Using Cone-Beam Computed Tomography to Assess Changes in Alveolar Bone Width around Dental Implants at Native and Reconstructed Bone Sites: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement of the Alveolar Bone Width
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sodek, J.; McKee, M.D. Molecular and cellular biology of alveolar bone. Periodontol. 2000 2000, 24, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.I.; Garant, P.R. Development and general structure of the periodontium. Periodontol. 2000 2000, 24, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R.; Marzola, A.; Bozzi, L.; Liljenberg, B.; Lindhe, J. Modeling and remodeling of human extraction sockets. J. Clin. Periodontol. 2008, 35, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Barone, A.; Ricci, M.; Tonelli, P.; Santini, S.; Covani, U. Tissue changes of extraction sockets in humans: A comparison of spontaneous healing vs. Ridge preservation with secondary soft tissue healing. Clin. Oral Implant. Res. 2013, 24, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Pietrokovski, J.; Massler, M. Alveolar ridge resorption following tooth extraction. J. Prosthet. Dent. 1967, 17, 21–27. [Google Scholar] [CrossRef]
- Atwood, D.A. Some clinical factors related to rate of resorption of residual ridges. J. Prosthet. Dent. 2001, 86, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ten Heggeler, J.M.; Slot, D.E.; Van der Weijden, G.A. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: A systematic review. Clin. Oral Implant. Res. 2011, 22, 779–788. [Google Scholar] [CrossRef]
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implant. Res. 2012, 23, 1–21. [Google Scholar] [CrossRef]
- Chappuis, V.; Araujo, M.G.; Buser, D. Clinical relevance of dimensional bone and soft tissue alterations post-extraction in esthetic sites. Periodontol. 2000 2017, 73, 73–83. [Google Scholar] [CrossRef]
- Chen, P.; Yu, S.; Zhu, G. The psychosocial impacts of implantation on the dental aesthetics of missing anterior teeth patients. Br. Dent. J. 2012, 213, E20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, R.; Bandeira, A.; Araujo, S.C.; Bragger, U.; Schimmel, M.; Leles, C.R. A parallel 3-group randomised clinical trial comparing different implant treatment options for the edentulous mandible: 1-year effects on dental patient-reported outcomes and chewing function. J. Oral Rehabil. 2020, 47, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Testori, T.; Weinstein, T.; Scutella, F.; Wang, H.L.; Zucchelli, G. Implant placement in the esthetic area: Criteria for positioning single and multiple implants. Periodontol. 2000 2018, 77, 176–196. [Google Scholar] [CrossRef] [PubMed]
- Asikainen, P.; Klemettil, E.; Vuilleminz, T.; Sutter, F.; Rainio, V.; Kotilainen, R. Titanium implants and lateral forces. An experimental study with sheep. Clin. Oral Implant. Res. 1997, 8, 465–468. [Google Scholar] [CrossRef]
- Akin-Nergiza, N.; Nergizb, I.; Schulzb, A.; Arpakc, N.; Niedermeierd, W. Reactions of peri-implant tissues to continuous loading of osseointegrated implants. Am. J. Orthod. Dentofac. Orthop. 1998, 114, 292–298. [Google Scholar] [CrossRef]
- Berglundh, T.; Abrahamsson, I.; Lindhe, J. Bone reactions to longstanding functional load at implants: An experimental study in dogs. J. Clin. Periodontol. 2005, 32, 925–932. [Google Scholar] [CrossRef]
- Lsidor, F. Clinical probing and radiographic assessment in relation to the histologic bone level at oral implants in monkeys. Clin. Oral Implant. Res. 1997, 8, 255–264. [Google Scholar] [CrossRef]
- Covani, U.; Cornelini, R.; Calvo-Guirado, J.L.; Tonelli, P.; Barone, A. Bone remodeling around implants placed in fresh extraction sockets. Int. J. Periodontics Restor. Dent. 2010, 30, 601–607. [Google Scholar]
- Araujo, M.G.; Wennström, J.L.; Lindhe, J. Modeling of the buccal and lingual bone walls of fresh extraction sites following implant installation. Clin. Oral Implant. Res. 2006, 17, 606–614. [Google Scholar] [CrossRef]
- Araújo, M.G.; Sukekava, F.; Wennström, J.L.; Lindhe, J. Ridge alterations following implant placement in fresh extraction sockets: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.G.; Sukekava, F.; Wennstrom, J.L.; Lindhe, J. Tissue modeling following implant placement in fresh extraction sockets. Clin. Oral Implant. Res. 2006, 17, 615–624. [Google Scholar] [CrossRef]
- Vera, C.; De Kok, I.J.; Chen, W.; Reside, G.; Tyndall, D.; Cooper, L.F. Evaluation of post-implant buccal bone resorption using cone beam computed tomography: A clinical pilot study. Int. J. Oral Maxillofac. Implant. 2012, 27, 1249–1257. [Google Scholar]
- Botticelli, D.; Berglundh, T.; Lindhe, J. Hard-tissue alterations following immediate implant placement in extraction sites. J. Clin. Periodontol. 2004, 31, 820–828. [Google Scholar] [CrossRef]
- Nalcaci, R.; Ozturk, F.; Sokucu, O. A comparison of two-dimensional radiography and three-dimensional computed tomography in angular cephalometric measurements. Dentomaxillofac. Radiol. 2010, 39, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vera, C.; De Kok, I.J.; Reinhold, D.; Limpiphipatanakorn, P.; Yap, A.K.; Tyndall, D.; Cooper, L.F. Evaluation of buccal alveolar bone dimension of maxillary anterior and premolar teeth: A cone beam computed tomography investigation. Int. J. Oral Maxillofac. Implant. 2012, 27, 1514–1519. [Google Scholar]
- Zhao, L.; Wei, Y.; Xu, T.; Zhang, B.; Hu, W.; Chung, K.H. Changes in alveolar process dimensions following extraction of molars with advanced periodontal disease: A clinical pilot study. Clin. Oral Implant. Res. 2019, 30, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Clementini, M.; Agostinelli, A.; Castelluzzo, W.; Cugnata, F.; Vignoletti, F.; De Sanctis, M. The effect of immediate implant placement on alveolar ridge preservation compared to spontaneous healing after tooth extraction: Radiographic results of a randomized controlled clinical trial. J. Clin. Periodontol. 2019, 46, 776–786. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Wang, H.L.; Yu, H. Initial bone volume changes after immediate implant placement associated with filling the gap using bovine bone in molar sites. Int. J. Oral Maxillofac. Implant. 2019, 34, 521–528. [Google Scholar] [CrossRef]
- Block, M.S.; Scoggin, Z.D.; Yu, Q. Assessment of bone width for implants in the posterior mandible. J. Oral Maxillofac. Surg. 2015, 73, 1715–1722. [Google Scholar] [CrossRef]
- Sasada, Y.; Cochran, D.L. Implant-abutment connections: A review of biologic consequences and peri-implantitis implications. Int. J. Oral Maxillofac. Implant. 2017, 32, 1296–1307. [Google Scholar] [CrossRef] [Green Version]
- Uraz, A.; Isler, S.C.; Cula, S.; Tunc, S.; Yalim, M.; Cetiner, D. Platform-switched implants vs platform-matched implants placed in different implant-abutment interface positions: A prospective randomized clinical and microbiological study. Clin. Implant. Dent. Relat. Res. 2020, 22, 59–68. [Google Scholar] [CrossRef]
- Thoma, D.S.; Payer, M.; Jakse, N.; Bienz, S.P.; Husler, J.; Schmidlin, P.R.; Jung, U.W.; Hammerle, C.H.F.; Jung, R.E. Randomized, controlled clinical two-centre study using xenogeneic block grafts loaded with recombinant human bone morphogenetic protein-2 or autogenous bone blocks for lateral ridge augmentation. J. Clin. Periodontol. 2018, 45, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Thoma, D.S.; Bienz, S.P.; Payer, M.; Husler, J.; Schmidlin, P.R.; Hammerle, C.H.F.; Jakse, N.; Jung, R.E. Randomized clinical study using xenograft blocks loaded with bone morphogenetic protein-2 or autogenous bone blocks for ridge augmentation-A three-dimensional analysis. Clin. Oral Implant. Res. 2019, 30, 872–881. [Google Scholar] [CrossRef]
- Naenni, N.; Bienz, S.P.; Munoz, F.; Hammerle, C.H.F.; Jung, R.E.; Thoma, D.S. Volumetric changes following ridge preservation or spontaneous healing and early implant placement with simultaneous guided bone regeneration. J. Clin. Periodontol. 2018, 45, 484–494. [Google Scholar] [CrossRef] [Green Version]
- Jung, R.E.; Sapata, V.M.; Hammerle, C.H.F.; Wu, H.; Hu, X.L.; Lin, Y. Combined use of xenogeneic bone substitute material covered with a native bilayer collagen membrane for alveolar ridge preservation: A randomized controlled clinical trial. Clin. Oral Implant. Res. 2018, 29, 522–529. [Google Scholar] [CrossRef]
- Tran, D.T.; Gay, I.C.; Diaz-Rodriguez, J.; Parthasarathy, K.; Weltman, R.; Friedman, L. Survival of dental implants placed in grafted and nongrafted bone: A retrospective study in a university setting. Int. J. Oral Maxillofac. Implant. 2016, 31, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Al-Abedalla, K.; Torres, J.; Cortes, A.R.; Wu, X.; Nader, S.A.; Daniel, N.; Tamimi, F. Bone augmented with allograft onlays for implant placement could be comparable with native bone. J. Oral Maxillofac. Surg. 2015, 73, 2108–2122. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Herzog, M.; Wolleb, K.; Ramel, C.F.; Thoma, D.S.; Hammerle, C.H. A randomized controlled clinical trial comparing small buccal dehiscence defects around dental implants treated with guided bone regeneration or left for spontaneous healing. Clin. Oral Implant. Res. 2017, 28, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izzetti, R.; Vitali, S.; Gabriele, M.; Caramella, D. Feasibility of a combination of intraoral uhfus and cbct in the study of peri-implantitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, e89–e94. [Google Scholar] [CrossRef] [PubMed]
- Bohner, L.O.L.; Mukai, E.; Oderich, E.; Porporatti, A.L.; Pacheco-Pereira, C.; Tortamano, P.; De Luca Canto, G. Comparative analysis of imaging techniques for diagnostic accuracy of peri-implant bone defects: A meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 432–440.e5. [Google Scholar] [CrossRef] [PubMed]
- De-Azevedo-Vaz, S.L.; Peyneau, P.D.; Ramirez-Sotelo, L.R.; Vasconcelos Kde, F.; Campos, P.S.; Haiter-Neto, F. Efficacy of a cone beam computed tomography metal artifact reduction algorithm for the detection of peri-implant fenestrations and dehiscences. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 550–556. [Google Scholar] [CrossRef]
- Schulze, R.K.; Berndt, D.; d’Hoedt, B. On cone-beam computed tomography artifacts induced by titanium implants. Clin. Oral Implant. Res. 2010, 21, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Benic, G.I.; Sancho-Puchades, M.; Jung, R.E.; Deyhle, H.; Hammerle, C.H. In vitro assessment of artifacts induced by titanium dental implants in cone beam computed tomography. Clin. Oral Implant. Res. 2013, 24, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Puchades, M.; Hammerle, C.H.; Benic, G.I. In vitro assessment of artifacts induced by titanium, titanium-zirconium and zirconium dioxide implants in cone-beam computed tomography. Clin. Oral Implant. Res. 2015, 26, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Schollchen, M.; Gauer, T.; Aarabi, G.; Assaf, A.T.; Rendenbach, C.; Beck-Broichsitter, B.; Semmusch, J.; Sedlacik, J.; Heiland, M.; et al. Artefacts in multimodal imaging of titanium, zirconium and binary titanium-zirconium alloy dental implants: An in vitro study. Dentomaxillofac. Radiol. 2017, 46, 20160267. [Google Scholar] [CrossRef] [Green Version]
- Parsa, A.; Ibrahim, N.; Hassan, B.; Syriopoulos, K.; van der Stelt, P. Assessment of metal artefact reduction around dental titanium implants in cone beam ct. Dentomaxillofac. Radiol. 2014, 43, 20140019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritter, L.; Elger, M.C.; Rothamel, D.; Fienitz, T.; Zinser, M.; Schwarz, F.; Zoller, J.E. Accuracy of peri-implant bone evaluation using cone beam ct, digital intra-oral radiographs and histology. Dentomaxillofac. Radiol. 2014, 43, 20130088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheridan, R.A.; Chiang, Y.C.; Decker, A.M.; Sutthiboonyapan, P.; Chan, H.L.; Wang, H.L. The effect of implant-induced artifacts on interpreting adjacent bone structures on cone-beam computed tomography scans. Implant. Dent. 2018, 27, 10–14. [Google Scholar] [CrossRef]
- Pelekos, G.; Acharya, A.; Tonetti, M.S.; Bornstein, M.M. Diagnostic performance of cone beam computed tomography in assessing peri-implant bone loss: A systematic review. Clin. Oral Implant. Res. 2018, 29, 443–464. [Google Scholar] [CrossRef]
- Lin, C.Y.; Pan, W.L.; Wang, H.L. Facial fenestration and dehiscence defects associated with immediate implant placement without flap elevation in anterior maxillary ridge: A preliminary cone beam computed tomography study. Int. J. Oral Maxillofac. Implant. 2018, 33, 1112–1118. [Google Scholar] [CrossRef]

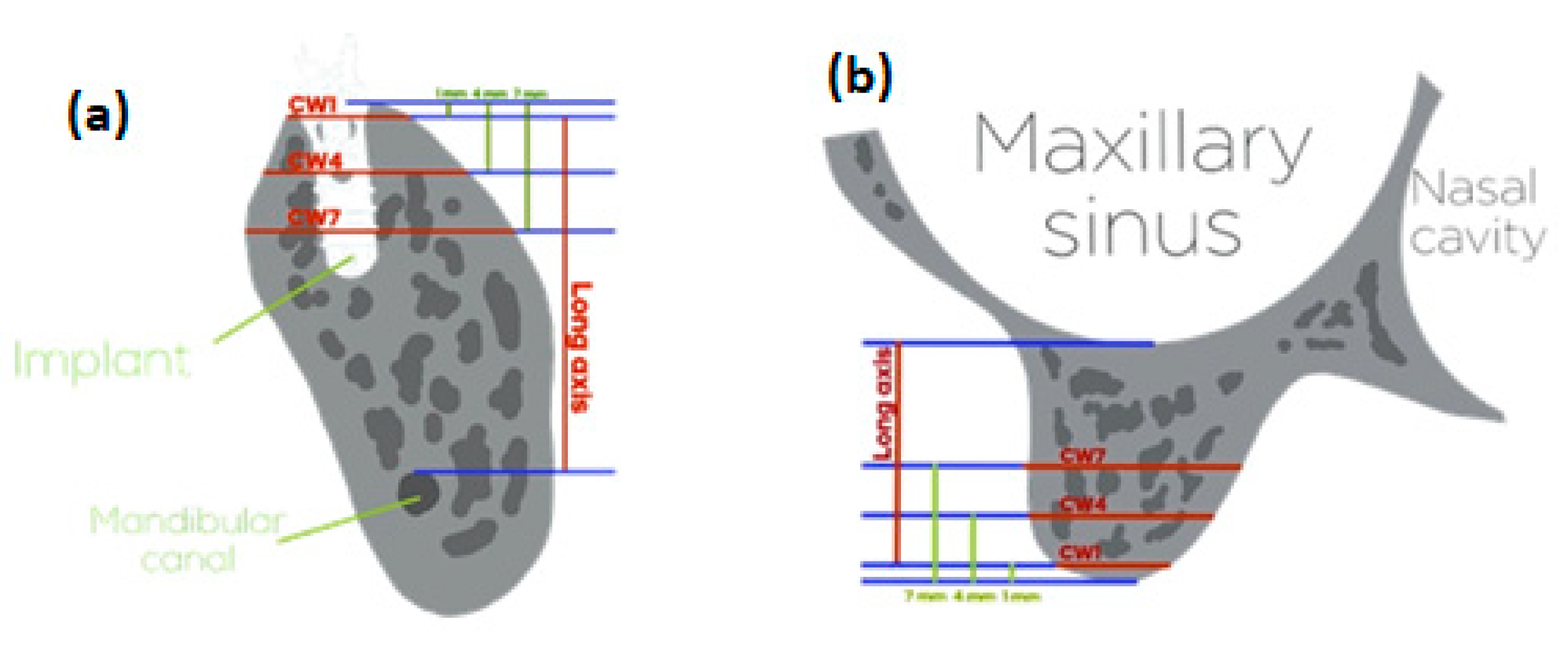
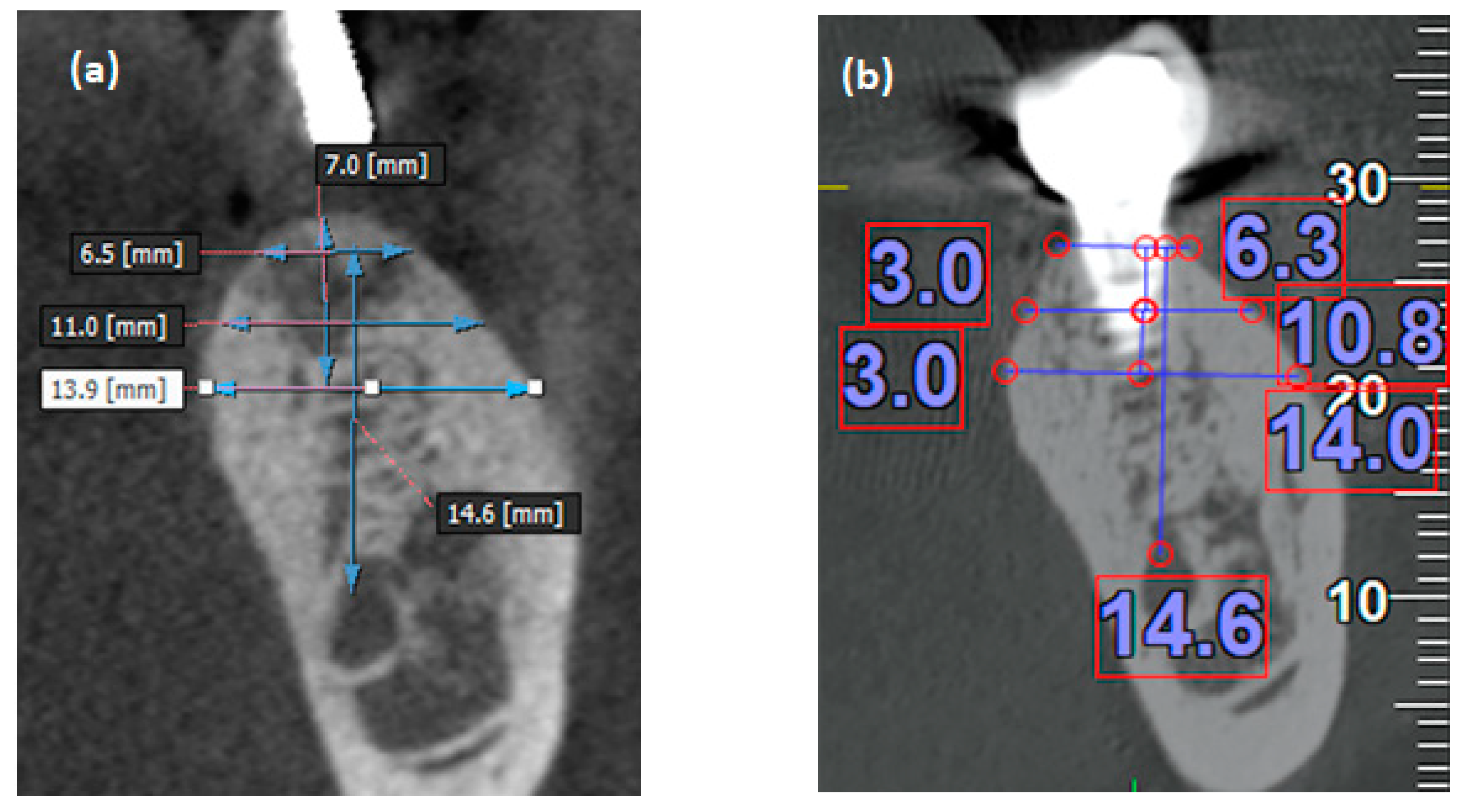

| Item | N (%) |
|---|---|
| Gender | |
| Male | 43 (43.4) |
| Female | 56 (56.6) |
| Age of surgery (mean ± SD, years) | 52.5 ± 11.4 |
| Follow-up period (mean ± SD, months) | 42.4 ± 32.3 |
| Implant sites | |
| Anterior | 6 (6) |
| Premolar | 27 (27.3) |
| Molar | 66 (66.7) |
| Implant arch | |
| Maxilla | 48 (48.5) |
| Mandible | 51 (51.5) |
| Brands of implants | |
| Straumann® | 63 (63.7) |
| MIS | 22 (22.2) |
| Biomet 3iTM | 13 (13.1) |
| Astra tech | 1 (1) |
| Implant lengths | |
| 8 mm | 3 (3) |
| 10 mm | 76 (76.8) |
| 11.0–12.0 mm | 20 (20.2) |
| Implant widths | |
| 3.25–3.75 mm | 20 (20.2) |
| 4.0–4.2 mm | 61 (61.6) |
| 4.8–5.0 mm | 18 (18.2) |
| Previous ridge augmentation or preservation | |
| Yes | 48 (48.5) |
| No | 51 (51.5) |
| Sub-Crestal Levels | Bone Widths (mm) | p-Value | ||
|---|---|---|---|---|
| Pre-surgery Mean ± SD | Post-surgery Mean ± SD | Change Mean ± SD | ||
| CW 1 | 6.98 ± 2.24 | 6.83 ± 2.02 | 0.15 ± 1.74 | 0.390 |
| CW 4 | 9.97 ± 2.64 | 9.58 ± 2.55 | 0.39 ± 1.12 | 0.0008 * |
| CW 7 | 11.33 ± 3.00 | 11.19 ± 2.90 | 0.14 ± 1.05 | 0.201 |
| Sub-Crestal Levels | Change of Bone Width (mm) | p-Value | |
|---|---|---|---|
| Maxilla Mean ± SD | Mandible Mean ± SD | ||
| CW 1 | 0.10 ± 2.05 | 0.19 ± 1.40 | 0.800 |
| CW 4 | 0.53 ± 1.17 | 0.26 ± 1.07 | 0.226 |
| CW 7 | 0.29 ± 1.30 | 0.00 ± 0.74 | 0.181 |
| Sub-Crestal Levels | Change of Bone Width (mm) | p-Value | |
|---|---|---|---|
| ARP/GBR Mean ± SD | Native bone Mean ± SD | ||
| CW 1 | −0.04 ± 1.72 | 0.34 ± 1.75 | 0.272 |
| CW 4 | 0.21 ± 1.17 | 0.56 ± 1.06 | 0.128 |
| CW 7 | 0.01 ± 1.12 | 0.25 ± 0.99 | 0.259 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, K.-F.; Lin, S.-W.; Lin, Y.-C.; Jeng, J.-H.; Huang, Y.-T.; Liu, P.-F.; Tseng, C.-J.; Chou, Y.-H. Using Cone-Beam Computed Tomography to Assess Changes in Alveolar Bone Width around Dental Implants at Native and Reconstructed Bone Sites: A Retrospective Cohort Study. J. Pers. Med. 2021, 11, 1011. https://doi.org/10.3390/jpm11101011
Hu K-F, Lin S-W, Lin Y-C, Jeng J-H, Huang Y-T, Liu P-F, Tseng C-J, Chou Y-H. Using Cone-Beam Computed Tomography to Assess Changes in Alveolar Bone Width around Dental Implants at Native and Reconstructed Bone Sites: A Retrospective Cohort Study. Journal of Personalized Medicine. 2021; 11(10):1011. https://doi.org/10.3390/jpm11101011
Chicago/Turabian StyleHu, Kai-Fang, Szu-Wei Lin, Ying-Chu Lin, Jiiang-Huei Jeng, Yu-Ting Huang, Pei-Feng Liu, Ching-Jiunn Tseng, and Yu-Hsiang Chou. 2021. "Using Cone-Beam Computed Tomography to Assess Changes in Alveolar Bone Width around Dental Implants at Native and Reconstructed Bone Sites: A Retrospective Cohort Study" Journal of Personalized Medicine 11, no. 10: 1011. https://doi.org/10.3390/jpm11101011
APA StyleHu, K.-F., Lin, S.-W., Lin, Y.-C., Jeng, J.-H., Huang, Y.-T., Liu, P.-F., Tseng, C.-J., & Chou, Y.-H. (2021). Using Cone-Beam Computed Tomography to Assess Changes in Alveolar Bone Width around Dental Implants at Native and Reconstructed Bone Sites: A Retrospective Cohort Study. Journal of Personalized Medicine, 11(10), 1011. https://doi.org/10.3390/jpm11101011








