What’s New in Cirrhotic Cardiomyopathy?—Review Article
Abstract
:1. Background
2. Methods
2.1. Epidemiology and Diagnostic Criteria
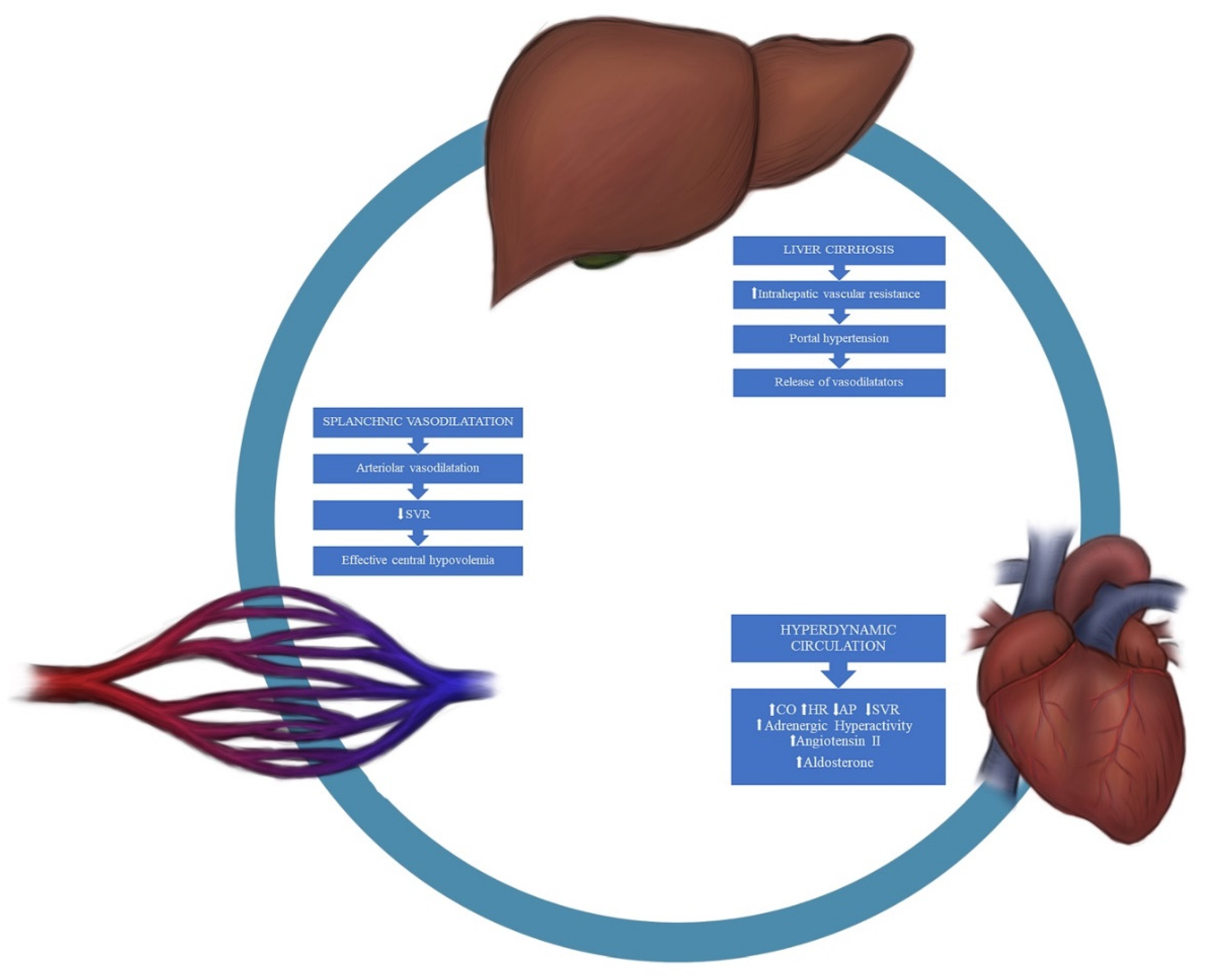
2.2. Pathophysiology
2.2.1. Cardiovascular Autonomic Dysfunction
2.2.2. Cell Membrane Modifications
2.2.3. Inflammation
2.2.4. Humoral Cardiodepressant Factors
2.3. Clinical Features
- -
- LA dilatation and increased LA volumes;
- -
- Increased LV diameters (but not volumes);
- -
- Increased thickness of the posterior wall of the LV and the interventricular septum;
- -
- Prolonged isovolumetric relaxation time (IVRT > 80 ms);
- -
- Prolonged deceleration time (DT > 200 ms);
- -
- E/A ratio < 1 (after age correction).
2.4. Biomarkers
2.5. Histopathology
2.6. Screening for CCM
2.7. ECG
2.8. Echocardiography
2.9. Biomarkers
2.10. Cardiopulmonary Exercise Test (CPET)
2.11. Magnetic Resonance Imaging (MRI)
2.12. CCM and Transjugular Intrahepatic Portosystemic Shunt
2.13. CCM and Liver Transplantation
3. Therapy
4. Gaps of Knowledge
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalski, H.J.; Abelmann, W.H. The cardiac output at rest in Laennec’s cirrhosis. J. Clin. Investig. 1953, 32, 1025–1033. [Google Scholar] [CrossRef]
- Gould, L.; Shariff, M.; Zahir, M.; Di Lieto, M. Cardiac hemodynamics in alcoholic patients with chronic liver disease and a presystolic gallop. J. Clin. Investig. 1969, 48, 860–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chayanupatkul, M.; Liangpunsakul, S. Cirrhotic cardiomyopathy: Review of pathophysiology and treatment. Hepatol. Int. 2014, 8, 308–315. [Google Scholar] [CrossRef]
- Rimbaş, R.C.; Baldea, S.M.; Guerra, R.D.G.A.; Visoiu, S.I.; Rimbaş, M.; Pop, C.S.; Vinereanu, D. New Definition Criteria of Myocardial Dysfunction in Patients with Liver Cirrhosis: A Speckle Tracking and Tissue Doppler Imaging Study. Ultrasound Med. Biol. 2018, 44, 562–574. [Google Scholar] [CrossRef]
- Gassanov, N.; Caglayan, E.; Semmo, N.; Massenkeil, G.; Er, F. Cirrhotic cardiomyopathy: A cardiologist’s perspective. World J. Gastroenterol. 2014, 20, 15492–15498. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, I.H.; Mahmood, K.; Naeem, M.; Vashwani, A.S.; Ziaullah, S. The heart matters when the liver shatters! Cirrhotic cardiomyopathy: Frequency, comparison, and correlation with severity of disease. Prz. Gastroenterol. 2016, 11, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izzy, M.; VanWagner, L.B.; Lin, G.; Altieri, M.; Findlay, J.Y.; Oh, J.K.; Watt, K.D.; Lee, S.S. Cirrhotic Cardiomyopathy Consortium. Redefining Cirrhotic Cardiomyopathy for the Modern Era. Hepatology 2020, 71, 334–345, Erratum in: Hepatology 2020, 72, 1161. [Google Scholar] [CrossRef]
- Rimbaș, R.C.; Rimbas, M.; Chitroceanu, A.M.; Luchian, L.M.; Pop, C.; Vinereanu, D. Cirrhotic Cardiomyopathy in the Era of Liver Transplantation: Time for Precise Stepwise Evaluation. J. Gastrointest. Liver Dis. 2020, 29, 665–675. [Google Scholar] [CrossRef]
- Myers, R.P.; Lee, S.S. Cirrhotic cardiomyopathy and liver transplantation. Liver Transplant. 2000, 6 (Suppl. 1), S44–S52. [Google Scholar] [CrossRef] [PubMed]
- Wong, F. Cirrhotic cardiomyopathy. Hepatol. Int. 2009, 3, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Acosta, F.; De La Morena, G.; Villegas, M.; Sansano, T.; Reche, M.; Beltran, R.; Roques, V.; Contreras, R.F.; Robles, R.; Bueno, F.S.; et al. Evaluation of cardiac function before and after liver transplantation. Transplant. Proc. 1999, 31, 2369–2370. [Google Scholar] [CrossRef]
- De, B.K.; Majumdar, D.; Das, D.; Biswas, P.K.; Mandal, S.K.; Ray, S.; Bandopadhyay, K.; Das, T.K.; Dasgupta, S.; Guru, S. Cardiac dysfunction in portal hypertension among patients with cirrhosis and non-cirrhotic portal fibrosis. J. Hepatol. 2003, 39, 315–319. [Google Scholar] [CrossRef]
- Bajaj, B.K.; Agarwal, M.P.; Ram, B.K. Autonomic neuropathy in patients with hepatic cirrhosis. Postgrad. Med. J. 2003, 79, 408–411. [Google Scholar] [CrossRef]
- Zardi, E.M.; Abbate, A.; Zardi, D.M.; Dobrina, A.; Margiotta, D.; Van Tassel, B.W.; Afeltra, A.; Sanyal, A.J. Cirrhotic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 539–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriksen, J.H.; Møller, S.; Ring-Larsen, H.; Christensen, N.J. The sympathetic nervous system in liver disease. J. Hepatol. 1998, 29, 328–341. [Google Scholar] [CrossRef]
- Moller, S.; Iversen, J.S.; Henriksen, J.H.; Bendtsen, F. ReducedReduced baroreflex sensitivity in alcoholic cirrhosis: Relations to hemodynamics and humoral systems. Am. J. Physiol. Circ. Physiol. 2007, 292, H2966–H2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Meddings, J.B.; Lee, S.S. Membrane physical properties determine cardiac beta-adrenergic receptor function in cirrhotic rats. Am. J. Physiol. Content 1994, 267 Pt 1, G87–G93. [Google Scholar] [CrossRef]
- Tahara, D.; Nakanishi, T.; Akazawa, S.; Yamaguchi, Y.; Yamamoto, H.; Akashi, M.; Chikuba, N.; Okuno, S.; Maeda, Y.; Kusumoto, Y. Lecithin-cholesterol acyltransferase and lipid transfer protein activities in liver disease. Metabolism 1993, 42, 19–23. [Google Scholar] [CrossRef]
- Ma, Z.; Lee, S.S.; Meddings, J.B. Effects of altered cardiac membrane fluidity on beta-adrenergic receptor signalling in rats with cirrhotic cardiomyopathy. J. Hepatol. 1997, 26, 904–912. [Google Scholar] [CrossRef]
- Keller, M.; Pignier, C.; Niggli, E.; Egger, M. Mechanisms of Na+-Ca2+ exchange inhibition by amphiphiles in cardiac myocytes: Importance of transbilayer movement. J. Membr. Biol. 2004, 198, 159–175. [Google Scholar] [CrossRef] [Green Version]
- Moreau, R.; Lebrec, D. Endogenous factors involved in the control of arterial tone in cirrhosis. J. Hepatol. 1995, 22, 370–376. [Google Scholar] [CrossRef]
- Ruiz-del-Árbol, L.; Serradilla, R. Cirrhotic cardiomyopathy. World J. Gastroenterol. 2015, 21, 11502–11521. [Google Scholar] [CrossRef]
- Wiest, R.; Lawson, M.; Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 2014, 60, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Bellot, P.; García-Pagán, J.C.; Francés, R.; Abraldes, J.G.; Navasa, M.; Pérez-Mateo, M.; Such, J.; Bosch, J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology 2010, 52, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D. Cytokine-induced modulation of cardiac function. Circ. Res. 2004, 95, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.; Giannone, F.A.; Napoli, L.; Tovoli, A.; Ricci, C.S.; Tufoni, M.; Caraceni, P. The endocannabinoid system in advanced liver cirrhosis: Pathophysiological implication and future perspectives. Liver Int. 2013, 33, 1298–1308. [Google Scholar] [CrossRef]
- Gaskari, S.A.; Liu, H.; Moezi, L.; Li, Y.; Baik, S.K.; Lee, S.S. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Br. J. Pharmacol. 2005, 146, 315–323. [Google Scholar] [CrossRef]
- van Obbergh, L.; Vallieres, Y.; Blaise, G. Cardiac modifications occurring in the ascitic rat with biliary cirrhosis are nitric oxide related. J. Hepatol. 1996, 24, 747–752. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Z.; Lee, S.S. Contribution of nitric oxide to the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Gastroenterology 2000, 118, 937–944. [Google Scholar] [CrossRef]
- Liu, H.; Song, D.; Lee, S.S. Role of heme oxygenase-carbon monoxide pathway in pathogenesis of cirrhotic cardiomyopathy in the rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 280, G68–G74. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Kubo, H.; Harris, D.M.; Mills, G.D.; Moyer, J.; Berretta, R.; Potts, S.T.; Marsh, J.D.; Houser, S.R. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ. Res. 2005, 97, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Zardi, E.M.; Zardi, D.M.; Chin, D.; Sonnino, C.; Dobrina, A.; Abbate, A. Cirrhotic cardiomyopathy in the pre- and post-liver transplantation phase. J. Cardiol. 2016, 67, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Karamchandani, K.; Wilson, R.; Baskin, S.; Bezinover, D. Acute heart failure after Orthotopic liver transplantation: A case series from one center. BMC Anesthesiol. 2018, 18, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moller, S.; Henriksen, J.H. Cardiovascular dysfunction in cirrhosis. Pathophysiological evidence of a cirrhotic cardiomyopathy. Scand. J. Gastroenterol. 2001, 36, 785–794. [Google Scholar] [CrossRef]
- Sonny, A.; Ibrahim, A.; Schuster, A.; Jaber, W.A.; Cywinski, J.B. Impact and persistence of cirrhotic cardiomyopathy after liver transplantation. Clin. Transplant. 2016, 30, 986–993. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Kazankov, K.; Holland-Fischer, P.; Andersen, N.H.; Torp, P.; Sloth, E.; Aagaard, N.K.; Vilstrup, H. Resting myocardial dysfunction in cirrhosis quantified by tissue Doppler imaging. Liver Int. 2011, 31, 534–540. [Google Scholar] [CrossRef]
- Karagiannakis, D.S.; Papatheodoridis, G.; Vlachogiannakos, J. Recent advances in cirrhotic cardiomyopathy. Dig. Dis. Sci. 2015, 60, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Hammami, R.; Boudabbous, M.; Jdidi, J.; Trabelsi, F.; Mroua, F.; Kallel, R.; Amouri, A.; Abid, D.; Tahri, N.; Abid, L.; et al. Cirrhotic cardiomyopathy: Is there any correlation between the stage of cardiac impairment and the severity of liver disease? Libyan J. Med. 2017, 12, 1283162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalheiro, F.; Rodrigues, C.; Adrego, T.; Viana, J.; Vieira, H.; Seco, C.; Pereira, L.; Pinto, F.; Eufrásio, A.; Bento, C.; et al. Diastolic Dysfunction in Liver Cirrhosis: Prognostic Predictor in Liver Transplantation? Transplant. Proc. 2016, 48, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Calicchia, A.; Ruffa, A.; Pellicori, P.; Riggio, O.; Giusto, M.; Gaudio, C.; Torromeo, C. Cardiac dysfunction in cirrhosis is not associated with the severity of liver disease. Eur. J. Intern. Med. 2013, 24, 172–176. [Google Scholar] [CrossRef]
- Glowczynska, R.; Galas, M.; Ołdakowska-Jedynak, U.; Peller, M.; Tomaniak, M.; Raszeja-Wyszomirska, J.; Milkiewicz, P.; Krawczyk, M.; Zieniewicz, K.; Opolski, G. Pretransplant QT Interval: The Relationship with Severity and Etiology of Liver Disease and Prognostic Value After Liver Transplantation. Ann. Transplant. 2018, 23, 622–630. [Google Scholar] [CrossRef]
- Bernardi, M.; Calandra, S.; Colantoni, A.; Trevisani, F.; Raimondo, M.L.; Sica, G.; Schepis, F.; Mandini, M.; Simoni, P.; Contin, M.; et al. Q-T interval prolongation in cirrhosis: Prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology 1998, 27, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Tieranu, E.; Donoiu, I.; Istrătoaie, O.; Găman, A.E.; Țieranu, L.M.; Gheonea, D.I.; Ciurea, T. Q-T Interval Prolongation in Patients with Liver Cirrhosis. Curr. Health Sci. J. 2018, 44, 274–279. [Google Scholar] [PubMed]
- Zambruni, A.; Di Micoli, A.; Lubisco, A.; Domenicali, M.; Trevisani, F.; Bernardi, M. QT interval correction in patients with cirrhosis. J. Cardiovasc. Electrophysiol. 2007, 18, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.H.; Fuglsang, S.; Bendtsen, F.; Christensen, E.; Møller, S. Dyssynchronous electrical and mechanical systole in patients with cirrhosis. J. Hepatol. 2002, 36, 513–520. [Google Scholar] [CrossRef]
- Henriksen, J.H.; Gøtze, J.P.; Fuglsang, S.; Christensen, E.; Bendtsen, F.; Møller, S. Increased circulating pro-brain natriuretic peptide (proBNP) and brain natriuretic peptide (BNP) in patients with cirrhosis: Relation to cardiovascular dysfunction and severity of disease. Gut 2003, 52, 1511–1517. [Google Scholar] [CrossRef]
- Licata, A.; Mazzola, A.; Ingrassia, D.; Calvaruso, V.; Cammà, C.; Craxì, A. Clinical implications of the hyperdynamic syndrome in cirrhosis. Eur. J. Intern. Med. 2014, 25, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Wiese, S.; Mortensen, C.; Gøtze, J.P.; Christensen, E.; Andersen, O.; Bendtsen, F.; Møller, S. Cardiac and proinflammatory markers predict prognosis in cirrhosis. Liver Int. 2014, 34, e19–e30. [Google Scholar] [CrossRef] [PubMed]
- Glowczynska, R.; Raszeja-Wyszomirska, J.; Janik, M.; Kostrzewa, K.; Zygmunt, M.; Zborowska, H.; Krawczyk, M.; Galas, M.; Niewiński, G.; Krawczyk, M.; et al. Troponin I Is Not a Predictor of Early Cardiovascular Morbidity in Liver Transplant Recipients. Transplant. Proc. 2018, 50, 2022–2026. [Google Scholar] [CrossRef]
- Zivlas, C.; Triposkiadis, F.; Psarras, S.; Giamouzis, G.; Skoularigis, I.; Chryssanthopoulos, S.; Kapelouzou, A.; Ramcharitar, S.; Barnes, E.; Papasteriadis, E.; et al. Left atrial volume index in patients with heart failure and severely impaired left ventricular systolic function: The role of established echocardiographic parameters, circulating cystatin C and galectin-3. Ther. Adv. Cardiovasc. Dis. 2017, 11, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Gehlken, C.; Suthahar, N.; Meijers, W.C.; de Boer, R.A. Galectin-3 in heart failure: An update of the last 3 years. Heart Fail Clin. 2018, 14, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.T.; Liu, H.; Lee, S.S. Cirrhotic Cardiomyopathy. Curr. Gastroenterol. Rep. 2020, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Lyssy, L.A.; Soos, M.P. Cirrhotic Cardiomyopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Abbas, W.A.; Kasem Ahmed, S.M.; Abdel Aal, A.M.; Mahmoud, A.A.; Abdelmalek, M.O.; Mekky, M.A.; Abozaid, M.A.; Ibrahim, A.K. Galactin-3 and brain natriuretic peptide versus conventional echocardiography in the early detection of cirrhotic cardiomyopathy. Turk. J. Gastroenterol. 2016, 27, 367–374. [Google Scholar] [CrossRef]
- Moller, S.; Wiese, S.; Halgreen, H.; Hove, J. Diastolic dysfunction in cirrhosis. Heart Fail. Rev. 2016, 21, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Harinstein, M.E.; Flaherty, J.D.; Ansari, A.H.; Robin, J.; Davidson, C.J.; Rossi, J.S.; Flamm, S.L.; Blei, A.T.; Bonow, R.O.; Abecassis, M.; et al. Predictive value of dobutamine stress echocardiography for coronary artery disease detection in liver transplant candidates. Am. J. Transplant. 2008, 8, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Zardi, E.M.; Giorgi, C.; Dobrina, A.; Vecile, E.; Zardi, D.M. Analogies and differences between cirrhotic cardiomyopathy and hepatopulmonary syndrome. Med. Res. Rev. 2021, 41, 739–753. [Google Scholar] [CrossRef]
- Wiese, S.; Hove, J.D.; Bendtsen, F.; Møller, S. Cirrhotic cardiomyopathy: Pathogenesis and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 2013, 11, 177–186. [Google Scholar] [CrossRef]
- Saner, F.H.; Neumann, T.; Canbay, A.; Treckmann, J.W.; Hartmann, M.; Goerlinger, K.; Bertram, S.; Beckebaum, S.; Cicinnati, V.; Paul, A. High brain-natriuretic peptide level predicts cirrhotic cardiomyopathy in liver transplant patients. Transplant. Int. 2011, 24, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Licata, A.; Corrao, S.; Petta, S.; Genco, C.; Cardillo, M.; Calvaruso, V.; Cabibbo, G.; Massenti, F.; Cammà, C.; Licata, G.; et al. NT pro BNP plasma level and atrial volume are linked to the severity of liver cirrhosis. PLoS ONE 2013, 8, e68364. [Google Scholar] [CrossRef]
- Epstein, S.K.; Freeman, R.B.; Khayat, A.; Unterborn, J.N.; Pratt, D.S.; Kaplan, M.M. Aerobic capacity is associated with 100-day outcome after hepatic transplantation. Liver Transplant. 2004, 10, 418–424. [Google Scholar] [CrossRef]
- Dharancy, S.; Lemyze, M.; Boleslawski, E.; Neviere, R.; Declerck, N.; Canva, V.; Wallaert, B.; Mathurin, P.; Pruvot, F.R. Impact of impaired aerobic capacity on liver transplant candidates. Transplantation 2008, 86, 1077–1083. [Google Scholar] [CrossRef]
- Prentis, J.M.; Manas, D.M.; Trenell, M.I.; Hudson, M.; Jones, D.J.; Snowden, C.P. Submaximal cardiopulmonary exercise testing predicts 90-day survival after liver transplantation. Liver Transplant. 2012, 18, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Mallett, S.V. Cirrhotic cardiomyopathy: Implications for the perioperative management of liver transplant patients. World J. Hepatol. 2015, 7, 507–520. [Google Scholar] [CrossRef]
- Baik, S.K.; Fouad, T.R.; Lee, S.S. Cirrhotic cardiomyopathy. Orphanet J. Rare Dis. 2007, 2, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altayar, O.; Lisker-Melman, M. Physiologic Adaptation or Cirrhotic Cardiomyopathy: It Is Time for New Definitions! J. Card. Fail. 2019, 25, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jayakumar, S.; Traboulsi, M.; Lee, S. Cirrhotic cardiomyopathy: Implications for liver transplantation. Liver Transplant. 2017, 23, 826–835. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Lee, S.S. Predicting cardiovascular complications after liver transplantation: 007 to the rescue? Liver Transplant. 2011, 17, 7–9. [Google Scholar] [CrossRef]
- Burtenshaw, A.J.; Isaac, J.L. The role of trans-oesophageal echocardiography for perioperative cardiovascular monitoring during orthotopic liver transplantation. Liver Transplant. 2006, 12, 1577–1583. [Google Scholar] [CrossRef]
- Siniscalchi, A.; Gamberini, L.; Laici, C.; Bardi, T.; Ercolani, G.; Lorenzini, L.; Faenza, S. Post reperfusion syndrome during liver transplantation: From pathophysiology to therapy and preventive strategies. World J. Gastroenterol. 2016, 22, 1551–1569. [Google Scholar] [CrossRef]
- Therapondos, G.; Flapan, A.D.; Plevris, J.N.; Hayes, P.C. Cardiac morbidity and mortality related to orthotopic liver transplantation. Liver Transplant. 2004, 10, 1441–1453. [Google Scholar] [CrossRef]
- Eimer, M.J.; Wright, J.M.; Wang, E.C.; Kulik, L.; Blei, A.; Flamm, S.; Beahan, M.; Bonow, R.O.; Abecassis, M.; Gheorghiade, M. Frequency and significance of acute heart failure following liver transplantation. Am. J. Cardiol. 2008, 101, 242–244. [Google Scholar] [CrossRef]
- Ripoll, C.; Yotti, R.; Bermejo, J.; Bañares, R. The heart in liver transplantation. J. Hepatol. 2011, 54, 810–822. [Google Scholar] [CrossRef] [Green Version]
- Torregrosa, M.; Torregrosa, M.; Aguadé, S.; Dos, L.; Segura, R.; Gónzalez, A.; Evangelista, A.; Castell, J.; Margarit, C.; Esteban, R.; et al. Cardiac alterations in cirrhosis: Reversibility after liver transplantation. J. Hepatol. 2005, 42, 68–74. [Google Scholar] [CrossRef]
- Liu, H.; Lee, S.S. What happens to cirrhotic cardiomyopathy after liver transplantation? Hepatology 2005, 42, 1203–1205. [Google Scholar] [CrossRef] [PubMed]
- Therapondos, G.; Flapan, A.D.; Dollinger, M.M.; Garden, O.J.; Plevris, J.N.; Hayes, P.C. Cardiac function after orthotopic liver transplantation and the effects of immunosuppression: A prospective randomized trial comparing cyclosporin (Neoral) and tacrolimus. Liver Transplant. 2002, 8, 690–700. [Google Scholar] [CrossRef]
- Karagiannakis, D.S.; Vlachogiannakos, J.; Anastasiadis, G.; Vafiadis-Zouboulis, I.; Ladas, S.D. Diastolic cardiac dysfunction is a predictor of dismal prognosis in patients with liver cirrhosis. Hepatol. Int. 2014, 8, 588–594. [Google Scholar] [CrossRef]
- Ruiz-del-Arbol, L.; Achécar, L.; Serradilla, R.; Rodríguez-Gandía, M.Á.; Rivero, M.; Garrido, E.; Natcher, J.J. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology 2013, 58, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Mittal, C.; Qureshi, W.; Singla, S.; Ahmad, U.; Huang, M.A. Pre-transplant left ventricular diastolic dysfunction is associated with post transplant acute graft rejection and graft failure. Dig. Dis. Sci. 2014, 59, 674–680. [Google Scholar] [CrossRef]
- Darstein, F.; König, C.; Hoppe-Lotichius, M.; Grimm, D.; Knapstein, J.; Mittler, J.; Zimmermann, A.; Otto, G.; Lang, H.; Galle, P.R.; et al. Preoperative left ventricular hypertrophy is associated with reduced patient survival after liver transplantation. Clin. Transplant. 2013, 28, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Izzy, M.; Oh, J.; Watt, K.D. Cirrhotic Cardiomyopathy After Transplantation: Neither the Transient Nor Innocent Bystander. Hepatology 2018, 68, 2008–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fede, G.; Privitera, G.; Tomaselli, T.; Spadaro, L.; Purrello, F. Cardiovascular dysfunction in patients with liver cirrhosis. Ann. Gastroenterol. 2015, 28, 31–40. [Google Scholar]
- VanWagner, L.B.; Ning, H.; Whitsett, M.; Levitsky, J.; Uttal, S.; Wilkins, J.T.; Abecassis, M.M.; Ladner, D.P.; Skaro, A.I.; Lloyd-Jones, D. A point-based prediction model for cardiovascular risk in orthotopic liver transplantation: The CAR-OLT score. Hepatology 2017, 66, 1968–1979; Epub 6 November 2017. [Google Scholar] [CrossRef] [PubMed]
- Zambruni, A.; Trevisani, F.; Di Micoli, A.; Savelli, F.; Berzigotti, A.; Bracci, E.; Caraceni, P.; Domenicali, M.; Felline, P.; Zoli, M.; et al. Effect of chronic beta-blockade on QT interval in patients with liver cirrhosis. J. Hepatol. 2008, 48, 415–421. [Google Scholar] [CrossRef] [PubMed]
| CCM Diagnostic Criteria | |
|---|---|
| I. Systolic Dysfunction | |
| 2005 criteria: (any of the following) | 2019 proposed criteria: (any of the following) |
|
|
| AND/OR II. Diastolic dysfunction | |
| 2005 criteria: (any of the following) | 2019 proposed criteria: (≥3 of the following) |
|
|
| IIIa. Supportive criteria (Not diagnostic!) | IIIb. Areas for further research (Validation required) |
| 2005 criteria: | 2019 proposed criteria: |
|
Our proposal:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodys-Pełka, A.; Kusztal, M.; Raszeja-Wyszomirska, J.; Główczyńska, R.; Grabowski, M. What’s New in Cirrhotic Cardiomyopathy?—Review Article. J. Pers. Med. 2021, 11, 1285. https://doi.org/10.3390/jpm11121285
Bodys-Pełka A, Kusztal M, Raszeja-Wyszomirska J, Główczyńska R, Grabowski M. What’s New in Cirrhotic Cardiomyopathy?—Review Article. Journal of Personalized Medicine. 2021; 11(12):1285. https://doi.org/10.3390/jpm11121285
Chicago/Turabian StyleBodys-Pełka, Aleksandra, Maciej Kusztal, Joanna Raszeja-Wyszomirska, Renata Główczyńska, and Marcin Grabowski. 2021. "What’s New in Cirrhotic Cardiomyopathy?—Review Article" Journal of Personalized Medicine 11, no. 12: 1285. https://doi.org/10.3390/jpm11121285
APA StyleBodys-Pełka, A., Kusztal, M., Raszeja-Wyszomirska, J., Główczyńska, R., & Grabowski, M. (2021). What’s New in Cirrhotic Cardiomyopathy?—Review Article. Journal of Personalized Medicine, 11(12), 1285. https://doi.org/10.3390/jpm11121285






