Associations between Lifestyle Behaviors and Quality of Life Differ Based on Multiple Sclerosis Phenotype
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Demographics and Clinical Outcomes
2.3. Lifestyle Behaviors
2.4. Outcome Measure
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics Based on Phenotype
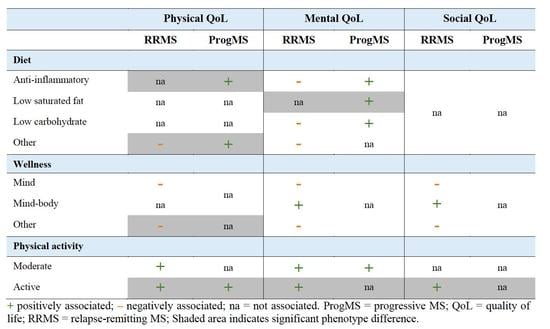
3.2. Associations between Lifestyle and Quality of Life Subdomains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and treatment of multiple sclerosis: A review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Brownlee, W.J.; Hardy, T.A.; Fazekas, F.; Miller, D.H. Diagnosis of multiple sclerosis: Progress and challenges. Lancet 2017, 389, 1336–1346. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurol. Clin. Neurophysiol. 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-González, I.; Martín-Rodríguez, A.; Conrad, R.; Pérez-San-Gregorio, M.Á. Quality of life in adults with multiple sclerosis: A systematic review. BMJ Open 2020, 10, e041249. [Google Scholar] [CrossRef] [PubMed]
- Evers, I.; Heerings, M.; de Roos, N.M.; Jongen, P.J.; Visser, L.H. Adherence to dietary guidelines is associated with better physical and mental quality of life: Results from a cross-sectional survey among 728 dutch ms patients. Nutr. Neurosci. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Mousavi-Shirazi-Fard, Z.; Mazloom, Z.; Izadi, S.; Fararouei, M. The effects of modified anti-inflammatory diet on fatigue, quality of life, and inflammatory biomarkers in relapsing-remitting multiple sclerosis patients: A randomized clinical trial. Int. J. Neurosci. 2021, 131, 657–665. [Google Scholar] [CrossRef]
- Hadgkiss, E.J.; Jelinek, G.A.; Weiland, T.J.; Pereira, N.G.; Marck, C.H.; van der Meer, D.M. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr. Neurosci. 2015, 18, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckmann, Y.; Türe, S.; Duman, S.U. Vitamin d deficiency and its association with fatigue and quality of life in multiple sclerosis patients. EPMA J. 2020, 11, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Simpson-Yap, S.; Jelinek, P.; Weiland, T.; Nag, N.; Neate, S.; Jelinek, G. Self-reported use of vitamin d supplements is associated with higher physical quality of life scores in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 49, 102760. [Google Scholar] [CrossRef]
- Jelinek, G.A.; Hadgkiss, E.J.; Weiland, T.J.; Pereira, N.G.; Marck, C.H.; van der Meer, D.M. Association of fish consumption and ω 3 supplementation with quality of life, disability and disease activity in an international cohort of people with multiple sclerosis. Int. J. Neurosci. 2013, 123, 792–800. [Google Scholar] [CrossRef]
- Simpson, R.; Booth, J.; Lawrence, M.; Byrne, S.; Mair, F.; Mercer, S. Mindfulness based interventions in multiple sclerosis-a systematic review. BMC Neurol. 2014, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Taylor, E.; Taylor-Piliae, R.E. The effects of tai chi on physical and psychosocial function among persons with multiple sclerosis: A systematic review. Complementary Ther. Med. 2017, 31, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Benito-Villalvilla, D.; de Uralde-Villanueva, L.; Ríos-León, M.; Álvarez-Melcón, A.C.; Martín-Casas, P. Effectiveness of motor imagery in patients with multiple sclerosis: A systematic review. Rev. Neurol. 2021, 72, 157–167. [Google Scholar] [PubMed]
- Ghahfarrokhi, M.M.; Banitalebi, E.; Negaresh, R.; Motl, R.W. Home-based exercise training in multiple sclerosis: A systematic review with implications for future research. Mult. Scler. Relat. Disord. 2021, 55, 103177. [Google Scholar] [CrossRef] [PubMed]
- Alphonsus, K.B.; Su, Y.; D’Arcy, C. The effect of exercise, yoga and physiotherapy on the quality of life of people with multiple sclerosis: Systematic review and meta-analysis. Complementary Ther. Med. 2019, 43, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.J.; Watson, C. Characteristics, burden of illness, and physical functioning of patients with relapsing-remitting and secondary progressive multiple sclerosis: A cross-sectional us survey. Neuropsychiatr. Dis. Treat. 2017, 13, 1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rooney, S.; Wood, L.; Moffat, F.; Paul, L. Prevalence of fatigue and its association with clinical features in progressive and non-progressive forms of multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 28, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Becker, H.; Stuifbergen, A. Comparing health promotion and quality of life in people with progressive versus nonprogressive multiple sclerosis. Int. J. MS Care 2020, 22, 239–246. [Google Scholar] [CrossRef]
- WHO. Body Mass Index. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 10 December 2020).
- Hohol, M.; Orav, E.; Weiner, H. Disease steps in multiple sclerosis: A simple approach to evaluate disease progression. Neurol. Clin. Neurophysiol. 1995, 45, 251–255. [Google Scholar] [CrossRef]
- Amireault, S.; Godin, G. The godin-shephard leisure-time physical activity questionnaire: Validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept. Mot. Ski. 2015, 120, 604–622. [Google Scholar] [CrossRef]
- Cella, D.; Lai, J.-S.; Nowinski, C.; Victorson, D.; Peterman, A.; Miller, D.; Bethoux, F.; Heinemann, A.; Rubin, S.; Cavazos, J. Neuro-qol: Brief measures of health-related quality of life for clinical research in neurology. Neurology 2012, 78, 1860–1867. [Google Scholar] [CrossRef] [Green Version]
- Rezapour, A.; Kia, A.A.; Goodarzi, S.; Hasoumi, M.; Motlagh, S.N.; Vahedi, S. The impact of disease characteristics on multiple sclerosis patients’ quality of life. Epidemiol. Health 2017, 39, e2017008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, K.L.; Simpson, S., Jr.; Jelinek, G.A.; Neate, S.L.; De Livera, A.M.; Brown, C.R.; O’Kearney, E.; Marck, C.H.; Weiland, T.J. Longitudinal associations of modifiable lifestyle factors with positive depression-screen over 2.5-years in an international cohort of people living with multiple sclerosis. Front. Psychiatry 2018, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Wahls, T.L.; Titcomb, T.J.; Bisht, B.; Eyck, P.T.; Rubenstein, L.M.; Carr, L.J.; Darling, W.G.; Hoth, K.F.; Kamholz, J.; Snetselaar, L.G. Impact of the swank and wahls elimination dietary interventions on fatigue and quality of life in relapsing-remitting multiple sclerosis: The waves randomized parallel-arm clinical trial. Mult. Scler. J.–Exp. Transl. Clin. 2021, 7, 5399. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, V.A.; Filippini, G.; Di Pietrantonj, C.; Asokan, G.V.; Robak, E.W.; Whamond, L.; Robinson, S.A. Vitamin d for the management of multiple sclerosis. Cochrane Database Syst. Rev. 2018, 9, CD008422. [Google Scholar] [CrossRef] [PubMed]
- Shohani, M.; Kazemi, F.; Rahmati, S.; Azami, M. The effect of yoga on the quality of life and fatigue in patients with multiple sclerosis: A systematic review and meta-analysis of randomized clinical trials. Complementary Ther. Clin. Pract. 2020, 39, 101087. [Google Scholar] [CrossRef]
- Dauwan, M.; Begemann, M.J.; Slot, M.I.; Lee, E.H.; Scheltens, P.; Sommer, I.E. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: A transdiagnostic systematic review and meta-analysis of randomized controlled trials. J. Neuron. 2021, 268, 1222–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Lifestyle | Category | Inclusions |
|---|---|---|
| Diet | Anti-inflammatory | Anti-inflammatory, fasting/calorie restriction, gluten-free, Mediterranean |
| Low-saturated fats | Jelinek, Swank, low-fat, ovo-lactovegetarian, vegetarian, vegan, lacto-vegetarian, Ornish, Pritikin, pescatarian | |
| Low-carbohydrate | Atkins, ketogenic, paleo, Wahls, low-carbohydrate | |
| Other | Organic, low sodium, low sugar, semi-vegetarian | |
| Supplements | Vitamin D | Vitamin D |
| Omega-3 | Omega-3, DHA or EPA fatty acid, fish-oil, flaxseed/flaxseed oil | |
| Wellness | Mind | Meditation, mindfulness, guided imagery, relaxation exercise, stress management |
| Mind-body | Tai chi, yoga, qigong, exercise therapy | |
| Other | Acupuncture, Ayurveda, biofeedback, brain training, chelation therapy, chiropractic/osteopathic manipulation, cognitive behavioral therapy, craniosacral therapy, deep breathing exercises, energy healing, hypnosis, massage, naturopathy, progressive relaxation, reflexology, traditional healing | |
| Physical activity | Sedentary Moderate Active | leisure activity score <14 leisure activity score 14–23 leisure activity score >23 |
| (A) | |||||||
| Demographics | RRMS n = 750 | ProgMS n = 358 | p | Lifestyle Behaviors | RRMS n = 750 | ProgMS n = 358 | p |
| n (%) | n (%) | n (%) | n (%) | ||||
| Age, years (Mean, SD) | 49.9 (11) | 58.3 (9) | <0.001 | Diet | |||
| Sex | Anti-inflammatory | ||||||
| Male | 126 (17%) | 113 (32%) | Ref. | Not used | 464 (62%) | 245 (68%) | Ref. |
| Female | 621 (83%) | 244 (68%) | <0.001 | Used/helpful | 286 (38%) | 113 (32%) | <0.05 |
| Country of birth | Low saturated fat | ||||||
| US | 634 (85%) | 308 (86%) | Ref. | Not used | 601 (80%) | 300 (84%) | Ref. |
| Other | 114 (15%) | 50 (14%) | 0.59 | Used/helpful | 149 (20%) | 58 (16%) | 0.16 |
| Ethnicity | Low carbohydrate | ||||||
| Caucasian | 688 (92%) | 337 (95%) | Ref. | Not used | 576 (77%) | 280 (78%) | Ref. |
| Other/mixed | 56 (8%) | 18 (5%) | 0.15 | Used/helpful | 174 (23%) | 78 (22%) | 0.61 |
| University degree | Other | ||||||
| Yes | 525 (70%) | 245 (68%) | Ref. | Not used | 500 (67%) | 252 (70%) | Ref. |
| No | 222 (30%) | 113 (32%) | 0.55 | Used/helpful | 250 (33%) | 106 (30%) | 0.23 |
| Partnered | Supplements | ||||||
| Yes | 530 (71%) | 274 (77%) | Ref. | Vitamin D | |||
| No | 214 (29%) | 84 (24%) | 0.06 | No | 108 (14%) | 65 (18%) | Ref. |
| Paid employment | Yes | 642 (86%) | 293 (82%) | 0.10 | |||
| Yes | 402 (54%) | 94 (27%) | Ref. | Omega-3 | |||
| No | 338 (46%) | 260 (74%) | <0.001 | Not used | 461 (61%) | 210 (59%) | Ref. |
| Household income (USD) | Used/helpful | 289 (39%) | 148 (41%) | 0.36 | |||
| ≤$50,000 | 144 (35%) | 67 (42%) | Ref. | Wellness | |||
| $50,001–100,000 | 121 (29%) | 49 (31%) | 0.56 | Mind | |||
| ≥$100,001 | 146 (35%) | 44 (28%) | 0.06 | Not used | 473 (63%) | 231 (65%) | Ref. |
| BMI | Used/helpful | 277 (37%) | 127 (36%) | 0.65 | |||
| Under/healthy | 331 (44%) | 166 (47%) | Ref. | Mind-body | |||
| Overweight | 204 (27%) | 103 (29%) | 0.99 | Not used | 456 (61%) | 224 (63%) | Ref. |
| Obese | 212 (28%) | 86 (24%) | 0.19 | Used/helpful | 294 (39%) | 134 (37%) | 0.58 |
| PDDS | Other | ||||||
| Normal/mild | 448 (60%) | 36 (10%) | Ref. | Not used | 249 (33%) | 132 (37%) | Ref. |
| Moderate | 252 (34%) | 186 (52%) | <0.001 | Used/helpful | 501 (67%) | 226 (63%) | 0.24 |
| Severe | 50 (7%) | 135 (38%) | <0.001 | Physical activity | |||
| MS duration (years) | 11.5 (9.1) | 16.1 (10.3) | <0.001 | Sedentary | 225 (30%) | 154 (43%) | Ref. |
| Moderate | 150 (20%) | 84 (24%) | 0.25 | ||||
| Active | 375 (50%) | 120 (34%) | <0.001 | ||||
| (B) | |||||||
| QoL Subdomains | RRMS n = 750 | ProgMS n = 358 | p | ||||
| Mean (SD) T-score | Mean (SD) T-score | ||||||
| Physical | |||||||
| Mobility | 48.0 (9.0) | 38.30 (7.5) | <0.001 | ||||
| Fine motor | 46.4 (8.7) | 41.0 (9.2) | <0.001 | ||||
| Fatigue | 51.8 (9.5) | 53.3 (8.5) | <0.05 | ||||
| Sleep disturbance | 53.8 (9.0) | 53.4 (7.8) | 0.433 | ||||
| Mental | |||||||
| Anxiety | 51.8 (8.6) | 50.6 (7.6) | <0.05 | ||||
| Depression | 47.7 (8.3) | 48.4 (7.9) | 0.185 | ||||
| Positive affect | 52.5 (8.2) | 51.1 (8.1) | <0.01 | ||||
| Emotional dyscontrol | 48.6 (9.8) | 48.1 (9.2) | 0.394 | ||||
| Stigma | 49.0 (7.8) | 52.7 (6.9) | <0.001 | ||||
| Cognitive function | 45.8 (10.7) | 46.6 (10.2) | 0.259 | ||||
| Communication a | 22.1 (3.4) | 21.9 (3.5) | 0.390 | ||||
| Social | |||||||
| Participation | 46.8 (7.9) | 43.7 (6.4) | <0.001 | ||||
| Satisfaction | 45.3 (7.0) | 42.0 (5.5) | <0.001 | ||||
| (A) | ||||||||
| Diet | Anti-Inflammatory | Low Carbohydrate | Low-Saturated Fat | Other | ||||
| QoL | RRMS | ProgMS | RRMS | ProgMS | RRMS | ProgMS | RRMS | ProgMS |
| Physical | ||||||||
| Mobility | −0.09 (−0.97, 0.79) | 1.51 (0.17, 2.84) * | −0.34 (−1.35, 0.67) | −0.77 (−0.73, 2.28) | 0.10 (−0.97, 1.18) | 0.62 (−1.09, 2.34) | −0.64 (−1.56, 0.27) | 1.42 (0.07, 2.78)* |
| Fine motor | −1.06 (−2.26, 0.14) | 0.69 (−1.12, 2.50) | −1.09 (−2.47, 0.28) | −0.84 (−2.87, 1.19) | 0.35 (−1.11, 1.81) | 1.42 (−0.90, 3.74) | −1.24 (−2.50, −0.01) | −0.20 (−2.05, 1.64) |
| Fatigue | −0.28 (−1.57, 1.00) | −0.02 (−1.96, 1.92) | 0.83 (−0.64, 2.30) | −0.93 (−3.11, 1.24) | 0.59 (−0.97, 2.15) | −1.53 (−4.01, 0.95) | 1.02 (−0.31, 2.34) | −1.51 (−3.48, 0.47) |
| Sleep disturbance | 0.41 (−0.81, 1.64) | 0.38 (−1.47, 2.23) | −0.16 (−1.56, 1.24) | 0.92 (−1.15, 2.99) | 0.15 (−1.33, 1.64) | 0.00 (−2.36, 2.37) | 0.16 (−0.21, 2.32) | 1.01 (−0.87, 2.89) |
| Mental | ||||||||
| Anxiety | 0.08 (−1.11, 1.29) | −0.01 (−1.81, 1.80) | 0.03 (−1.34, 1.40) | −0.50 (−2.53, 1.53) | 0.89 (−0.56, 2.34) | 1.36 (−0.94, 3.65) | 0.69 (−0.55, 1.93) | 0.08 (−1.76, 1.93) |
| Depression | 0.24 (−0.94, 1.42) | −1.08 (−2.85, 0.70) | −0.11 (−1.46, 1.24) | −1.31 (−3.31, 0.68) | 0.38 (−1.05, 1.81) | 1.50 (−0.75, 3.76) | 1.03 (−0.19, 2.25) | −0.72 (−2.53, 1.09) |
| Positive affect | 0.45 (−0.73, 1.63) | 2.05 (0.27, 3.83) | 0.54 (−0.81, 1.88) | 2.22 (0.23, 4.21) | 0.14 (−1.29, 1.57) | −0.03 (−2.31, 2.25) | −0.17 (−1.39, 1.05) | 1.55 (−0.27, 3.36) |
| Emotional dyscontrol | 0.51 (−0.87, 1.91) | −0.08 (−2.17, 2.02) | 0.20 (−1.38, 1.79) | −1.73 (−4.08, 0.62) | 0.63 (−1.05, 2.32) | −0.28 (−2.97, 2.40) | 0.91 (−0.53, 2.34) | −0.00 (−2.14, 2.13) |
| Stigma | 1.26 (0.22, 2.30) | −0.12 (−1.68, 1.44) | 1.23 (0.04, 2.41) | 0.38 (−1.37, 2.13) | 0.55 (−0.71, 1.80) | 0.25 (−1.76, 2.25) | 1.36 (0.29, 2.43) | −0.08 (−1.68, 1.52) |
| Cognitive function | −0.77 (−2.27, 0.72) | −0.32 (−2.58, 1.94) | −1.04 (−2.75, 0.67) | 0.23 (−2.30, 2.77) | −1.04 (−2.85, 0.77) | 1.64 (−1.25, 4.53) | −1.92 (−3.46, −0.37) | 0.50 (−1.80, 2.79) |
| Communication a | −0.20 (−0.68, 0.29) | 0.07 (−0.66, 0.80) | −0.03 (−0.58, 0.52) | −0.06 (−0.88, 0.76) | −0.30 (−0.88, 0.29) | 1.23 (0.31, 2.15)* | −0.48 (−0.98, 0.02) | 0.26 (−0.48, 1.00) |
| Social | ||||||||
| Participation | 0.27 (−0.73, 1.27) | −0.00 (−1.51, 1.51) | −0.49 (−1.63, 0.66) | −0.28 (−1.98, 1.42) | −0.53 (−1.75, 0.69) | 0.38 (−1.54, 2.31) | −1.04 (−2.07, 0.01) | 0.50 (−1.03, 2.04) |
| Satisfaction | 0.28 (−0.62, 1.18) | 0.77 (−0.59, 2.13) | −0.27 (−1.29, 0.76) | 0.05 (−1.48, 1.57) | 0.15 (−0.95, 1.24) | 0.58 (−1.16, 2.33) | −0.20 (−1.13, 0.73) | 0.51 (−0.87, 1.90) |
| (B) | ||||||||
| Wellness | Mind | Mind−Body | Other | |||||
| QoL | RRMS | ProgMS | RRMS | ProgMS | RRMS | ProgMS | ||
| Physical | ||||||||
| Mobility | −0.82 (−1.71, 0.07) | 0.29 (−1.02, 1.61) | 0.20 (−0.69, 1.09) | 0.95 (−0.34, 2.24) | −0.89 (−1.80, 0.03) | 0.48 (−0.81, 1.78) | ||
| Fine motor | −1.70 (−2.90, −0.49) | 0.09 (−1.68, 1.86) | −0.34 (−1.54, 0.87) | 0.57 (−1.18, 2.32) | −1.75 (−2.99, −0.51) | 0.64 (−1.11, 2.39) * | ||
| Fatigue | 1.39 (0.10, 2.68) | 1.26 (−0.64, 3.16) | −0.83 (−2.12, 0.45) | −1.46 (−3.33, 0.41) | 2.35 (1.03, 3.68) | 0.85 (−1.03, 2.72) | ||
| Sleep disturbance | 1.09 (−0.13, 2.31) | 1.72 (−0.09, 3.53) | −1.16 (−2.38, 0.07) | −0.31 (−2.09, 1.47) | 1.72 (0.46, 2.99) | 0.57 (−1.22, 2.34) | ||
| Mental | ||||||||
| Anxiety | 2.30 (1.10, 3.49) | 1.57 (−0.20, 3.33) | −1.00 (−2.20, 0.20) | −0.62 (−2.36, 1.12) | 1.87 (0.63, 3.11) | 0.42 (−1.32, 2.16) | ||
| Depression | 0.95 (−0.23, 2.13) | 1.28 (−0.46, 3.02) | −1.06 (−2.24, 0.12) | −0.39 (−0.22, 3.20) | 0.92 (−0.30, 2.14) | 0.23 (−1.49, 1.95) | ||
| Positive affect | −0.40 (−1.58, 0.78) | −0.73 (−2.48, 1.02) | 1.44 (0.27, 2.62) | 1.49 (−0.22, 3.20) | −0.56 (−1.80, 0.66) | 0.11 (−1.61, 1.84) | ||
| Emotional dyscontrol | 2.03 (0.64, 3.42) | 1.58 (−0.47, 3.63) | −1.63 (−3.02, −0.25) | 0.50 (−1.51, 2.52) | 2.16 (0.72, 3.59) | 1.16 (−0.87, 3.17) | ||
| Stigma | 1.04 (0.01, 2.08) | 0.86 (−0.68, 2.40) | 0.15 (−0.89, 1.19) | −0.40 (−1.91, 1.11) | 1.11 (0.04, 2.19) | 0.23 (−1.29, 1.75) | ||
| Cognitive function | −1.67 (−3.16, −1.17) | −1.38 (−3.59, 0.84) | 0.76 (−0.74, 2.26) | 0.26 (−1.92, 2.43) | −3.20 (−4.74, −1.67) | −0.89 (−3.06, 1.28) | ||
| Communicationa | −0.53 (−1.01, −0.05) | −0.42 (−1.13, 0.29) | 0.01 (−0.47, 0.50) | 0.12 (−0.58, 0.82) | −0.56 (−1.06, −0.07) | −0.39 (−1.10, 0.31) | ||
| Social | ||||||||
| Participation | −1.29(−2.29, −0.28) | −0.89 (−2.37, 0.59) | 1.29 (0.29, 2.29) | 1.26 (−0.20, 2.71) | −1.52 (−2.55, −0.49) | −0.99 (−2.45, 0.47) | ||
| Satisfaction | −0.99 (−1.90, −0.09) | −0.62 (−1.95, 0.71) | 0.83 (−0.08, 1.72) | 1.11 (−0.20, 2.42) | −1.24 (−2.18, −0.31) | −0.48 (−1.80, 0.83) | ||
| (C) | ||||||||
| Supplements | Physical Activity | |||||||
| Vitamin D | Omega−3 | Moderate | Active | |||||
| QoL | RRMS | ProgMS | RRMS | ProgMS | RRMS | ProgMS | RRMS | ProgMS |
| Physical | ||||||||
| Mobility | 0.01 (−1.22, 1.24) | 0.40 (−1.20, 2.01) | 0.35 (−0.52, 1.25) | 0.33 (−0.94, 1.60) | 1.41 (0.19, 2.63) | −0.31 (−1.88, 1.25) | 3.07 (2.04, 4.09) | 1.49 (0.05, 2.94) |
| Fine motor | −0.39 (−2.05, 1.28) | 1.80 (−0.37, 3.97) | −0.34 (−1.54, 0.86) | 0.76 (−0.96, 2.47) | 0.93 (−0.74, 2.61) | −0.77 (−2.92, 1.38) | 2.45 (1.05, 3.86) | −0.71 (−2.70, 1.27) * |
| Fatigue | −1.57 (−3.35, 0.21) | −0.92 (−3.24, 1.41) | −0.61 (−1.90, 0.67) | −0.91 (−2.75, 0.92) | −1.48 (−3.25, 0.29) | −1.35 (−3.63, 0.93) | −4.14 (−5.63, −2.65) | −2.11 (−4.21, −0.01) |
| Sleep disturbance | −0.52 (−2.22, 1.17) | −0.52 (−2.73, 1.70) | −0.39 (−1.61, 0.83) | −0.74 (−2.49, 1.01) | −1.20 (−2.91, 0.51) | −1.64 (−3.84, 0.55) | −2.37 (−3.80, −0.94) | −1.54 (−3.56, 0.48) |
| Mental | ||||||||
| Anxiety | 0.12 (−1.53, 1.79) | −1.05 (−3.22, 1.12) | 0.39 (−0.89, 1.50) | −0.48 (−2.19, 1.23) | −1.97 (−3.64, −0.31) | −1.66 (−3.79, 0.48) | −2.92 (−4.32, −1.52) | 0.37 (−1.60, 2.34) * |
| Depression | −0.42 (−2.05, 1.22) | −0.28 (−2.41, 1.85) | 0.38 (−0.80, 1.55) | 0.05 (−1.63, 1.73) | −2.02 (−3.65, −0.39) | −1.47 (−3.56, 0.62) | −3.67 (−5.04, −2.30) | −0.50 (−2.43, 1.43) * |
| Positive affect | 0.37 (−1.26, 2.01) | −0.82 (−2.95, 1.31) | −0.09 (−1.26, 1.09) | −0.05 (−1.74, 1.63) | 2.51 (0.89, 4.13) | 2.61 (0.52, 4.69) | 4.35 (2.99, 5.71) | 0.59 (−1.32, 2.50) * |
| Emotional dyscontrol | −0.28 (−2.20, 1.65) | −1.09 (−3.60, 1.42) | 0.43 (−0.95, 1.82) | 0.30 (−1.68, 2.28) | −1.27 (−3.41, 0.66) | −2.15 (−4.63, 0.33) | −3.11 (−4.73, −1.49) | 0.05 (−2.33, 2.24) * |
| Stigma | −0.61 (−2.05, 0.83) | −1.28 (−3.15, 0.60) | 0.41 (−0.61, 1.45) | −0.23 (−1.71, 1.25) | −2.30 (−3.73, −0.86) | −1.51 (−3.36, 0.33) | −3.11 (−4.32, −1.90) | −0.30 (−2.01, 1.40) * |
| Cognitive function | 1.15 (−0.92, 3.23) | 2.00 (−0.70, 4.70) | −0.07 (−1.56, 1.42) | 1.39 (−0.75, 3.52) | 1.49 (−0.60, 3.57) | 3.17 (0.50, 5.85) | 2.85 (1.10, 4.60) | 1.11 (−1.37, 3.57) |
| Communication a | 0.12 (−0.56, 0.78) | 0.73 (−0.14, 1.60) | −0.28 (−0.76, 0.20) | 0.42 (−0.26, 1.11) | 0.37 (−0.30, 1.04) | 0.93 (0.07, 1.79) | 0.80 (0.23, 1.36) | 0.30 (−0.49, 1.10) |
| Social | ||||||||
| Social participation | 0.69 (−0.70, 2.08) | 0.82 (−1.00, 2.63) | −0.06 (−1.06, 0.94) | 0.44 (−0.99, 1.87) | 1.29 (−0.10, 2.70) | 0.95 (−0.83, 2.74) | 2.97 (1.81, 4.14) | 0.80 (−0.84, 2.45) * |
| Social satisfaction | 1.01 (−0.24, 2.26) | 0.32 (−1.31, 1.95) | 0.73 (−0.16, 1.64) | 0.10 (−1.19, 1.39) | 1.64 (0.41, 2.89) | 1.49 (−0.09, 3.08) | 3.71 (2.67, 4.75) | 1.20 (−0.25, 2.66) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nag, N.; Yu, M.; Jelinek, G.A.; Simpson-Yap, S.; Neate, S.L.; Schmidt, H.K. Associations between Lifestyle Behaviors and Quality of Life Differ Based on Multiple Sclerosis Phenotype. J. Pers. Med. 2021, 11, 1218. https://doi.org/10.3390/jpm11111218
Nag N, Yu M, Jelinek GA, Simpson-Yap S, Neate SL, Schmidt HK. Associations between Lifestyle Behaviors and Quality of Life Differ Based on Multiple Sclerosis Phenotype. Journal of Personalized Medicine. 2021; 11(11):1218. https://doi.org/10.3390/jpm11111218
Chicago/Turabian StyleNag, Nupur, Maggie Yu, George A. Jelinek, Steve Simpson-Yap, Sandra L. Neate, and Hollie K. Schmidt. 2021. "Associations between Lifestyle Behaviors and Quality of Life Differ Based on Multiple Sclerosis Phenotype" Journal of Personalized Medicine 11, no. 11: 1218. https://doi.org/10.3390/jpm11111218
APA StyleNag, N., Yu, M., Jelinek, G. A., Simpson-Yap, S., Neate, S. L., & Schmidt, H. K. (2021). Associations between Lifestyle Behaviors and Quality of Life Differ Based on Multiple Sclerosis Phenotype. Journal of Personalized Medicine, 11(11), 1218. https://doi.org/10.3390/jpm11111218