1. Introduction
The vast majority of pituitary adenomas, recently renamed pituitary neuroendocrine tumors (PitNETs) [
1,
2], are benign and are easily treatable with already established therapeutic options—surgery; conventional medical treatments, including somatostatin receptor ligands and dopamine receptor type 2 agonists; and sometimes radiotherapy (RT) [
3]. However, PitNETs may also prove to be aggressive and, very rarely, to metastasize, in the latter case being called pituitary carcinomas [
1]. Both pituitary carcinomas and aggressive PitNETs lead to increased morbidity and mortality, and are very difficult to manage. The European Society of Endocrinology (ESE) guidelines on aggressive PitNETs and pituitary carcinomas recommend temozolomide, an oral alkylating agent, to be used after the failure of surgery, conventional medical treatments, and radiotherapy [
3]. Unfortunately, in the ESE survey, the largest series on the use of temozolomide, this treatment led to a partial or complete radiological response in only 37% of cases, and to stable disease in 33% of cases. Moreover, after treatment ceased, progressive disease was noted in 25, 40, and 48% of patients initially responding to treatment with a complete response, partial response, and stable disease, respectively [
4], and a second course of temozolomide proved to be effective only in rare cases [
4,
5,
6]. Once temozolomide has failed, no other treatment option is formally recommended, because of a lack of sufficient evidence regarding efficacy in aggressive PitNETs, including carcinomas. Recently, the use of immune checkpoint inhibitors (ICIs) has raised hope, but so far only two cases of corticotroph tumors (one carcinoma treated with combined ipilimumab, an anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and nivolumab, an anti-programmed cell death protein 1 (PD-1), which showed a partial response [
7], and one aggressive corticotroph tumor treated with pembrolizumab alone, an anti-PD-1, which showed progressive disease [
8]) are reported in the literature.
Here, we report two cases. First, we present the response of a functioning corticotroph carcinoma to a different dosing regimen of combined immunotherapy with ipilimumab and nivolumab than the one already reported in literature (chosen based on a better tolerance with comparable efficacy of this regimen in other cancers [
9,
10,
11]). Second, we present the first reported case of an aggressive prolactinoma treated with immunotherapy.
2. Patients and Methods
Here, we present a case series describing the clinical management of one corticotroph carcinoma and one aggressive prolactinoma, with an emphasis on the effect of immunotherapy with ipilimumab (1 mg/kg every 3 weeks) and nivolumab (3 mg/kg every 3 weeks), followed by maintenance treatment with nivolumab alone in the case of the corticotroph carcinoma (3 mg/kg every 2 weeks). The cases were treated in two different French centers, Lille and Limoges, where they had been followed since 2001 and 2012, respectively. For both cases, the indication of immunotherapy was validated after a tumor board multidisciplinary discussion conducted at the national level (HYPOCare multidisciplinary reunion conducted by the French Reference Center for Rare Pituitary Diseases HYPO). Clinical, radiological, and pathological data were collected retrospectively from the patients’ medical records. All of the hormonal assays, immunohistochemistry, and methylation analyses were performed with commercially available kits. The study is in accordance with the Ethics Committee of Lille University Hospital and Limoges University Hospital. Informed consent was obtained from each patient after a full explanation of the purpose and nature of all of the procedures used, as well as for publication of the article along with accompanying images.
3. Patient #1
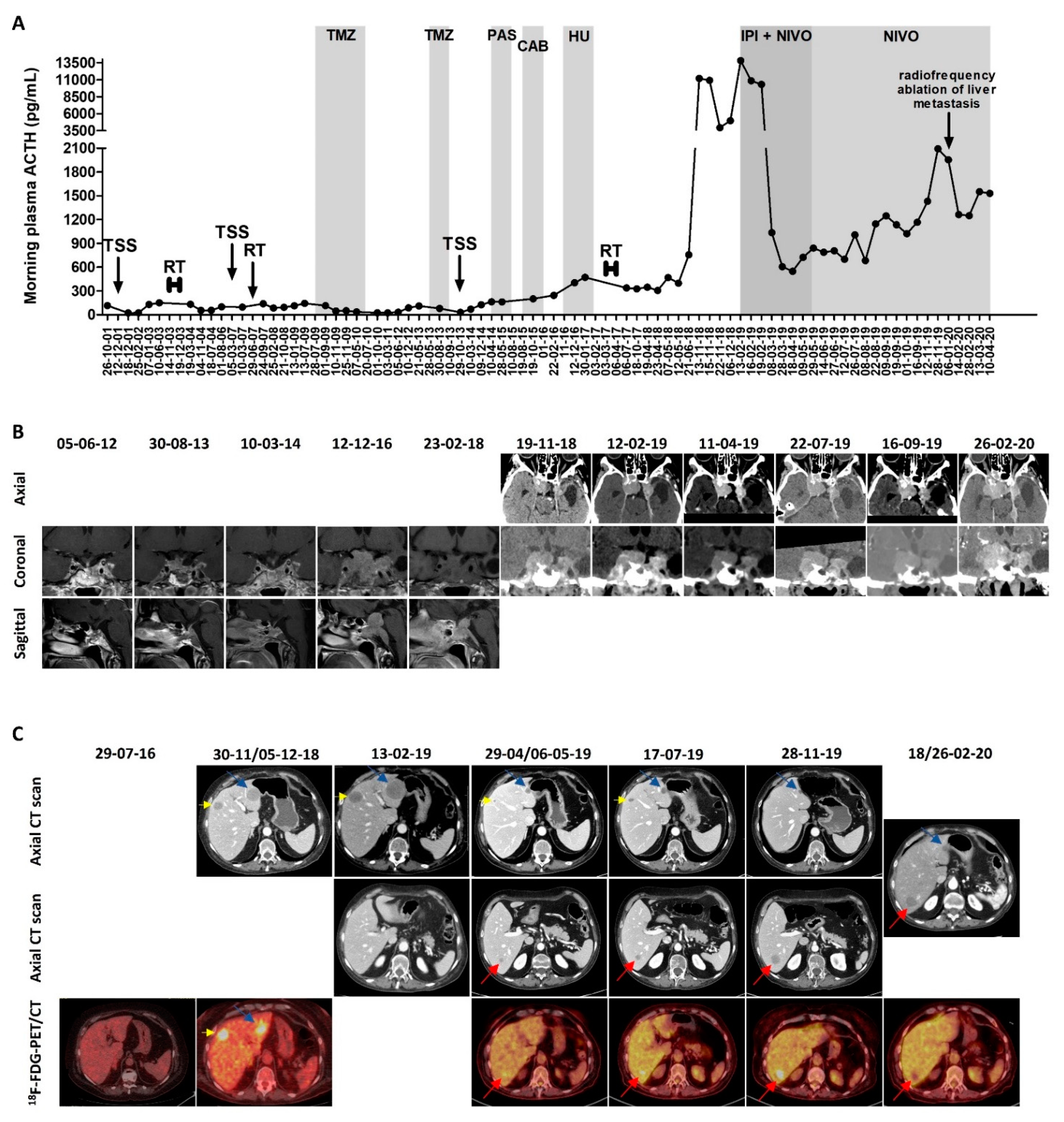
A 42-year-old female presented in 2001 with headaches, diabetes, hypertension, and obesity, and was diagnosed with Cushing’s disease based on an elevated urinary free cortisol (UFC) level of 212 µg/24 h (N 20-110), elevated morning plasma adrenocorticotropic hormone (ACTH) of 115 pg/mL (N < 46), and invasive pituitary tumor measuring 25 × 23 × 20 mm. The first transsphenoidal surgery (TSS), which was subtotal as a result of left cavernous sinus invasion, revealed a tumor with a Ki-67 index of 2%, absence of mitosis, and negative p53; therefore, it was classified as a grade 2a corticotroph tumor.
The regrowth of the tumor residue required multiple additional treatments between 2003 and 2017, as shown in
Figure 1A, which shows the evolution of the plasma ACTH under the different treatments—fractionated RT (50 Gy in 28 fractions) in 2003; second TSS (rare mitosis) followed by Gamma knife radiosurgery (25 Gy) in 2007; 10 cycles of temozolomide 250–270 mg/day, 5 days every 4 to 5 weeks in 2009-2010 (with initial biochemical complete response and radiological partial response, but with relapse 3 years after treatment ceased; side effects: thrombocytopenia); three additional cycles of temozolomide 250 mg/day, 5 days every 5 weeks in 2013 (stopped due to radiological progressive disease and thrombocytopenia); third TSS (Ki-67 index 5%, 5 mitoses/10 high-power fields (HPFs), and positive p53–2%) in 2013; pasireotide 0.9 mg twice daily for 4 months in 2015 (ineffective); cabergoline 2 mg/week for 5 months in 2015-2016 (ineffective); hydroxyurea 1500 mg/day for 3 months in 2016–2017 (ineffective and causing pancytopenia); and fractionated RT (45 Gy in 25 fractions) in 2017.
In November 2018, the patient was admitted for asthenia, weight loss (10 kg), accentuation of melanoderma, right ptosis, and diplopia due to right third and sixth cranial nerve palsy. The plasma ACTH varied between 3957 and 11,191 pg/mL, compared with 398 pg/mL in May 2018. The pituitary computed tomography (CT) showed the global stability of the pituitary mass compared with the pituitary magnetic resonance imaging (MRI) performed in February 2018. The
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT showed an intense uptake by the pituitary mass, the skull (corresponding to one lytic occipital lesion), and three hepatic lesions; there was also a suspected T2 vertebral lesion. The abdominopelvic CT showed five hepatic lesions, with maximal diameters of 31, 22, 12, 7, and 5 mm. A liver biopsy was performed and showed the presence of a corticotroph tumor metastasis (immunohistochemistry: Ki-67 index 10%, positive p53–7%, and absent programmed death ligand 1 (PD-L1) expression), confirming the diagnosis of pituitary carcinoma. The pituitary and thoraco-abdominopelvic CT repeated in February 2020 showed the progression of the pituitary mass (anteroposterior diameter: 37 mm vs. 32 mm) and of the two largest liver metastases (maximal diameters of 45, 30, 12, 7, and 6 mm). The evolution of the pituitary mass and of the liver metastases is presented in
Figure 1B,C, respectively.
Immunotherapy with ipilimumab (1 mg/kg) and nivolumab (3 mg/kg) every 3 weeks was initiated in February 2019, and was administered for five cycles. A very rapid and significant decrease of plasma ACTH was observed after the first cycle, from 13,813 to 1036 pg/mL. The plasma ACTH continued to further decrease (nadir of 549 pg/mL after three cycles) and remained < 1000 pg/mL during the five cycles of combined immunotherapy. Regarding the radiological response, after three and four cycles, the pituitary mass decreased in size from 37 × 32 × 41 mm to 29 × 23 × 42 mm, while the five known liver metastases decreased in size from 45 to 14 mm, from 30 to 13 mm, from 12 to 5 mm, or were not visible anymore (for the smallest ones). The 18F-FDG-PET/CT showed a very positive metabolic response for the known metastases and the disappearance of the suspect T2 vertebral lesion, but the appearance of a new focal liver uptake was noted, corresponding to a new 11 mm lesion on the CT (described only afterwards on the CT). A concomitant improvement of melanoderma and right ptosis were observed, the diplopia disappeared, and the body weight stabilized. The patient did not report any side effects from the combined immunotherapy other than asthenia on the day of administration.
Since May 2019, the patient received maintenance treatment with nivolumab alone (3 mg/kg every 2 weeks). After the fourth cycle of nivolumab, the right ptosis worsened again, concomitantly with a progressive increase of plasma ACTH (1147 pg/mL, 1248 pg/mL, 1431 pg/mL, and 2094 pg/mL after the fourth, seventh, eleventh, and twelfth cycle in November 2019, respectively). The increase in the pituitary mass (from 29 × 23 × 42 to 29 × 25 × 45 mm in July 2019) was initially considered to be non-significant, however the pituitary CT performed in February 2020 confirmed the local progression (38 × 28 × 54 mm). Regarding the liver metastases, in November 2019, the thoraco-abdominopelvic CT showed the progression of the liver metastasis that became visible after the start of the immunotherapy (22 mm vs. 14 mm in July and 11 mm in May), whereas the other liver metastases were stable, measuring 12, 11, and 4 mm. Radiofrequency ablation of this liver metastasis was performed in January 2020, and 1-2 months later, the concurrent stigma of the radiofrequency ablation on the CT scan, a complete metabolic response of the hepatic lesion on the 18F-FDG-PET/CT, and a decrease of plasma ACTH were observed. The three other liver metastases remained stable, measuring 12, 8, and 4 mm. At the last follow-up in April 2020, the plasma ACTH was stable (1530 pg/mL) in comparison with March, but higher than in February (1250 pg/mL). The patient died four days later of an unknown cause. As potential side-effects of nivolumab, the patient reported asthenia, anorexia, and progressive weight loss (5 kg).
Of note, for the hypercortisolism, the patient also received ketoconazole and mitotane (before the biochemical remission induced by the 10 cycles of temozolomide), and after relapse, metyrapone 1000-3000 mg/day from August 2015 to December 2018 (side-effects: abdominal pain, hair loss, and hirsutism), and mitotane up to 2000 mg/day since February 2017 (side-effects: anorexia and vertigo).
4. Patient #2
A 60-year-old male presented in August 2012 with visual disturbances and bitemporal hemianopsia, and was diagnosed with a prolactinoma based on the presence of an invasive pituitary tumor measuring 36 × 31 × 26 mm, and a prolactin level of 5130 ng/mL (N 2.30-14.7).
Figure 2A presents the evolution of the prolactin levels under the different treatments, while
Figure 2B presents the evolution of the pituitary mass.
Cabergoline 0.5 mg × 3/week was started, and then increased to 0.5 mg daily from January 2013, enabling a decrease of prolactin to 1330 ng/mL, concurrent with a 50% decrease in tumor volume in March 2013. Cabergoline was further increased and maintained at 1 mg daily from June 2013. Despite an initial further decrease in the tumor volume and recovery of visual signs (nadir prolactin level of 787 ng/mL), the patient presented with the recurrence of the left temporal hemianopsia in December 2015. The pituitary MRI showed rapid tumor regrowth between July and December 2015 (from 7 × 19 × 17 mm to 30 × 25 × 25 mm). The TSS performed in January 2016 was subtotal as a result of left cavernous sinus invasion, and revealed a proliferative tumor (Ki-67 index of 10%, 5 mitosis/10 HPFs), and was therefore classified as a grade 2b prolactinoma. Adjuvant fractionated RT (50.4 Gy in 30 fractions) was performed in 2016.
Following the first TSS, the patient was kept on 1 mg of cabergoline daily. At the end of 2018, the patient presented again with rapid tumor progression, with optic chiasm compression, for which a second TSS was performed in January 2019 (Ki-67 index 10–11%, 1 mitosis/10 HPFs). After surgery, cabergoline was further increased to a maximum of 10.5 mg/week and pasireotide 0.6–0.9 mg twice daily was administered for two months (ineffective and resulting in QT interval prolongation). The administration of temozolomide was decided, but due to the rapid tumor progression with a worsening of the visual field, decreasing visual acuity, and involvement of the left third cranial nerve, before starting temozolomide, a third TSS was performed in June 2019 (Ki-67 index 25%, >20 mitosis/10 HPFs). Neither the 18F-FDG PET/CT performed in June 2019 nor the medullary MRI performed in August 2019 found any metastasis.
The cabergoline was interrupted in July 2019, being considered ineffective given the rapid progression despite very high doses, and six cycles of 220–290 mg of temozolomide per day for 5 out of 28 days were administered starting at the end of July 2019. The tumor progressed under temozolomide, with worsening of the left cavernous sinus syndrome (diplopia, ptosis, mydriasis, and trigeminal neuralgia, for which methylprednisolone up to 80 mg daily was administered), a significant increase in the tumor size seen on the MRI performed in September 2019 (39 × 43 × 27 mm compared with 20 × 20 × 23 in June 2019), and a concurrent significant increase in prolactin levels. Then, 1 mg of cabergoline daily was reintroduced, enabling a decrease in prolactin levels, which, nevertheless, remained higher compared with the moment when the temozolomide was started. An improvement in the left cavernous sinus syndrome was also noted (possibly due to the methylprednisolone as well), but with the MRI performed in December 2019 not showing any regression in the pituitary mass (38 × 42 × 26 mm).
Given the inefficacy of temozolomide, the administration of immunotherapy with ipilimumab (1 mg/kg) and nivolumab (3 mg/kg) every three weeks was decided. The administration of methylprednisolone was progressively decreased, and stopped at the end of December 2019 in order to start the combined immunotherapy, while cabergoline was maintained at 1 mg daily. Unfortunately, the combined immunotherapy resulted in rapid progression, with the prolactin levels increasing from 4410 ng/mL before initiation to 9840 ng/mL after the first two cycles, and with the size of the pituitary tumor increasing from 38 × 42 × 26 mm in December 2019 to 53 × 57 × 44 mm in March 2020. Left ptosis also reoccurred. After the two cycles, the patient also presented severe side-effects (grade 3–4 diarrhea, and grade 1 nausea and vomiting), and therefore the combined immunotherapy was stopped. Starting at the end of March, 15 mg/kg of bevacizumab was administered every three weeks, with, so far, a stabilization of prolactin levels (~8830 ng/mL in April and May 2020), a stable disease shown in the MRI, and good tolerance (minimal epistaxis and grade 1 hypertension).
Of note, the somatostatin receptor type 5 (SST5) immunohistochemistry performed in January 2020 on the tumor block from January 2019 showed the absence of staining, which may partly explain the lack of an effect of pasireotide [
12]. At the same time, as a potential marker of response to temozolomide, O6-methylguanine-DNA methyltransferase (MGMT) immunohistochemistry was performed, but was not interpretable, while the methylation analysis revealed a weak methylation of the MGMT promoter (average between 9 and 12%). On the tumor block from June 2019, genetic analysis was performed, as follows: (1) next-generation DNA-sequencing in January 2020—FoundationOne® companion diagnostic (CDx; Foundation Medicine, Inc., Cambridge, MA, USA)—revealing the absence of alterations with a known clinical and/or therapeutic impact (absence of microsatellite instability, low mutational burden (1 mutation/Mb), amplification of the
HGF gene, deletion of the
CDKN2A/B gene, mutation (p.Ser514*) of the
BCORL1 gene, mutation (p.Asn2fs*52) of the
FLCN gene, and mutation (p.Lys666Glu) of the
SF3B1 gene), and (2) next-generation RNA-sequencing in March 2020—Archer
® FusionPlex
® CTL Panel (ArcherDX, Inc., Boulder, CO, USA)—revealing the absence of mutations or of fusion transcripts.
5. Discussion
ICIs block the inhibitory signals of T lymphocyte function and/or activation, and, more precisely, they block CTLA-4 or PD-1, which are found on T lymphocytes or its ligand, programmed death ligand 1 (PD-L1), which is found on antigen-presenting cells and on tumor cells. These inhibitory signals would otherwise enable tumors to evade immune response. By blocking them, ICIs enhance T lymphocyte function and reactivate antitumor immune responses [
13,
14,
15].
Recent studies on the tumor microenvironment of PitNETs [
16] have demonstrated in these tumors, and especially in functioning PitNETs, the presence of tumor-infiltrating lymphocytes [
13,
17,
18] and of PD-L1, a potential biomarker of response to ICIs [
13,
17]. These findings have raised the hope that ICIs might be effective in these tumors. ICIs might be especially promising for patients who previously received conventional chemotherapies, such as temozolomide, because the administration of conventional chemotherapies can induce somatic hypermutations that will render ICIs more effective [
7]. A recent in silico analysis of the immune tumor microenvironment of PitNETs also revealed that functioning corticotroph tumors had higher CD8+ T lymphocyte infiltration than somatotroph, lactotroph, thyreotroph, and non-functioning PitNETs, suggesting that functioning corticotroph tumors may be more amenable to ICIs than other PitNET subtypes [
19].
So far, only two cases of corticotroph tumors have been treated with ICIs [
7,
8].
Table 1 summarizes their findings, together with the findings from our two cases. The two cases that showed a partial response were both carcinomas that had been previously treated with multiple conventional chemotherapies (including multiple courses), in comparison with the two cases that resulted in progressive disease, which were both aggressive pituitary tumors that had been previously treated with only one course of temozolomide. Although we did not perform genetic analyses before the start of the ICIs (and neither did the authors of the corticotroph tumor that showed progressive disease), a higher mutational burden would be expected in the two responders, and the liver metastasis of the responsive corticotroph carcinoma reported in the literature did indeed show somatic hypermutations [
7]. However, it is worth mentioning that the aggressive corticotroph tumor that showed the progressive disease had a mismatch-repair deficiency (as did the liver metastasis from the other reported case), which, in other cancers, is associated with responsiveness to anti-PD-1 therapy [
8]. Other than a less important mutational burden, additional possible reasons for ICI inefficacy in this case consists of using monotherapy instead of combined ICIs (less effective) and of the presence of high levels of cortisol during ICI administration, given that glucocorticoids have immunomodulating effects (the hypercortisolism was controlled in the other two cases). Moreover, this tumor did not express PD-L1 in the immunohistochemistry, but the predictive value of the PD-L1 expression still needs to be proven in pituitary tumors, especially given the fact that its predictive value has not been consistent across studies in other cancers [
8]. Importantly, the liver metastasis that was biopsied in patient #1 did not express PD-L1 either, but proved to have an excellent response to ICIs, as did the liver metastasis from the other corticotroph carcinoma, which also only had <1% positive PD-L1 staining [
7]. Therefore, the lack of PD-L1 expression should not preclude the indication of immunotherapy. Interestingly, both in our case and in the case of the corticotroph carcinoma previously reported [
7], the liver metastases showed a greater decrease in size compared with the pituitary masses, which may be at least partly explained by a different composition of the tumor microenvironment between the liver and the pituitary (as two different anatomic sites), but also between primary and secondary lesions in general. In addition, given the rapid and significant biochemical response seen in the two responders (an ~10-fold decrease in ACTH levels within 1 week [
7], and after the first cycle in patient #1), the biochemical response might prove to be an early and easy-to-perform marker of response to ICIs.
As limited experience is available on the use of ICIs in pituitary tumors, we think that response to treatment should be evaluated on a case-by-case basis. Given the regression of the pituitary mass and of all five pre-existing liver metastases, we consider patient #1 to be a responder to combined ipilimumab and nivolumab, instead of classifying the response as progressive disease based on the new liver metastasis that appeared after the four cycles of combined immunotherapy, as we could have done based on the iRECIST guidelines [
20]. Although this last metastasis continued to grow on maintenance nivolumab and was finally treated by radiofrequency ablation, and the pituitary mass slowly progressed, nivolumab alone continued to be effective on the rest of the metastases. Therefore, in the case of a dissociate response (which might reflect a distinct evolution of the genetic/epigenetic landscape or of the tumor microenvironment), in which only one or very few of the lesions progress, the use of complementary local therapies such as radiofrequency ablation or surgery might be tried in combination with continued immunotherapy, instead of considering the immunotherapy ineffective and ceasing it. Additionally, the reintroduction of double immunotherapy might also prove useful in the future.
Moreover, besides a complete/partial response, stable disease, and progressive disease, one should be aware of two additional patterns of tumor response when using immunotherapy, namely: pseudoprogression (i.e., apparent increase in the tumor burden initially, followed by delayed tumor shrinkage, which could lead to the premature cessation of effective immunotherapy) [
20,
21] and hyperprogressive disease (i.e., accelerated tumor progression following the introduction of ICIs) [
22,
23]. Hyperprogressive disease was shown to be associated with older age—in a study including 131 patients treated with anti-PD-1/PD-L1 therapy, 7 out of 36 patients ≥65 years old (19%) were classified as having hyperprogressive disease, compared with 5 out of the 95 patients ≤64 years old (5%),
p = 0.01, of which only one patient was <55 years old [
22]. Patient #2 from the current study, who was 68 years old when immunotherapy was introduced, presented with rapid progression following ICIs initiation, but it is difficult to say whether it was the cessation of temozolomide, the natural history of the disease, or the introduction of ICIs that led to this evolution.
Regarding the side-effects of ICIs, combined immunotherapy, besides being more effective, also increases treatment-related adverse events [
17]. In the four pituitary cases reported so far, the most frequently affected was the gastrointestinal tract, with patient #2 from the current study being the only one experiencing severe treatment-related adverse events. Regarding ICIs-related endocrinopathies, both of our patients had already substituted central hypothyroidism at the time immunotherapy started (for which the replacement therapy was maintained) and had type 2 diabetes mellitus (which remained well controlled during immunotherapy). No new endocrinopathies were diagnosed during immunotherapy.
6. Conclusions
So far, there is not enough data to enable any formal conclusion on the efficacy of ICIs in pituitary carcinomas and aggressive PitNETs, or on which subgroups of patients will prove to respond, but ICIs appear to be a promising therapeutic option. At the moment, there are two clinical trials running on combined ipilimumab and nivolumab, namely: NCT04042753, a clinical trial dedicated to pituitary carcinomas and aggressive PitNETs; and NCT02834013, a basket trial that also accepts pituitary carcinomas. Hopefully, their results will provide valuable information on the efficacy of ICIs in these tumors. At the same time, potential leads for the improvement of ICI efficacy emerging from studies on other cancers, such as the combination of ICIs with drugs targeting angiogenesis [
24,
25] or with radiotherapy [
26,
27], will potentially be transferable to pituitary carcinomas and aggressive pituitary tumors as well and will result in better outcomes for the patients treated with such therapies.
Author Contributions
Conceptualization, C.D., M.D.I., H.S., A.S.N., S.G., E.D., R.A., L.M., C.C. and G.R.; Data curation, C.D., M.D.I., H.S., A.S.N., S.G., E.D., R.A., L.M., C.C. and G.R.; Writing (original draft preparation), C.D. and M.D.I.; Writing (review and editing), C.D., M.D.I., H.S., A.S.N., S.G., E.D., R.A., L.M., C.C. and G.R.; Visualization, C.D., M.D.I., A.S.N., C.C. and G.R.; SUPERVISION, C.C. and G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Gustavo Soto-Ares (neuroradiology) and François Dubois (neuro-oncology) from Lille University Hospital for their involvement in patient care.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Asa, S.L.; Casar-Borota, O.; Chanson, P.; Delgrange, E.; Earls, P.; Ezzat, S.; Grossman, A.; Ikeda, H.; Inoshita, N.; Karavitaki, N.; et al. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): An International Pituitary Pathology Club proposal. Endocr. Relat. Cancer 2017, 24, C5–C8. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Vasiljevic, A.; Jaffrain-Rea, M.L.; Ansorge, O.; Asioli, S.; Barresi, V.; Chinezu, L.; Gardiman, M.P.; Lania, A.; Lapshina, A.M.; et al. A standardised diagnostic approach to pituitary neuroendocrine tumours (PitNETs): A European Pituitary Pathology Group (EPPG) proposal. Virchows Arch. 2019, 475, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Burman, P.; McCormack, A.; Heaney, A.; Petersenn, S.; Popovic, V.; Trouillas, J.; Dekkers, O.M. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur. J. Endocrinol. 2018, 178, G1–G24. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.; Dekkers, O.M.; Petersenn, S.; Popovic, V.; Trouillas, J.; Raverot, G.; Burman, P. Treatment of aggressive pituitary tumours and carcinomas: Results of a European Society of Endocrinology (ESE) survey 2016. Eur. J. Endocrinol. 2018, 178, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, D.; Schrøder, H.D.; Berinder, K.; Maiter, D.; Hoybye, C.; Ragnarsson, O.; Feldt-Rasmussen, U.; Krogh Rasmussen, Å.; van der Lely, A.; Petersson, M.; et al. Tumoral MGMT content predicts survival in patients with aggressive pituitary tumors and pituitary carcinomas given treatment with temozolomide. Endocrine 2018, 62, 737–739. [Google Scholar] [CrossRef]
- Lasolle, H.; Cortet, C.; Castinetti, F.; Cloix, L.; Caron, P.; Delemer, B.; Desailloud, R.; Jublanc, C.; Lebrun-Frenay, C.; Sadoul, J.-L.; et al. Temozolomide treatment can improve overall survival in aggressive pituitary tumors and pituitary carcinomas. Eur. J. Endocrinol. 2017, 176, 769–777. [Google Scholar] [CrossRef]
- Lin, A.L.; Jonsson, P.; Tabar, V.; Yang, T.J.; Cuaron, J.; Beal, K.; Cohen, M.; Postow, M.; Rosenblum, M.; Shia, J.; et al. Marked Response of a Hypermutated ACTH-Secreting Pituitary Carcinoma to Ipilimumab and Nivolumab. J. Clin. Endocrinol. Metab. 2018, 103, 3925–3930. [Google Scholar] [CrossRef] [Green Version]
- Caccese, M.; Barbot, M.; Ceccato, F.; Padovan, M.; Gardiman, M.P.; Fassan, M.; Denaro, L.; Emanuelli, E.; D’Avella, D.; Scaroni, C.; et al. Rapid disease progression in patient with mismatch-repair deficiency pituitary ACTH-secreting adenoma treated with checkpoint inhibitor pembrolizumab. Anticancer Drugs 2020, 31, 199–204. [Google Scholar] [CrossRef]
- Lebbé, C.; Meyer, N.; Mortier, L.; Marquez-Rodas, I.; Robert, C.; Rutkowski, P.; Menzies, A.M.; Eigentler, T.; Ascierto, P.A.; Smylie, M.; et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination With Ipilimumab in Patients With Advanced Melanoma: Results From the Phase IIIb/IV CheckMate 511 Trial. J. Clin. Oncol. 2019, 37, 867–875. [Google Scholar] [CrossRef]
- Gu, L.; Khadaroo, P.A.; Su, H.; Kong, L.; Chen, L.; Wang, X.; Li, X.; Zhu, H.; Zhong, X.; Pan, J.; et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): A systematic review and meta-analysis. BMC Cancer 2019, 19, 559. [Google Scholar] [CrossRef] [Green Version]
- Hammers, H.J.; Plimack, E.R.; Infante, J.R.; Rini, B.I.; McDermott, D.F.; Lewis, L.D.; Voss, M.H.; Sharma, P.; Pal, S.K.; Razak, A.R.A.; et al. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J. Clin. Oncol. 2017, 35, 3851–3858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasolle, H.; Ilie, M.D.; Raverot, G. Aggressive prolactinomas: How to manage? Pituitary 2020, 23, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Bi, W.L.; Greenwald, N.F.; Du, Z.; Agar, N.Y.R.; Kaiser, U.B.; Woodmansee, W.W.; Reardon, D.A.; Freeman, G.J.; Fecci, P.E.; et al. Increased expression of programmed death ligand 1 (PD-L1) in human pituitary tumors. Oncotarget 2016, 7, 76565–76576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postow, M.A. Managing Immune Checkpoint-Blocking Antibody Side Effects. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 76–83. [Google Scholar] [CrossRef]
- Castinetti, F.; Albarel, F.; Archambeaud, F.; Bertherat, J.; Bouillet, B.; Buffier, P.; Briet, C.; Cariou, B.; Caron, P.; Chabre, O.; et al. French Endocrine Society Guidance on endocrine side effects of immunotherapy. Endocr. Relat. Cancer 2019, 26, G1–G18. [Google Scholar] [CrossRef] [Green Version]
- Ilie, M.D.; Vasiljevic, A.; Raverot, G.; Bertolino, P. The Microenvironment of Pituitary Tumors—Biological and Therapeutic Implications. Cancers 2019, 11, 1605. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Wang, T.; Yang, Y.; Yao, K.; Li, Z.; Li, Y.M.; Yan, C.-X. The expression profile of PD-L1 and CD8+ lymphocyte in pituitary adenomas indicating for immunotherapy. J. Neurooncol. 2018, 139, 89–95. [Google Scholar] [CrossRef]
- Lu, J.-Q.; Adam, B.; Jack, A.S.; Lam, A.; Broad, R.W.; Chik, C.L. Immune Cell Infiltrates in Pituitary Adenomas: More Macrophages in Larger Adenomas and More T Cells in Growth Hormone Adenomas. Endocr. Pathol. 2015, 26, 263–272. [Google Scholar] [CrossRef]
- Yeung, J.T.; Vesely, M.D.; Miyagishima, D.F. In silico analysis of the immunological landscape of pituitary adenomas. J. Neurooncol. 2020, 147, 595–598. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.-S.; Wang, E.L.; Xu, W.-F.; Yamada, S.; Yoshimoto, K.; Qian, Z.R.; Shi, L.; Liu, L.-L.; Li, X.-H. Overexpression of DNA (Cytosine-5)-Methyltransferase 1 (DNMT1) And DNA (Cytosine-5)-Methyltransferase 3A (DNMT3A) Is Associated with Aggressive Behavior and Hypermethylation of Tumor Suppressor Genes in Human Pituitary Adenomas. Med. Sci. Monit. 2018, 24, 4841–4850. [Google Scholar] [CrossRef] [PubMed]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.-C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adashek, J.J.; Subbiah, I.M.; Matos, I.; Garralda, E.; Menta, A.K.; Ganeshan, D.M.; Subbiah, V. Hyperprogression and Immunotherapy: Fact, Fiction, or Alternative Fact? Trends Cancer 2020, 6, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Ramjiawan, R.R.; Griffioen, A.W.; Duda, D.G. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017, 20, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Hodi, F.S. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin. Cancer Res. 2019, 25, 5449–5457. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Invest. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Wilky, B.A. Immune checkpoint inhibitors: The linchpins of modern immunotherapy. Immunol. Rev. 2019, 290, 6–23. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).