google Scholar] [CrossRef]
- Hays, S.A.; Ruiz, A.; Bethea, T.; Khodaparast, N.; Carmel, J.B.; Rennaker, R.L.; Kilgard, M.P. Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol. Aging 2016, 43, 111–118. [Google Scholar] [CrossRef]
- Khodaparast, N.; Kilgard, M.P.; Casavant, R.; Ruiz, A.; Qureshi, I.; Ganzer, P.D.; Rennaker, R.L.; Hays, S.A. Vagus nerve stimulation during rehabilitative training improves forelimb recovery after chronic ischemic stroke in rats. Neurorehabil. Neural Repair 2015, 30, 676–684. [Google Scholar] [CrossRef]
- Pruitt, D.T.; Schmid, A.N.; Kim, L.L.; Abe, C.M.; Trieu, J.L.; Choua, C. Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury. J. Neurotrauma 2016, 33, 871–879. [Google Scholar] [CrossRef]
- Suthana, N.; Fried, I. Deep brain stimulation for enhancement of learning and memory. Neuroimage 2014, 85, 996–1002. [Google Scholar] [CrossRef]
- Zuo, Y.; Smith, D.C.; Jensen, R.A. Vagus nerve stimulation potentiates hippocampal LTP in freely-moving rats. Physiol. Behav. 2007, 90, 583–589. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.A.; Alves, S.; Sheridan, M.A. Vagal regulation and internalizing psychopathology among adolescents exposed to childhood adversity. Dev. Psychobiol. 2014, 56, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zheng, C.; Sato, T.; Kawada, T.; Sugimachi, M.; Sunagawa, K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004, 109, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R.; Pearlman, A.; Hyman, R. Beneficial effects of vagal stimulation and bradycardia during experimental acute myocardial ischemia. Circulation 1974, 49, 943–947. [Google Scholar] [CrossRef]
- Kent, K.M.; Smith, E.R.; Redwood, D.R.; Epstein, S.E. Electrical stability of acutely ischemic myocardium: Influences to heart rate and vagal stimulation. Circulation 1973, 47, 291–298. [Google Scholar] [CrossRef]
- Bohning, D.E.; Lomarev, M.P.; Denslow, S.; Nahas, Z.; Shastri, A.; George, M.S. Vagus Nerve Stimulation (VNS) synchronized BOLD-fMRI. Radiology 2001, 36, 470–479. [Google Scholar]
- Chae, J.H.; Nahas, Z.; Lomarev, M.; Denslow, S.; Lorberbaum, J.P.; Bohning, D.E.; George, M.S. A review of functional neuroimaging studies of Vagus Nerve Stimulation (VNS). J. Psychiatr. Res. 2003, 37, 443–455. [Google Scholar] [CrossRef]
- Smith, M.A.; Makino, S.; Kvetnansky, R.; Post, R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNA in the hippocampus. J. Neurosci. 1995, 15, 1768–1777. [Google Scholar] [CrossRef]
- Diamond, D.M.; Fleshner, M.; Ingersoll, N.; Rose, G.M. Psychological stress impairs spatial working memory: Relevance to electrophysiological studies of hippocampal function. Behav. Neurosci. 1996, 110, 661–672. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Krey, L.; McEwen, B. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. J. Neurosci. 1985, 5, 1221–1226. [Google Scholar] [CrossRef]
- Woolley, C.S.; Gould, E.; McEwen, B.S. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990, 531, 225–231. [Google Scholar] [CrossRef]
- Elzinga, B.M.; Bermond, B.; van Dyck, R. The relationship between dissociative proneness and alexithymia. Psychother. Psychosom. 2002, 71, 104–111. [Google Scholar] [CrossRef]
- Bremner, J.D.; Vermetten, E. The hippocampus and post-traumatic stress disorders. In The Clinical Neurobiology of the Hippocampus: An Integrative View; Bartsch, T., Ed.; Oxford University Press: Oxford, UK, 2012; pp. 262–272. [Google Scholar]
- Bremner, J.D. Structural changes in the brain in depression and relationship to symptom recurrence. CNS Spectr. 2002, 7, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D. Alterations in brain structure and function associated with posttraumatic stress disorder. Semin. Clin. Neuropsychiatry 1999, 4, 249–255. [Google Scholar]
- Sheline, Y.I.; Wang, P.; Gado, M.; Csernansky, J.; Vannier, M. Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. USA 1996, 93, 3908–3913. [Google Scholar] [CrossRef]
- LeDoux, J.E. The Emotional Brain: The Mysterious Underpinnings of Emotional Life; Simon & Schuster: New York, NY, USA, 1996. [Google Scholar]
- Quirk, G.J. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn. Mem. 2002, 9, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D.; Staib, L.; Kaloupek, D.; Southwick, S.M.; Soufer, R.; Charney, D.S. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biol. Psychiatry 1999, 45, 806–816. [Google Scholar] [CrossRef]
- Britton, J.C.; Phan, K.L.; Taylor, S.F.; Fig, L.M.; Liberzon, I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol. Psychiatry 2005, 57, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.M.; McNally, R.J.; Kosslyn, S.M.; Thompson, W.L.; Rauch, S.L.; Alpert, N.M.; Metzger, L.J.; Lasko, N.B.; Orr, S.P.; Pitman, R.K. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am. J. Psychiatry 1999, 156, 575–584. [Google Scholar] [PubMed]
- Shin, L.M.; Kosslyn, S.M.; McNally, R.J.; Alpert, N.M.; Thompson, W.L.; Rauch, S.L.; Macklin, M.L.; Pitman, R.K. Visual imagery and perception in posttraumatic stress disorder: A positron emission tomographic investigation. Arch. Gen. Psychiatry 1997, 54, 233–237. [Google Scholar] [CrossRef]
- Shin, L.M.; Orr, S.P.; Carson, M.A.; Rauch, S.L.; Macklin, M.L.; Lasko, N.B.; Peters, P.M.; Metzger, L.J.; Dougherty, D.D.; Cannistraro, P.A.; et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch. Gen. Psychiatry 2004, 61, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Fonzo, G.A.; Simmons, A.N.; Thorp, S.R.; Norman, S.B.; Paulus, M.P.; Stein, M.B. Blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol. Psychiatry 2010, 68, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.L.; Britton, J.C.; Taylor, S.F.; Fig, L.M.; Liberzon, I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch. Gen. Psychiatry 2006, 63, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wu, M.T.; Hsu, C.C.; Ker, J.H. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: A functional MRI study. Neurosci. Lett. 2004, 370, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.M.; Whalen, P.J.; Pitman, R.K.; Bush, G.; Macklin, M.L.; Lasko, N.B.; Orr, S.P.; McInerney, S.C.; Rauch, S.L. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol. Psychiatry 2001, 50, 932–942. [Google Scholar] [CrossRef]
- Hopper, J.W.; Frewen, P.A.; van der Kolk, B.A.; Lanius, R.A. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J. Trauma. Stress 2007, 20, 713–725. [Google Scholar] [CrossRef]
- Hou, C.; Liu, J.; Wang, K.; Li, L.; Liang, M.; He, Z.; Liu, Y.; Zhang, Y.; Li, W.; Jiang, T. Brain responses to symptom provocation and trauma-related short-term memory recall in coal mining accident survivors with acute severe PTSD. Brain Res. 2007, 1144, 165–174. [Google Scholar] [CrossRef]
- Lanius, R.A.; Williamson, P.C.; Hopper, J.; Densmore, M.; Boksman, K.; Gupta, M.A.; Neufeld, R.W.; Gati, J.S.; Menon, R.S. Recall of emotional states in posttraumatic stress disorder: An fMRI investigation. Biol. Psychiatry 2003, 53, 204–210. [Google Scholar] [CrossRef]
- Lanius, R.A.; Williamson, P.C.; Densmore, M.; Boksman, K.; Gupta, M.A.; Neufeld, R.W.; Gati, J.S.; Menon, R.S. Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. Am. J. Psychiatry 2001, 158, 1920–1922. [Google Scholar] [CrossRef]
- Liberzon, I.; Taylor, S.F.; Amdur, R.; Jung, T.D.; Chamberlain, K.R.; Minoshima, S.; Koeppe, R.A.; Fig, L.M. Brain activation in PTSD in response to trauma-related stimuli. Biol. Psychiatry 1999, 45, 817–826. [Google Scholar] [CrossRef]
- Liberzon, I.; Britton, J.C.; Phan, K.L. Neural correlates of traumatic recall in posttraumatic stress disorder. Stress 2003, 6, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.M.; Wright, C.I.; Cannistraro, P.A.; Wedig, M.M.; McMullin, K.; Martis, B.; Macklin, M.L.; Lasko, N.B.; Cavanagh, S.R.; Krangel, T.S.; et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch. Gen. Psychiatry 2005, 62, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Mayberg, H.S.; Liotti, M.; Brannan, S.K.; McGinnis, S.; Mahurin, R.K.; Jerabek, P.A.; Silva, J.A.; Tekell, J.L.; Martin, C.C.; Lancaster, J.L.; et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am. J. Psychiatry 1999, 156, 675–682. [Google Scholar] [PubMed]
- Sheline, Y.I.; Barcha, D.M.; Price, J.L.; Rundleb, M.M.; Vaishnavib, S.N.; Snyderb, A.Z.; Mintun, M.A.; Wanga, S.; Coalson, R.S.; Raichle, M.E. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. USA 2009, 106, 1942–1947. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Simpson, J.R.J.; Todd, R.D.; Reich, T.; Vannier, M.; Raichle, M.E. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997, 386, 824–827. [Google Scholar] [CrossRef]
- Simmons, A.N.; Paulus, M.P.; Thorp, S.R.; Matthews, S.C.; Norman, S.B.; Stein, M.B. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol. Psychiatry 2008, 64, 681–690. [Google Scholar] [CrossRef]
- Rauch, S.L.; van der Kolk, B.A.; Fisler, R.E.; Alpert, N.M.; Orr, S.P.; Savage, C.R.; Fischman, A.J.; Jenike, M.A.; Pitman, R.K. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch. Gen. Psychiatry 1996, 53, 380–387. [Google Scholar] [CrossRef]
- Admon, R.; Lubin, G.; Stern, O.; Rosenberg, K.; Sela, L.; Ben-Ami, H.; Hendler, T. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proc. Natl. Acad. Sci. USA 2009, 106, 14120–14125. [Google Scholar] [CrossRef]
- Bremner, J.D.; Vermetten, E.; Schmahl, C.; Vaccarino, V.; Vythilingam, M.; Afzal, N.; Grillon, C.; Charney, D.S. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual abuse-related posttraumatic stress disorder. Psychol. Med. 2005, 35, 791–806. [Google Scholar] [CrossRef]
- Rauch, S.L.; Shin, L.M.; Wright, C.I. Neuroimaging studies of amygdala function in anxiety disorders. Ann. N. Y. Acad. Sci. 2003, 985, 389–410. [Google Scholar] [CrossRef]
- Protopopescu, X.; Pan, H.; Tuescher, O.; Cloitre, M.; Goldstein, M.; Engelien, W.; Epstein, J.; Yang, Y.; Gorman, J.; LeDoux, J.; et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol. Psychiatry 2005, 57, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.A.; Kim, S.H.; Chung, S.K.; Chae, J.H.; Yang, D.W.; Sohn, H.S.; Jeong, J. Alterations in cerebral perfusion in posttraumatic stress disorder patients without re-exposure to accident-related stimuli. Clin. Neurophysiol. 2006, 117, 637–642. [Google Scholar] [CrossRef]
- Felmingham, K.L.; Williams, L.M.; Kemp, A.H.; Rennie, C.; Gordon, E.; Bryant, R.A. Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in posttraumatic stress disorder. Psychiatry Res. 2009, 173, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Semple, W.E.; Goyer, P.; McCormick, R.; Donovan, B.; Muzic, R.F.; Rugle, L.; McCutcheon, K.; Lewis, C.; Liebling, D.; Kowaliw, S.; et al. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared to controls. Psychiatry 2000, 63, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Felmingham, K.L.; Kemp, A.H.; Barton, M.; Peduto, A.S.; Rennie, C.; Gordon, E.; Williams, L.M. Neural networks of information processing in posttraumatic stress disorder: A functional magnetic resonance imaging study. Biol. Psychiatry 2005, 58, 111–118. [Google Scholar] [CrossRef]
- Armony, J.L.; Corbo, V.; Clement, M.H.; Brunet, A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am. J. Psychiatry 2005, 162, 1961–1963. [Google Scholar] [CrossRef]
- Bryant, R.A.; Kemp, A.H.; Felmingham, K.L.; Liddell, B.; Olivieri, G.; Peduto, A.; Gordon, E.; Williams, L.M. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum. Brain Mapp. 2008, 29, 517–523. [Google Scholar] [CrossRef]
- Kemp, A.H.; Felmingham, K.; Das, P.; Hughes, G.; Peduto, A.S.; Bryant, R.A.; Williams, L.M. Influence of comorbid depression on fear in posttraumatic stress disorder: An fMRI study. Psychiatry Res. 2007, 155, 265–269. [Google Scholar] [CrossRef]
- Kemp, A.H.; Felmingham, K.L.; Falconer, E.; Liddell, B.J.; Bryant, R.A.; Williams, L.M. Heterogeneity of non-conscious fear perception in posttraumatic stress disorder as a function of physiological arousal: An fMRI study. Psychiatry Res. 2009, 174, 158–161. [Google Scholar] [CrossRef]
- Rauch, S.L.; Whalen, P.J.; Shin, L.M.; McInerney, S.C.; Macklin, M.L.; Lasko, N.B.; Orr, S.P.; Pitman, R.K. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biol. Psychiatry 2000, 47, 769–776. [Google Scholar] [CrossRef]
- Brohawn, K.H.; Offringa, R.; Pfaff, D.L.; Hughes, K.C.; Shin, L.M. The neural correlates of emotional memory in posttraumatic stress disorder. Biol. Psychiatry 2010, 68, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, M.; Sepede, G.; Mingoia, G.; Catani, C.; Ferretti, A.; Merla, A.; Del Gratta, C.; Romani, G.L.; Babiloni, C. Elevated response of human amygdala to neutral stimuli in mild post traumatic stress disorder: Neural correlates of generalized emotional response. Neuroscience 2010, 168, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Pissiota, A.; Frans, O.; Fernandez, M.; Von Knorring, L.; Fischer, H.; Fredrikson, M. Neurofunctional correlates of posttraumatic stress disorder: A PET symptom provocation study. Eur. Arch. Psychiatry Clin. Neurosci. 2002, 252, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Milad, M.R.; Pitman, R.K.; Ellis, C.B.; Gold, A.L.; Shin, L.M.; Lasko, N.B.; Zeidan, M.A.; Handwerger, K.; Orr, S.P.; Rauch, S.L. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry 2009, 66, 1075–1082. [Google Scholar] [CrossRef]
- Drevets, W.C.; Raichle, M.E. Neuroanatomical circuits in depression: Implications for treatment mechanisms. Psychopharmacol. Bull. 1992, 28, 261–274. [Google Scholar]
- Drevets, W.C.; Price, J.L.; Bardgett, M.E.; Reich, T.; Todd, R.D.; Raichle, M.E. Glucose metabolism in the amygdala in depression: Relationship to diagnostic subtype and plasma cortisol levels. Pharmacol. Biochem. Behav. 2002, 71, 431–447. [Google Scholar] [CrossRef]
- Saxena, S.; Brody, A.L.; Ho, M.L.; Zohrabi, N.; Maidment, K.M.; Baxter, L.R. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am. J. Psychiatry 2003, 160, 522–532. [Google Scholar] [CrossRef]
- Sheline, Y.I.; Barch, D.M.; Donnelly, J.M.; Ollinger, J.M.; Snyder, A.Z.; Mintun, M.A. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol. Psychiatry 2001, 50, 651–658. [Google Scholar] [CrossRef]
- Bremner, J.D.; Campanella, C. Effects of psychotherapy for psychological trauma on PTSD symptoms and the brain. In Posttraumatic Stress Disorder: From Neurobiology to Treatment; Bremner, J.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 413–420. [Google Scholar]
- Vermetten, E.; Vythilingam, M.; Southwick, S.M.; Charney, D.S.; Bremner, J.D. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry 2003, 54, 693–702. [Google Scholar] [CrossRef]
- Letizia, B.; Andrea, F.; Paolo, C. Neuroanatomical changes after eye movement desensitization and reprocessing (EMDR) treatment in posttraumatic stress disorder. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 475–476. [Google Scholar] [CrossRef]
- Bremner, J.D.; Mletzko, T.; Welter, S.; Quinn, S.; Williams, C.; Brummer, M.; Siddiq, S.; Reed, L.; Heim, C.M.; Nemeroff, C.B. Effects of phenytoin on memory, cognition and brain structure in posttraumatic stress disorder: A pilot study. J. Psychopharmacol. 2005, 19, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Fani, N.; Kitayama, N.; Ashraf, A.; Reed, L.; Afzal, N.; Jawed, F.; Bremner, J.D. Neuropsychological functioning in patients with posttraumatic stress disorder following short-term paroxetine treatment. Psychopharmacol. Bull. 2009, 42, 53–68. [Google Scholar] [PubMed]
- Fani, N.; Ashraf, A.; Afzal, N.; Jawed, F.; Kitayama, N.; Reed, L.; Bremner, J.D. Increased neural response to trauma scripts in posttraumatic stress disorder following paroxetine treatment: A pilot study. Neurosci. Lett. 2011, 491, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Brody, A.L.; Saxena, S.; Stoessel, P.; Gillies, L.A.; Fairbanks, L.A.; Alborzian, S.; Phelps, M.E.; Huang, S.C.; Wu, H.M.; Ho, M.L.; et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: Preliminary findings. Arch. Gen. Psychiatry 2001, 58, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.D.; Vythilingam, M.; Vermetten, E.; Charney, D.S. Effects of antidepressant treatment on neural correlates of emotional and neutral declarative verbal memory in depression. J. Affect. Disord. 2007, 101, 99–111. [Google Scholar] [CrossRef][Green Version]
- Drevets, W.C.; Bogers, W.; Raichle, M.E. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur. Neuropsychopharmacol. 2002, 12, 527–544. [Google Scholar] [CrossRef]
- Kennedy, S.H.; Evans, K.R.; Kruger, S.; Mayberg, H.S.; Meyer, J.H.; McCann, S.; Arifuzzman, A.I.; Houle, S.; Vaccarino, F.J. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am. J. Psychiatry 2001, 158, 899–905. [Google Scholar] [CrossRef]
- Vythilingam, M.; Vermetten, E.; Anderson, G.M.; Luckenbaugh, D.; Anderson, E.R.; Snow, J.; Staib, L.H.; Charney, D.S.; Bremner, J.D. Hippocampal volume, memory and cortisol status in major depressive disorder: Effects of treatment. Biol. Psychiatry 2004, 56, 101–112. [Google Scholar] [CrossRef]
- Henry, T.R. Therapeutic mechanisms of vagus nerve stimulation. Neurology 2002, 59, S3–S14. [Google Scholar] [CrossRef]
- Henry, T.R.; Bakay, R.A.; Votaw, J.R.; Pennell, P.B.; Epstein, C.M.; Faber, T.L.; Grafton, S.T.; Hoffman, J.M. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia 1998, 39, 983–990. [Google Scholar] [CrossRef]
- Conway, C.R.; Sheline, Y.I.; Chibnall, J.T.; Bucholz, R.D.; Price, J.L.; Gangwani, S.; Mintun, M.A. Brain blood-flow change with acute vagus nerve stimulation in treatment-refractory major depressive disorder. Brain Stimul. 2012, 5, 163–171. [Google Scholar] [CrossRef]
- Fang, J.; Egorova, N.; Rong, P.; Liu, J.; Hong, Y.; Fan, Y.; Wang, X.; Wang, H.; Yu, Y.; Ma, Y.; et al. Early cortical biomarkers of longitudinal transcutaneous vagus nerve stimulation treatment success in depression. Neuroimage Clin. 2017, 14, 105–111. [Google Scholar] [CrossRef]
- Liu, J.; Fang, J.; Wang, Z.; Rong, P.; Hong, Y.; Fan, Y.; Wang, X.; Park, J.; Jin, Y.; Liu, C.; et al. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J. Affect. Disord. 2016, 205, 319–326. [Google Scholar] [CrossRef]
- Lomarev, M.; Denslow, S.; Nahas, Z.; Chae, J.-H.; George, M.S.; Bohning, D.E. Vagus nerve stimulation (VNS): Synchronized BOLD fMRI suggests that VNS in depressed adults has frequency and/or dose dependent effects at rest and during a simple task. J. Psychiatr. Res. 2002, 36, 219–227. [Google Scholar] [CrossRef]
- Van Laere, K.; Vonck, K.; Boon, P.; Versijpt, J.; Dierckx, R. Perfusion SPECT changes after acute and chronic vagus nerve stimulation in relation to prestimulus condition and long-term efficacy. J. Nucl. Med. 2002, 43, 733–744. [Google Scholar] [PubMed]
- Bremner, J.D.; Wittbrodt, M.T.; Gurel, N.Z.; Nye, J.; Alam, A.; Vaccarino, V.; Ladd, S.L.; Shallenberger, L.H.; Huang, M.; Ko, Y.-Y.; et al. Brain correlates of non-invasive Vagal Nerve Stimulation in stress. In Proceedings of the NYC Neuromodulation/NANS Conference, New York, NY, USA, 24–26 August 2018; p. 14. [Google Scholar]
- Bremner, J.D.; Rapaport, M.H. Vagus Nerve Stimulation: Back to the future. Am. J. Psychiatry 2017, 174, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Yakunina, N.; Kim, S.S.; Nam, E.-C. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation 2017, 20, 290–300. [Google Scholar] [CrossRef]
- Redgrave, J.; Day, D.; Leung, H.; Ali, A.; Lindert, R.; Majid, A. Safety and tolerability of transcutaneous vagus nerve stimulation in humans: A systematic review. Brain Stimul. 2018, 11, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menachem, E.; Revesz, D.; Simon, B.J.; Silberstein, S. Surgically implanted and non-invasive vagus nerve stimulation: A review of efficacy, safety and tolerability. Eur. J. Neurol. 2015, 22, 1260–1268. [Google Scholar] [CrossRef]
- Nonis, R.; D’Ostilio, K.; Schoenen, J.; Magis, D. Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: An electrophysiological study in healthy volunteers. Cephalagia 2017, 37, 1285–1293. [Google Scholar] [CrossRef]
- Usami, K.; Kawai, K.; Sonoo, M.; Saito, N. Scalp-recorded evoked potentials as a marker for afferent nerve impulse in clinical vagus nerve stimulation. Brain Stimul. 2013, 6, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Yoo, P.B.; Lubock, N.B.; Hincapie, J.G.; Ruble, S.B.; Hamann, J.J.; Grill, W.M. High-resolution measurement of electrically-evoked vagus nerve activity in the anesthetized dog. J. Neural Eng. 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Fallgatter, A.J.; Neuhauser, B.; Herrmann, M.J.; Ehlis, A.-C.; Wagener, A.; Scheuerpflug, P.; Reiners, K.; Riederer, P. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J. Neural Transm. 2003, 110, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Frangos, E.; Ellrich, E.; Komisaruk, B.R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015, 8, 624–636. [Google Scholar] [CrossRef]
- Badran, B.W.; Dowdle, L.T.; Mithoefer, O.J.; LaBate, N.T.; Coatsworth, J.; Brown, J.C.; DeVries, W.H.; Austelle, C.W.; McTeague, L.M.; George, M.S. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul. 2018, 11, 492–500. [Google Scholar] [CrossRef]
- Frangos, E.; Komisaruk, B.R. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain Stimul. 2017, 10, 19–27. [Google Scholar] [CrossRef]
- Clancy, J.A.; Mary, D.A.; Witte, K.K.; Greenwood, J.P.; Deuchars, S.A.; Deuchars, J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014, 7, 871–877. [Google Scholar] [CrossRef]
- Badran, B.W.; Mithoefer, O.J.; Summer, C.E.; LaBate, N.T.; Glusman, C.E.; Badran, A.W.; DeVries, W.H.; Summers, P.M.; Austelle, C.W.; McTeague, L.M.; et al. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul. 2018, 11, 699–708. [Google Scholar] [CrossRef]
- Warren, C.M.; Tona, K.D.; Ouwerkerk, L.; van Paridon, J.; Poletiek, F.; van Steenbergen, H.; Bosch, J.A.; Nieuwenhuis, S. The neuromodulatory and hormonal effects of transcutaneous vagus nerve stimulation as evidenced by salivary alpha amylase, salivary cortisol, pupil diameter, and the P3 event-related potential. Brain Stimul. 2019, 12, 635–642. [Google Scholar] [CrossRef]
- Burger, A.M.; Verkuil, B.; Fenlon, H.; Thijs, L.; Cools, H.C.; Miller, I.; Vervliet, B.; Van Diest, I. Mixed evidence for the potential of non-invasive transcutaneous vagal nerve stimulation to improve the extinction and retention of fear. Behav. Res. Ther. 2017, 97, 64–74. [Google Scholar] [CrossRef]
- Verkuil, B.; Burger, A.M. Transcutaneous vagus nerve stimulation does not affect attention to fearful faces in high worriers. Behav. Res. Ther. 2019, 113, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Gurel, N.Z.; Huang, M.; Wittbrodt, M.T.; Jung, H.; Ladd, S.L.; Shandhi, M.H.; Ko, Y.-A.; Shallenberger, L.; Nye, J.A.; Pearce, B.; et al. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul. 2020, 13, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Gurel, N.Z.; Gazi, A.H.; Scott, K.L.; Wittbrodt, M.T.; Shah, A.J.; Vaccarino, V.; Bremner, J.D.; Inan, O.T. Timing considerations for noninvasive Vagal Nerve Stimulation in clinical studies. AMIA Annu. Symp. Proc. 2020, 2019, 1061–1070. [Google Scholar] [PubMed]
- Gurel, N.Z.; Wittbrodt, W.T.; Jung, H.; Ladd, S.L.; Shah, A.J.; Vaccarino, V.; Bremner, J.D.; Inan, O.T. Automatic detection of target engagement in transcutaneous cervical Vagal Nerve Stimulation for traumatic stress triggers. IEEE J. Biomed. Health Inform. 2020, 24, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Brock, C.; Brock, B.; Aziz, Q.; Møller, H.J.; Pfeiffer Jensen, M.; Drewes, A.M.; Farmer, A.D. Transcutaneous cervical vagal nerve stimulation modulates cardiac vagal tone and tumor necrosis factor-alpha. Neurogastroenterol. Motil. 2017, 29, e12999. [Google Scholar] [CrossRef]
- Lerman, I.; Hauger, R.; Sorkin, L.; Proudfoot, J.; Davis, B.; Huang, A.; Lam, K.; Simon, B.; Baker, D.G. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: A randomized, blinded, healthy control pilot trial. Neuromodulation 2016, 19, 283–290. [Google Scholar] [CrossRef]
- Tarn, J.; Legg, S.; Mitchell, S.; Simon, B.; Ng, W.-F. The effects of noninvasive vagus nerve stimulation on fatigue and immune responses in patients with primary Sjögren’s Syndrome. Neuromodulation 2019, 22, 580–585. [Google Scholar] [CrossRef]
- Milev, R.V.; Giacobbe, P.; Kennedy, S.H.; Blumberger, D.M.; Daskalakis, Z.J.; Downar, J.; Modirrousta, M.; Patry, S.; Vila-Rodriguez, F.; Lam, R.W.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 4. Neurostimulation Treatments. Can. J. Psychiatry 2016, 61, 561–575. [Google Scholar] [CrossRef]
- Feldman, R.L.; Dunner, D.L.; Muller, J.S.; Stone, D.A. Medicare patient experience with vagus nerve stimulation for treatment-resistant depression. J. Med. Econ. 2013, 16, 63–74. [Google Scholar] [CrossRef]
- Hasan, A.; Wolff-Menzler, C.; Pfeiffer, S.; Falkai, P.; Weidinger, E.; Jobst, A.; Hoell, I.; Malchow, B.; Yeganeh-Doost, P.; Strube, W.; et al. Transcutaneous noninvasive vagus nerve stimulation (tVNS) in the treatment of schizophrenia: A bicentric randomized controlled pilot study. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 256, 589–600. [Google Scholar] [CrossRef]
- D’Urso, G.; Brunoni, A.R.; Mazzaferro, M.P.; Anastasia, A.; de Bartolomeis, A.; Mantovani, A. Transcranial direct current stimulation for obsessive-compulsive disorder: A randomized, controlled, partial crossover trial. Depress. Anxiety 2016, 33, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Rong, P.; Liu, J.; Wang, L.; Liu, R.; Fang, J.; Zhao, J.; Zhao, Y.; Wang, H.; Vangel, M.; Sun, S.; et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J. Affect. Disord. 2016, 195, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.G.; Porges, E.C.; Lewis, G.F.; Williamson, J.B. Non-invasive Vagal Nerve Stimulation effects on hyperarousal and autonomic state in patients with posttraumatic stress disorder and history of mild traumatic brain injury: Preliminary evidence. Front. Med. 2017, 4, 124. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Ward, H.E.; Ninan, P.T.; Pollack, M.; Nahas, Z.; Anderson, B.; Kose, S.; Howland, R.H.; Goodman, W.K.; Ballenger, J.C. A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimul. 2008, 1, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Grazzi, L.; Egeo, G.; Padovan, A.; Liebler, E.; Bussone, G. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: An open-label study. J. Headache Pain 2015, 16, 61. [Google Scholar] [CrossRef]
- Nesbitt, A.D.; Marin, J.C.A.; Tomkins, E.; Ruttledge, M.H.; Goadsby, P.J. Non-invasive vagus nerve stimulation for the treatment of cluster headache: A case series. J. Headache Pain 2013, 14. [Google Scholar] [CrossRef]
- Gaul, C.; Magis, D.; Liebler, E.J.; Straube, A. Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: A post hoc analysis of the randomized, controlled PREVA Study. J. Headache Pain 2017, 18, 22. [Google Scholar] [CrossRef]
- Rosell, J.; Colominas, J.; Riu, P.; Pallas-Areny, R.; Webster, J.G. Skin impedance from 1 Hz to 1 MHz. IEEE Trans. Biomed. Eng. 1988, 35, 649–651. [Google Scholar] [CrossRef]
- Gazi, A.H.; Gurel, N.Z.; Richardson, J.L.S.; Wittbrodt, M.T.; Shah, A.J.; Vaccarino, V.; Bremner, J.D.; Inan, O.T. Investigating digital cardiovascular biomarker responses to transcutaneous cervical vagus nerve stimulation: State-space modeling, prediction, and simulation. JMIR hHealth uHealth 2020. [Google Scholar] [CrossRef]
- Wittbrodt, M.T.; Gurel, N.Z.; Nye, J.A.; Ladd, S.; Shandhi, M.M.H.; Huang, M.; Shah, A.J.; Pearce, B.D.; Alam, Z.S.; Rapaport, M.H.; et al. Non-invasive vagal nerve stimulation decreases brain activity during trauma scripts. Brain Stimul. 2020, 13, 1333–1348. [Google Scholar] [CrossRef]
- Pimple, P.; Lima, B.B.; Hammadah, M.; Wilmot, K.; Ramadan, R.; Levantsevych, O.; Sullivan, S.; Kim, J.H.; Kaseer, B.; Shah, A.J.; et al. Psychological distress and subsequent cardiovascular events in individuals with coronary artery disease. J. Am. Hear. Assoc. 2019, 8, e011866. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.B.; Hammadah, M.; Pearce, B.D.; Shah, A.; Moazzami, K.; Kim, J.H.; Sullivan, S.; Levantsevych, O.; Lewis, T.T.; Weng, L.; et al. Association of posttraumatic stress disorder with mental stress-induced myocardial ischemia in adults after myocardial infarction. JAMA Netw. Open 2020, 3, e202734. [Google Scholar] [CrossRef] [PubMed]
- Pimple, P.; Shah, A.; Rooks, C.; Bremner, J.D.; Nye, J.; Ibeanu, I.; Murrah, N.; Shallenberger, L.; Kelley, M.; Raggi, P.; et al. Association between anger and mental stress-induced myocardial ischemia. Am. Heart J. 2015, 169, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Gurel, N.Z.; Mobashir, H.S.; Bremner, J.D.; Vaccarino, V.; Ladd, S.L.; Shah, A.; Inan, O.T. Toward closed-loop transcutaneous vagus nerve stimulation using peripheral cardiovascular physiological biomarkers: A proof-of-concept study. IEEE Body Sens. Netw. 2018. [Google Scholar] [CrossRef]
- Szeska, C.; Richter, J.; Wendt, J.; Weymar, M.; Hamm, A.O. Promoting long-term inhibition of human fear responses by non-invasive transcutaneous vagus nerve stimulation during extinction training. Sci. Rep. 2020, 10, 1529. [Google Scholar] [CrossRef] [PubMed]
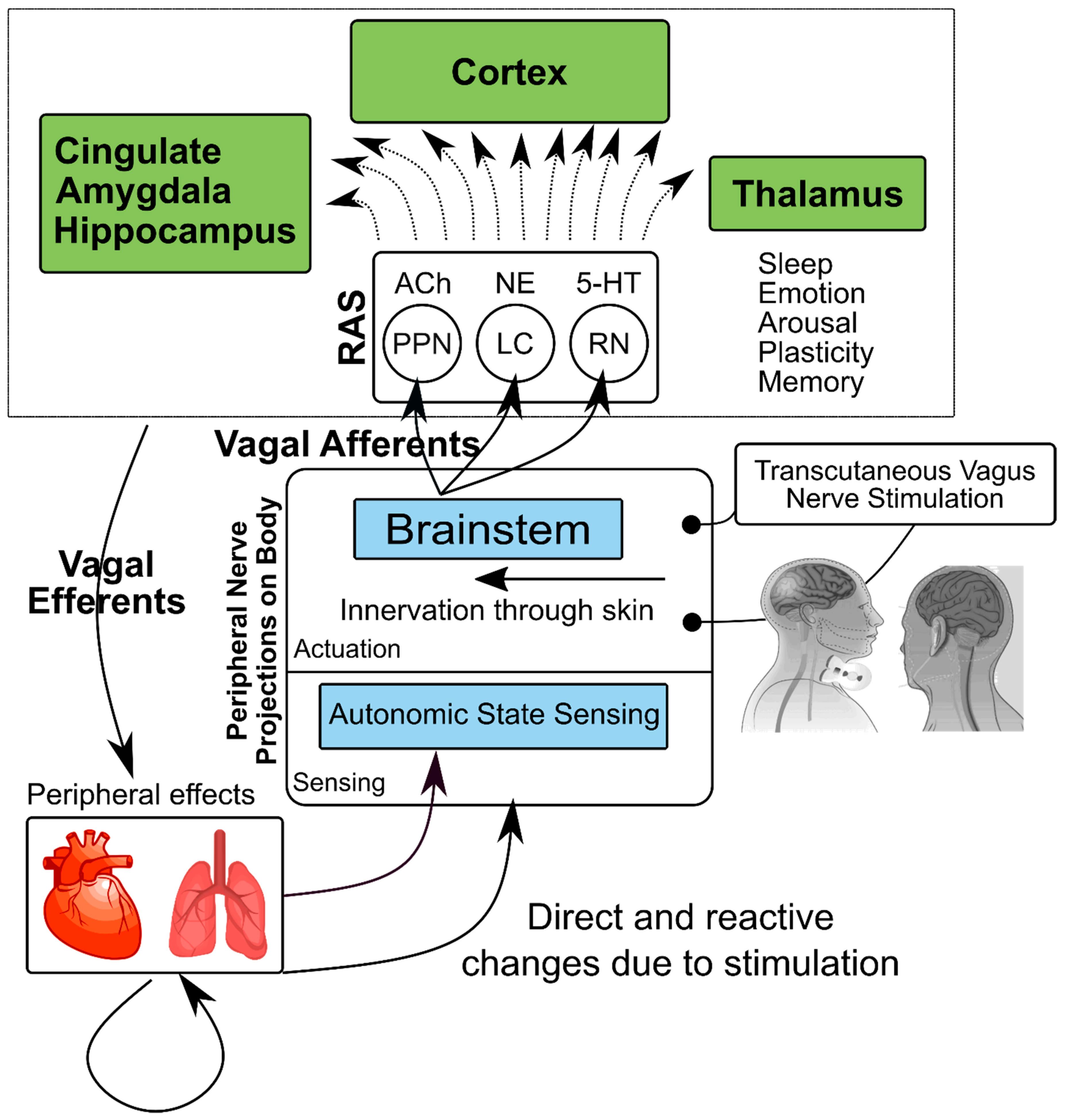
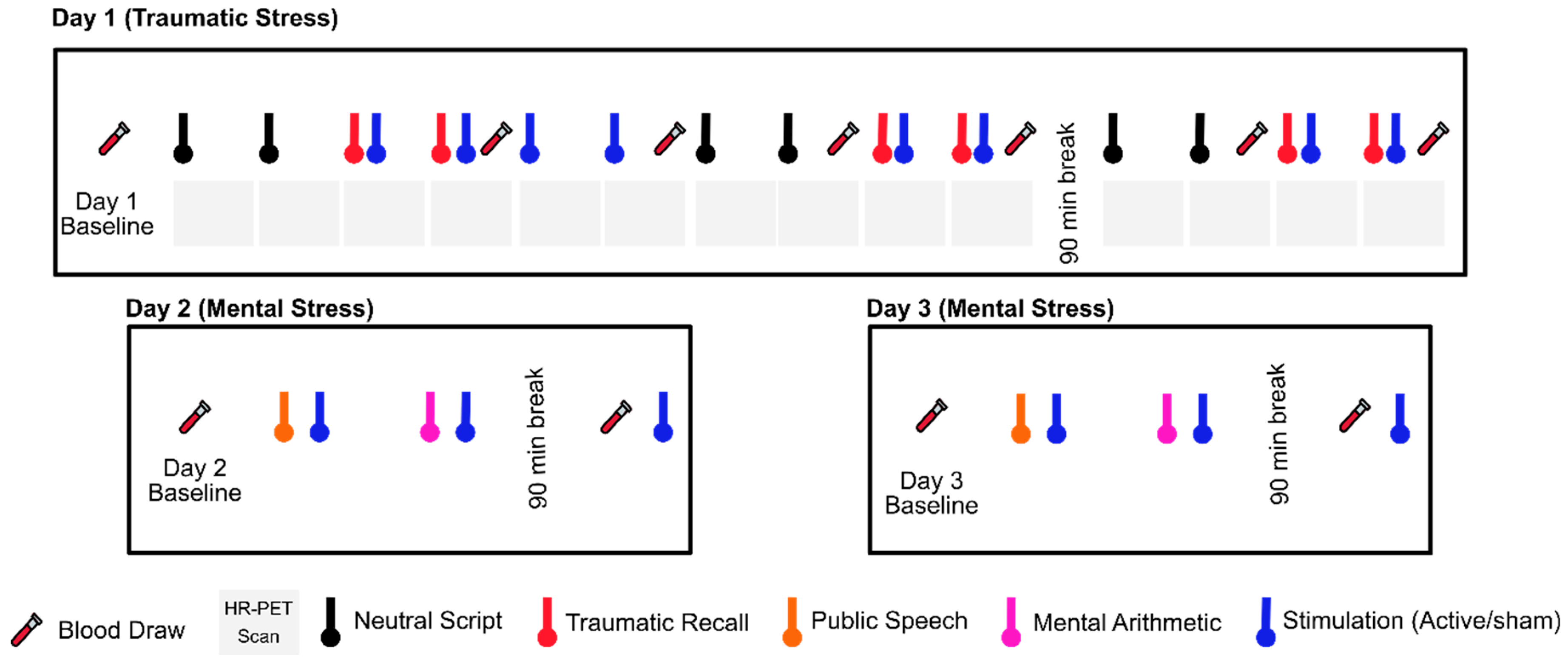
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bremner, J.D.; Gurel, N.Z.; Wittbrodt, M.T.; Shandhi, M.H.; Rapaport, M.H.; Nye, J.A.; Pearce, B.D.; Vaccarino, V.; Shah, A.J.; Park, J.; et al. Application of Noninvasive Vagal Nerve Stimulation to Stress-Related Psychiatric Disorders. J. Pers. Med. 2020, 10, 119. https://doi.org/10.3390/jpm10030119
Bremner JD, Gurel NZ, Wittbrodt MT, Shandhi MH, Rapaport MH, Nye JA, Pearce BD, Vaccarino V, Shah AJ, Park J, et al. Application of Noninvasive Vagal Nerve Stimulation to Stress-Related Psychiatric Disorders. Journal of Personalized Medicine. 2020; 10(3):119. https://doi.org/10.3390/jpm10030119
Chicago/Turabian StyleBremner, James Douglas, Nil Z. Gurel, Matthew T. Wittbrodt, Mobashir H. Shandhi, Mark H. Rapaport, Jonathon A. Nye, Bradley D. Pearce, Viola Vaccarino, Amit J. Shah, Jeanie Park, and et al. 2020. "Application of Noninvasive Vagal Nerve Stimulation to Stress-Related Psychiatric Disorders" Journal of Personalized Medicine 10, no. 3: 119. https://doi.org/10.3390/jpm10030119
APA StyleBremner, J. D., Gurel, N. Z., Wittbrodt, M. T., Shandhi, M. H., Rapaport, M. H., Nye, J. A., Pearce, B. D., Vaccarino, V., Shah, A. J., Park, J., Bikson, M., & Inan, O. T. (2020). Application of Noninvasive Vagal Nerve Stimulation to Stress-Related Psychiatric Disorders. Journal of Personalized Medicine, 10(3), 119. https://doi.org/10.3390/jpm10030119




