Interaction of Polygenetic Variants for Gestational Diabetes Mellitus Risk with Breastfeeding and Korean Balanced Diet to Influence Type 2 Diabetes Risk in Later Life in a Large Hospital-Based Cohort
Abstract
:1. Introduction
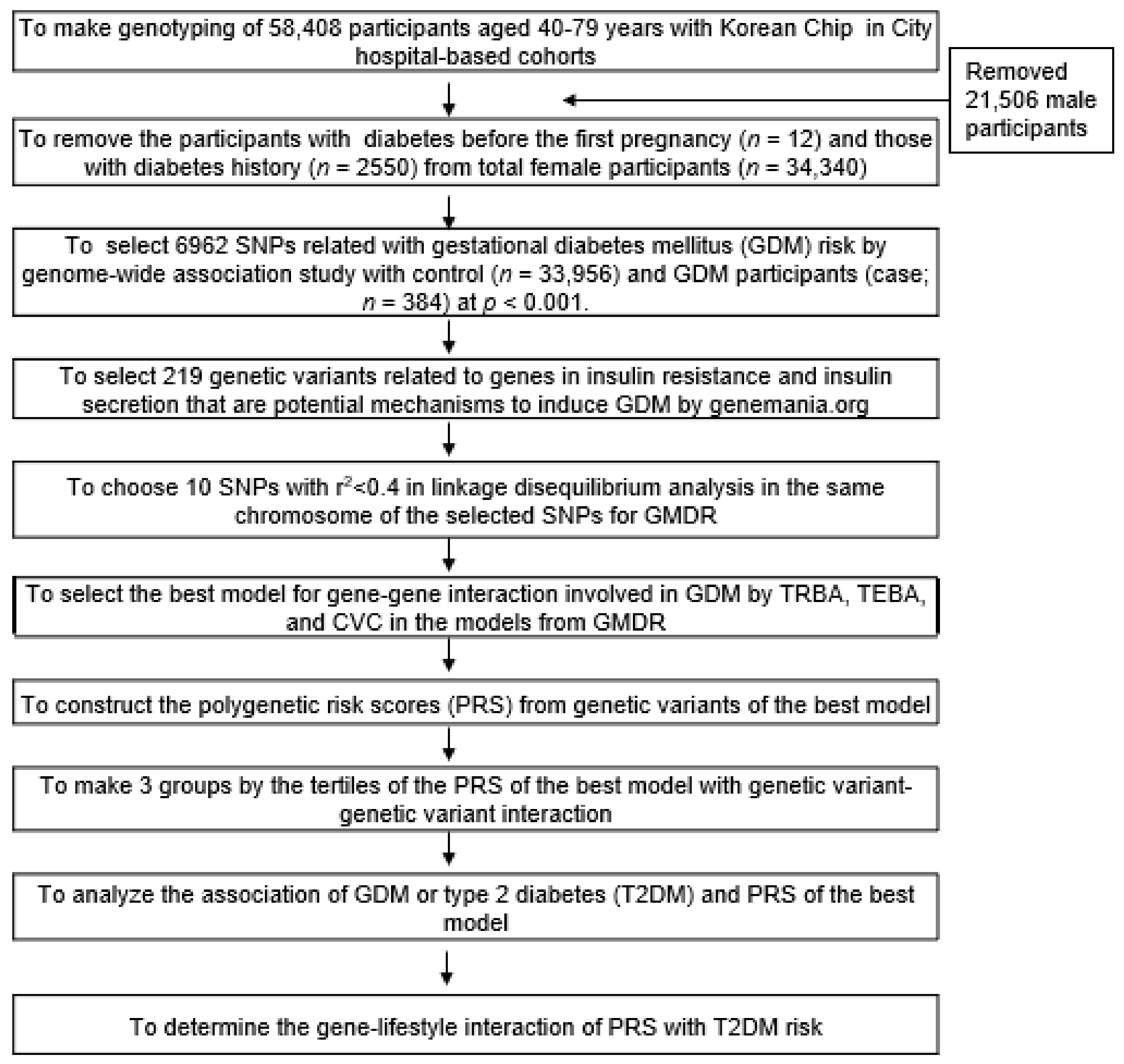
2. Methods
2.1. Participants
2.2. Definition of GDM and T2DM
2.3. General Characteristics and Anthropometric and Biochemical Measurements
2.4. Food Intakes Using a Semi-Quantitative Food Frequency Questionnaire (SQFFQ) and Dietary Pattern Analysis
2.5. Genotyping DNA and Its Quality Control
2.6. Best Models for Genetic Variant-Genetic Variant Interactions by Generalized Multifactor Dimensionality Reduction (GMDR)
2.7. Statistical Analyses
3. Results
3.1. Daily Nutrient Intake
3.2. Genetic Variants Associated with the GDM Risk and Its Best Model of Gene-Gene Interactions
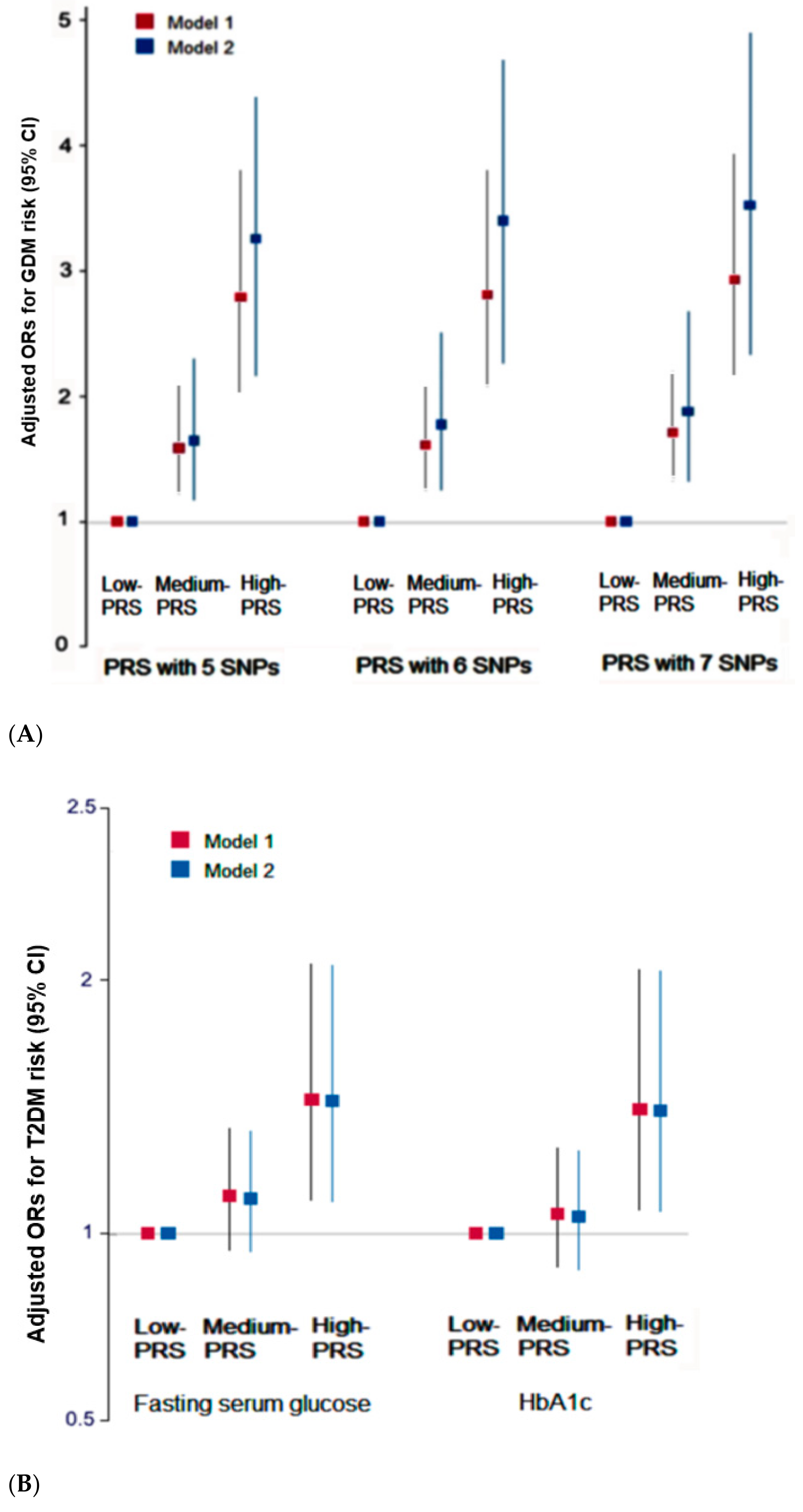
3.3. Association of the PRS with the 5-SNP Model with GDM and T2DM Risk
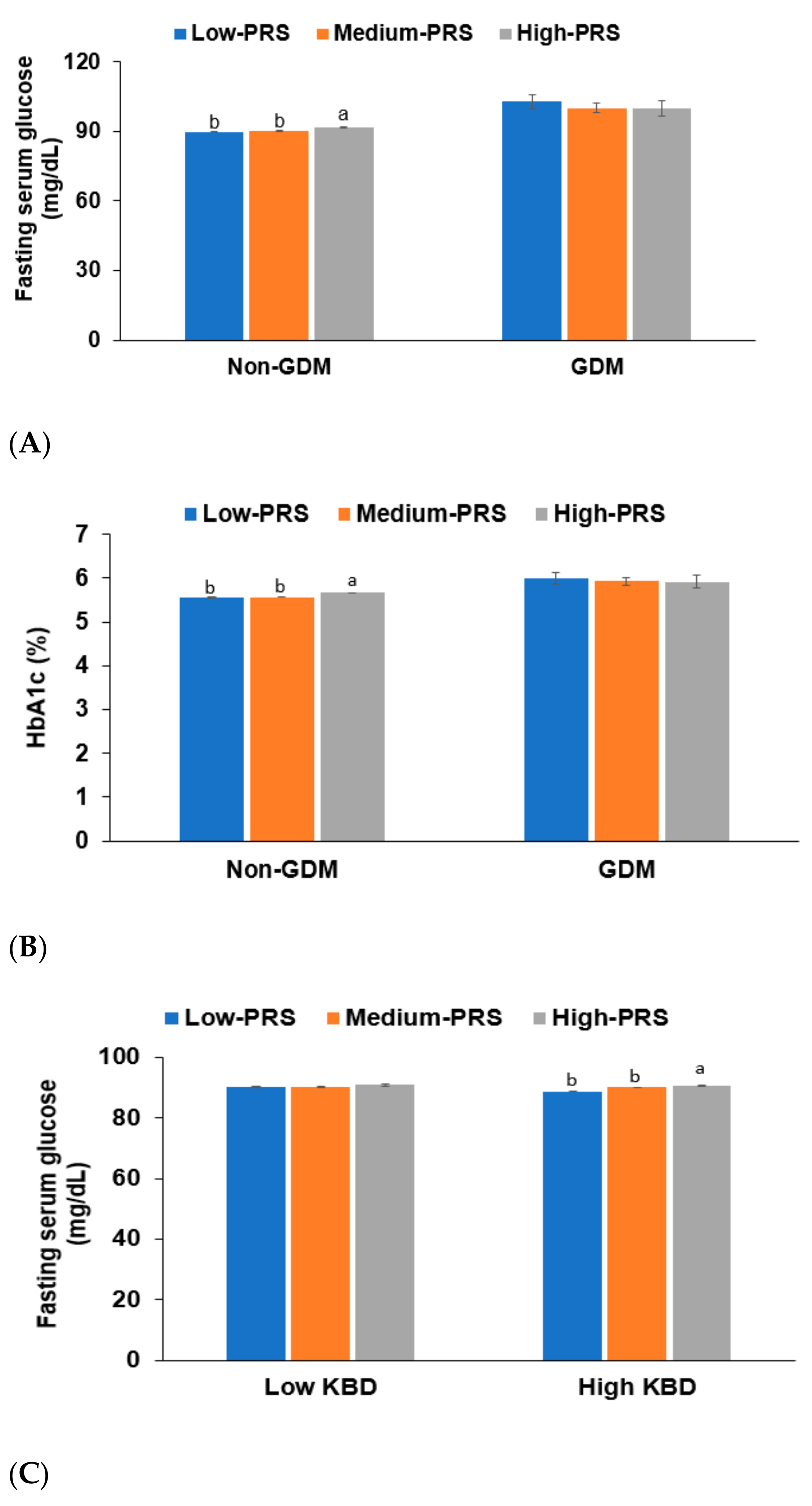
3.4. Interaction between PRS, GDM, and Dietary Patterns for T2DM Risk
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Shi, L.; Zhang, D.; Chao, S.M. Influence of Acculturation on Risk for Gestational Diabetes among Asian Women. Prev. Chronic Dis. 2019, 16, 190212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kim, M.H.; Kim, S.H. Early gestational weight gains within current recommendations result in increased risk of gestational diabetes mellitus among Korean women. Diabetes Metab. Res. Rev. 2014, 30, 716–725. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.Y.; Baik, S.H.; Woo, J.T.; Kwon, Y.J.; Daily, J.W.; Park, Y.M.; Yang, J.H.; Kim, S.H. Gestational diabetes is associated with high energy and saturated fat intakes and with low plasma visfatin and adiponectin levels independent of prepregnancy BMI. Eur. J. Clin. Nutr. 2013, 67, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Ching, S.M.; Ramachandran, V.; Yee, A.; Hoo, F.K.; Chia, Y.C.; Wan Sulaiman, W.A.; Suppiah, S.; Mohamed, M.H.; Veettil, S.K. Prevalence and risk factors of gestational diabetes mellitus in Asia: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2018, 18, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Park, J.Y.; Lee, J.H.; Kim, S.H. Plasma levels of lysine, tyrosine, and valine during pregnancy are independent risk factors of insulin resistance and gestational diabetes. Metab. Syndr. Relat. Disord. 2015, 13, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Yoon, H.K.; Ryu, H.M.; Han, Y.J.; Lee, S.W.; Park, B.K.; Park, S.Y.; Yim, C.H.; Kim, S.H. Maternal vitamin D deficiency in early pregnancy is not associated with gestational diabetes mellitus development or pregnancy outcomes in Korean pregnant women in a prospective study. J. Nutr. Sci. Vitaminol. 2014, 60, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Chon, S.; Park, S.Y.; Yun, S.; Baik, S.H.; Woo, J.T.; Rhee, S.Y.; Pak, Y.K.; Kim, S.H. Association of aryl hydrocarbon receptor transactivating activity, a potential biomarker for persistent organic pollutants, with the risk of gestational diabetes mellitus. Sci. Rep. 2021, 11, 3185. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, S.H. Women with rigorously managed overt diabetes during pregnancy do not experience adverse infant outcomes but do remain at serious risk of postpartum diabetes. Endocr. J. 2015, 62, 319–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Yu, W.; Hu, C. Genetics of Type 2 Diabetes: Insights into the Pathogenesis and Its Clinical Application. BioMed Res. Int. 2014, 2014, 926713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.S.; Kim, B.C.; Daily, J.W.; Park, S. High genetic risk scores for impaired insulin secretory capacity doubles the risk for type 2 diabetes in Asians and is exacerbated by Western-type diets. Diabetes Metab. Res. Rev. 2018, 34, e2944. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.C.; Kang, S. Interaction effect of PGC−1α rs10517030 variants and energy intake in the risk of type 2 diabetes in middle-aged adults. Eur. J. Clin. Nutr. 2017, 71, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Liu, M.; Kang, S. Alcohol Intake Interacts with CDKAL1, HHEX, and OAS3 Genetic Variants, Associated with the Risk of Type 2 Diabetes by Lowering Insulin Secretion in Korean Adults. Alcohol Clin. Exp. Res. 2018, 42, 2326–2336. [Google Scholar] [CrossRef]
- Hong, K.W.; Kim, S.H.; Zhang, X.; Park, S. Interactions among the variants of insulin-related genes and nutrients increase the risk of type 2 diabetes. Nutr. Res. 2018, 51, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.; Shuldiner, A.R. The genetic interface between gestational diabetes and type 2 diabetes. J. Matern.-Fetal Neonatal Med. 2012, 25, 36–40. [Google Scholar] [CrossRef]
- Kawai, V.K.; Levinson, R.T.; Adefurin, A.; Kurnik, D.; Collier, S.P.; Conway, D.; Stein, C.M. A genetic risk score that includes common type 2 diabetes risk variants is associated with gestational diabetes. Clin. Endocrinol. 2017, 87, 149–155. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cheong, H.S.; Park, B.L.; Baik, S.H.; Park, S.; Lee, S.W.; Kim, M.H.; Chung, J.H.; Choi, J.S.; Kim, M.Y.; et al. Melatonin receptor 1 B polymorphisms associated with the risk of gestational diabetes mellitus. BMC Med. Genet. 2011, 12, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, S.H.; Kim, S.H.; Cho, Y.M.; Go, M.J.; Cho, Y.S.; Choi, S.H.; Moon, M.K.; Jung, H.S.; Shin, H.D.; Kang, H.M.; et al. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes 2012, 61, 531–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noury, A.E.; Azmy, O.; Alsharnoubi, J.; Salama, S.; Okasha, A.; Gouda, W. Variants of CDKAL1 rs7754840 (G/C) and CDKN2A/2B rs10811661 (C/T) with gestational diabetes: Insignificant association. BMC Res. Notes 2018, 11, 181. [Google Scholar] [CrossRef] [Green Version]
- Anghebem-Oliveira, M.I.; Martins, B.R.; Alberton, D.; Ramos, E.A.S.; Picheth, G.; Rego, F.G.M. Type 2 diabetes-associated genetic variants of FTO, LEPR, PPARg, and TCF7L2 in gestational diabetes in a Brazilian population. Arch. Endocrinol. Metab. 2017, 61, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Saucedo, R.; Valencia, J.; Gutierrez, C.; Basurto, L.; Hernandez, M.; Puello, E.; Rico, G.; Vega, G.; Zarate, A. Gene variants in the FTO gene are associated with adiponectin and TNF-alpha levels in gestational diabetes mellitus. Diabetol. Metab. Syndr. 2017, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Jin, H.S.; Park, S. Protein and fat intake interacts with the haplotype of PTPN11_rs11066325, RPH3A_rs886477, and OAS3_rs2072134 to modulate serum HDL concentrations in middle-aged people. Clin. Nutr. 2020, 39, 942–949. [Google Scholar] [CrossRef]
- Park, S.; Ahn, J.; Lee, B.K. Self-rated Subjective Health Status Is Strongly Associated with Sociodemographic Factors, Lifestyle, Nutrient Intakes, and Biochemical Indices, but Not Smoking Status: KNHANES 2007–2012. J. Korean Med. Sci. 2015, 30, 1279–1287. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Yang, H.J.; Kim, M.J.; Hur, H.J.; Kim, S.-H.; Kim, M.-S. Interactions between Polygenic Risk Scores, Dietary Pattern, and Menarche Age with the Obesity Risk in a Large Hospital-Based Cohort. 2021; 13, 3772. [Google Scholar]
- Kim, Y.; Han, B.G. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, 1350. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S. A Western-style diet interacts with genetic variants of the LDL receptor to hyper-LDL cholesterolemia in Korean adults. Public Health Nutr. 2020, 24, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Park, S. Association between polygenetic risk scores related to sarcopenia risk and their interactions with regular exercise in a large cohort of Korean adults. Clin. Nutr. 2021, 40, 5355–5364. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, M.K. Relationship of sodium intake with obesity among Korean children and adolescents: Korea National Health and Nutrition Examination Survey. Br. J. Nutr. 2016, 115, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Rabbee, N.; Speed, T.P. A genotype calling algorithm for affymetrix SNP arrays. Bioinformatics 2006, 22, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Uma Jyothi, K.; Reddy, B.M. Gene-gene and gene-environment interactions in the etiology of type 2 diabetes mellitus in the population of Hyderabad, India. Meta Gene 2015, 5, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.D.; Park, B.L.; Shin, H.J.; Kim, J.Y.; Park, S.; Kim, B.; Kim, S.-H. Association of KCNQ1 Polymorphisms with the Gestational Diabetes Mellitus in Korean Women. J. Clin. Endocrinol. Metab. 2010, 95, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Cui, L.; Tam, W.H.; Ma, R.C.W.; Wang, C.C. Genetic variants associated with gestational diabetes mellitus: A meta-analysis and subgroup analysis. Sci. Rep. 2016, 6, 30539. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Zhang, L.; Chen, T.; Shi, A.; Xie, K.; Li, Z.; Xu, J.; Chen, Z.; Ji, C.; Wen, J. Genetic Susceptibility to Gestational Diabetes Mellitus in a Chinese Population. Front. Endocrinol. 2020, 11, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powe, C.E.; Nodzenski, M.; Talbot, O.; Allard, C.; Briggs, C.; Leya, M.V.; Perron, P.; Bouchard, L.; Florez, J.C.; Scholtens, D.M.; et al. Genetic Determinants of Glycemic Traits and the Risk of Gestational Diabetes Mellitus. Diabetes 2018, 67, 2703–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Kim, M.Y.; Yang, J.H.; Park, S.Y.; Yim, C.H.; Han, K.O.; Yoon, H.K.; Park, S. Nutritional risk factors of early development of postpartum prediabetes and diabetes in women with gestational diabetes mellitus. Nutrition 2011, 27, 782–788. [Google Scholar] [CrossRef]
- Ley, S.H.; Chavarro, J.E.; Li, M.; Bao, W.; Hinkle, S.N.; Wander, P.L.; Rich-Edwards, J.; Olsen, S.; Vaag, A.; Damm, P.; et al. Lactation Duration and Long-term Risk for Incident Type 2 Diabetes in Women with a History of Gestational Diabetes Mellitus. Diabetes Care 2020, 43, 793–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jannasch, F.; Kröger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [Green Version]

| Parameters | Control 1 (n = 33,956) | GDM 2 (n = 384) | ORs and 95% CI for GDM 3 |
|---|---|---|---|
| Age (<55 years) 4 | 53.0 ± 0.05 | 49.2 ± 0.45 *** | 0.434 (0.310–0.607) |
| Education (Number, %) | |||
| <high school | 6773 (20.2) | 44 (11.5) *** | 1 |
| High school | 7678 (22.9) | 55 (14.4) | 0.915 (0.546–1.534) |
| ≥College more | 19,078 (56.9) | 284 (74.1) | 1.446 (1.017–2.381) |
| Income (Number, %) | |||
| Low (<$2000) | 3688 (11.0) | 19 (5.1) *** | 1 |
| Medium ($2000–4000) | 14,686 (43.8) | 153 (40.0) | 1.103 (0.675–1.803) |
| High (>$4000) | 15,189 (45.3) | 211 (54.9) | 0.968 (0.586–1.600) |
| Age at first pregnancy (<25 years) | 25.2 ± 0.02 | 26.4 ± 0.21 *** | 1.170 (1.068 1.282) |
| BMI at age 20 (<25 kg/m2) | 20.3 ± 0.08 | 22.2 ± 0.70 *** | 7.600 (1.938–29.80) |
| Large baby (number, %) | 326 (0.97) | 55 (14.3) *** | 2.085 (1.393–3.120) |
| Age born LGA (<29 years) | 27.8 ± 0.11 | 29.1 ± 0.63 * | 1.451 (0.544–3.868) |
| Children number (≤1) | 161 (1.47) | 223 (0.86) | 0.830 (0.667–1.033) |
| Menarche age (<15 years) | 15.0 ± 0.05 | 15.2 ± 0.47 | 0.838 (0.612–1.147) |
| Breast feeding (yes %) | 31,088 (86.4) | 301 (78.8) *** | |
| <1 year | 25,218 (69.1) | 212 (55.2) *** | 0.920 (0.821–1.031) 5 |
| ≥1 year | 16,292 (44.6) | 104 (27.1) *** | 0.887 (0.806–0.976) 5 |
| Metabolic syndrome (n, %) | 322 (1.01) | 62 (1.22) | 1.764 (1.279–2.434) |
| BMI (<25 kg/m2) | 23.5 ± 0.02 | 23.5 ± 0.19 | 1.193 (0.879–1.620) |
| Waist circumference (cm) 6 | 78.2 ± 0.03 | 78.7 ± 0.25 | 1.345 (0.933–1.939) |
| Fasting serum glucose (<126 mg/dL) | 90.9 ± 0.07 | 101 ± 0.66 *** | 8.420 (6.452–10.99) |
| HbA1C (<6.5%) | 5.59 ± 0.00 | 5.97 ± 0.03 *** | 9.229 (6.368–13.38) |
| Type 2 diabetes (n, %) | 2550 (7.6) | 88 (22.9) *** | 4.746 (3.314–6.796) |
| Serum total cholesterol (<230 mg/dL) | 201 ± 0.20 | 199 ± 1.83 | 1.152 (0.886–1.498) |
| Serum LDL (<130 mg/dL) | 119.2 ± 1.52 | 118.0 ± 11.2 | 1.076 (0.794–1.458) |
| Serum HDL (mg/dL) 7 | 56.4 ± 0.07 | 56.0 ± 0.67 | 1.083 (0.864–1.358) |
| Serum TG (<150 mg/dL) | 112 ± 0.39 | 117 ± 3.68 | 1.163 (0.893–1.515) |
| SBP (<130 mmHg) | 121 ± 0.08 | 121 ± 0.73 | 1.167 (0.913–1.491) |
| DBP (<90 mmHg) | 74.3 ± 0.05 | 73.9 ± 0.44 | 1.221 (0.907–1.644) |
| Nutrients | Control 1 | GDM 2 | ||
|---|---|---|---|---|
| No T2DM (n = 33,956) | T2DM (n = 2550) | No T2DM (n = 296) | T2DM (n = 88) | |
| Energy (EER %) | 99.0 3 ± 0.03 a | 98.5 ± 0.11 b | 98.3 ± 0.31 b | 98.1 ± 0.59 b# |
| Carbohydrate (energy %) | 72.0 ± 0.04 ab | 72.1 ± 0.14 ab | 71.2 ± 0.40 b | 72.9 ± 0.76 a |
| Dietary fiber (g) | 5.7 ± 0.01 | 5.6 ± 0.05 | 5.4 ± 0.13 | 5.8 ± 0.24 |
| Fat (energy %) | 13.7 ± 0.03 ab | 13.4 ± 0.11 b | 14.3 ± 0.3 a | 12.9 ± 0.57 b## |
| Protein (energy %) | 13.4 ± 0.01 | 13.4 ± 0.05 | 13.5 ± 0.15 | 13.4 ± 0.29 |
| Sodium (mg/day) | 2337 ± 6.31 | 2344 ± 23.5 | 2232 ± 65.9 | 2449 ± 126 |
| Vitamin C (mg/day) | 110 ± 0.32 a | 105 ± 1.22 b | 99.7 ± 3.42 b | 108.5 ± 6.54 ab+ |
| KBD (≥70th percentiles) | 10,460 (29.7) | 295 (22.7) *** | 116 (34.0) | 18 (41.9) |
| WSD (≥70th percentiles) | 10,394 (29.5) | 343 (26.3) * | 135 (45.6) | 28 (31.8) * |
| RMD (≥70th percentiles) | 10,459 (29.7) | 316 (24.3) *** | 106 (31.1) | 10 (23.3) |
| Smoking (current + past) | 987 (2.91) | 103 (3.97) *** | 10 (3.39) | 5 (5.69) |
| Drinking (g/day) | 39.1 ± 1.27 a | 29.7 ± 3.83 b | 33.7 ± 10.7 b | 38.6 ± 20.5 a+ |
| Regular exercise (n, %) | 17,722 (52.2) | 1369 (53.7) | 156 (52.7) | 53 (60.2) |
| Chr 1 | SNP 2 | Position | Mi 3 | Ma 4 | OR and 95% CI 5 | p-Value Adjusted 6 | MAF 7 | p-Value for HWE 8 | Gene | Functional Consequence |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | rs6821589 | 89192792 | G | A | 2.86 (1.80–4.54) | 7.84 × 10−7 | 0.011 | 0.547 | PPM1K | intron |
| 4 | rs189428800 | 123779401 | A | G | 2.24 (1.45–3.45) | 2.74 × 10−5 | 0.015 | 0.774 | FGF2 | intron |
| 6 | rs7754840 | 20661250 | C | G | 1.16 (1.00–1.35) | 4.46 × 10−5 | 0.476 | 0.427 | CDKAL1 | intron |
| 7 | rs181540079 | 17370229 | C | T | 2.13 (1.37–3.31) | 8.47 × 10−5 | 0.015 | 0.498 | AHR | intron |
| 7 | rs11975504 | 151481965 | C | T | 1.73 (1.31–2.28) | 1.04 × 10−5 | 0.051 | 0.553 | PRKAG2 | intron |
| 9 | rs916855529 | 8721355 | G | A | 0.73 (0.62–0.86) | 1.28 × 10−5 | 0.397 | 0.160 | PTPRD | intron |
| 10 | rs2274034 | 6019248 | C | T | 0.72 (0.08–0.62) | 3.32 × 10−6 | 0.419 | 0.061 | IL15RA | 3′ UTR |
| 12 | rs148031082 | 80309656 | A | G | 2.48 (1.61–3.82) | 3.86 × 10−6 | 0.014 | 0.129 | PPP1R12A | intron |
| 13 | rs9589710 | 93967361 | T | C | 0.73 (0.62–0.86) | 1.81 × 10−5 | 0.364 | 0.782 | GPC6 | intron |
| 18 | rs80164908 | 7862077 | G | A | 1.42 (1.18–1.71) | 1.87 × 10−5 | 0.158 | 0.901 | PTPRM | intron |
| Model | Adjusted Age at First Pregnancy, and Weight at 20 | Adjusted Age at First Pregnancy, Weight at 20, Residence Area, Childbirth Experience, Education | ||||||
|---|---|---|---|---|---|---|---|---|
| TRBA | TEBA | p Value | CVC | TRBA | TEBA | p Value | CVC | |
| PTPRD_rs916855529 | 0.536 | 0.501 | 4 (0.828) | 7/10 | 0.532 | 0.514 | 6 (0.377) | 7/10 |
| GPC6_ rs9589710 plus model 1 | 0.558 | 0.529 | 9 (0.010) | 5/10 | 0.549 | 0.527 | 8 (0.055) | 7/10 |
| CDKAL1_rs7754840 plus model 2 | 0.581 | 0.550 | 8 (0.055) | 8/10 | 0.568 | 0.536 | 8 (0.055) | 7/10 |
| PRKAG2_ rs11975504 plus model 3 | 0.601 | 0.543 | 9 (0.011) | 5/10 | 0.587 | 0.547 | 9 (0.011) | 9/10 |
| PTPRM_rs80164908 plus model 4 | 0.624 | 0.555 | 10 (0.001) | 10/10 | 0.608 | 0.547 | 9 (0.011) | 10/10 |
| IL15RA_rs2274034 plus model 5 | 0.644 | 0.548 | 10 (0.001) | 9/10 | 0.626 | 0.546 | 10 (0.001) | 9/10 |
| AHR_rs181540079 plus model 6 | 0.663 | 0.557 | 10 (0.001) | 10/10 | 0.643 | 0.549 | 10 (0.001) | 10/10 |
| PPM1K_ rs6821589 plus model 7 | 0.677 | 0.563 | 10 (0.001) | 10/10 | 0.655 | 0.557 | 10 (0.001) | 10/10 |
| PPP1R12A_rs148031082 plus model 8 | 0.689 | 0.555 | 10 (0.001) | 9/10 | 0.666 | 0.544 | 10 (0.001) | 9/10 |
| FGF2_rs189428800 plus model 9 | 0.699 | 0.553 | 10 (0.001) | 10/10 | 0.674 | 0.544 | 10 (0.001) | 10/10 |
| Low-PRS | Medium-PRS | High-PRS | Interaction | |
|---|---|---|---|---|
| Non-GDM | 1 | 1.009 (0.805–1.264) | 1.362 (1.002–1.857) | 0.0004 for FSB |
| GDM | 1 | 1.429 (0.777–2.629) | 1.115 (0.502–2.477) | |
| Non-GDM | 1 | 1.005 (0.801–1.259) | 1.358 (1.003–1.851) | 0.023 for HbA1c |
| GDM | 1 | 1.252 (0.545–2.874) | 1.543 (0.567–4.203) | |
| Short period for BF (<1 year) | 1 | 1.226 (0.925–1.625) | 1.570 (1.049–2.349) | 0.216 |
| Long period for BF (≥1 year) | 1 | 1.046 (0.776–1.411) | 1.526 (0.991–2.351) | |
| Low intake of KBD (<70th percentile) | 1 | 0.998 (0.705–1.412) | 1.526 (0.934–2.494) | 0.030 |
| High intake of KBD (≥70th percentile) | 1 | 1.117 (0.907–1.375) | 1.503 (1.113–2.030) | |
| Low intake of WSD (<70th percentile) | 1 | 1.129 (0.920–1.387) | 1.623 (1.214–2.169) | 0.193 |
| High intake of WSD (≥70th percentile) | 1 | 1.373 (0.950–1.986) | 2.046 (1.240–3.377) | |
| Low intake of RMD (<70th percentile) | 1 | 1.138 (0.927–1.398) | 1.523 (1.128–2.055) | 0.234 |
| High intake of RMD (≥70th percentile) | 1 | 1.537 (1.092–2.163) | 2.048 (1.273–3.292) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S. Interaction of Polygenetic Variants for Gestational Diabetes Mellitus Risk with Breastfeeding and Korean Balanced Diet to Influence Type 2 Diabetes Risk in Later Life in a Large Hospital-Based Cohort. J. Pers. Med. 2021, 11, 1175. https://doi.org/10.3390/jpm11111175
Park S. Interaction of Polygenetic Variants for Gestational Diabetes Mellitus Risk with Breastfeeding and Korean Balanced Diet to Influence Type 2 Diabetes Risk in Later Life in a Large Hospital-Based Cohort. Journal of Personalized Medicine. 2021; 11(11):1175. https://doi.org/10.3390/jpm11111175
Chicago/Turabian StylePark, Sunmin. 2021. "Interaction of Polygenetic Variants for Gestational Diabetes Mellitus Risk with Breastfeeding and Korean Balanced Diet to Influence Type 2 Diabetes Risk in Later Life in a Large Hospital-Based Cohort" Journal of Personalized Medicine 11, no. 11: 1175. https://doi.org/10.3390/jpm11111175
APA StylePark, S. (2021). Interaction of Polygenetic Variants for Gestational Diabetes Mellitus Risk with Breastfeeding and Korean Balanced Diet to Influence Type 2 Diabetes Risk in Later Life in a Large Hospital-Based Cohort. Journal of Personalized Medicine, 11(11), 1175. https://doi.org/10.3390/jpm11111175






