Allogeneic Bone-Marrow Mesenchymal Stem Cell with Moldable Cryogel for Craniofacial Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Expansion of BMSCs from Rats
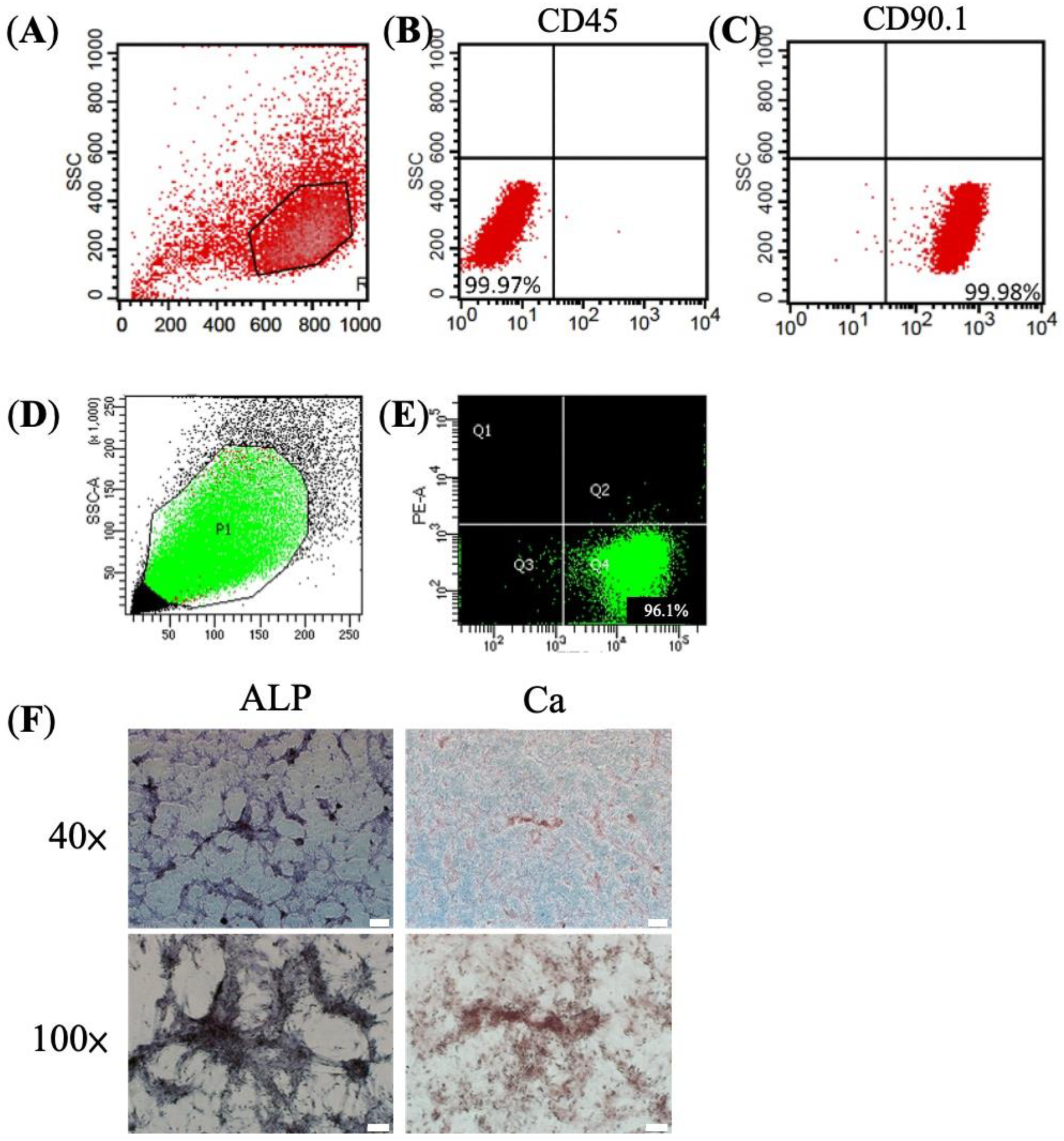
2.2. Flow Cytometry
2.3. Mixed Lymphocyte Reaction Assay (MLR)
2.4. Calcium (Ca2+) Stain and Alkaline Phosphatase (ALP) Stain
2.5. Cryogel Preparation
2.6. Preparation of Cell-Seeded Cryogel Scaffolds
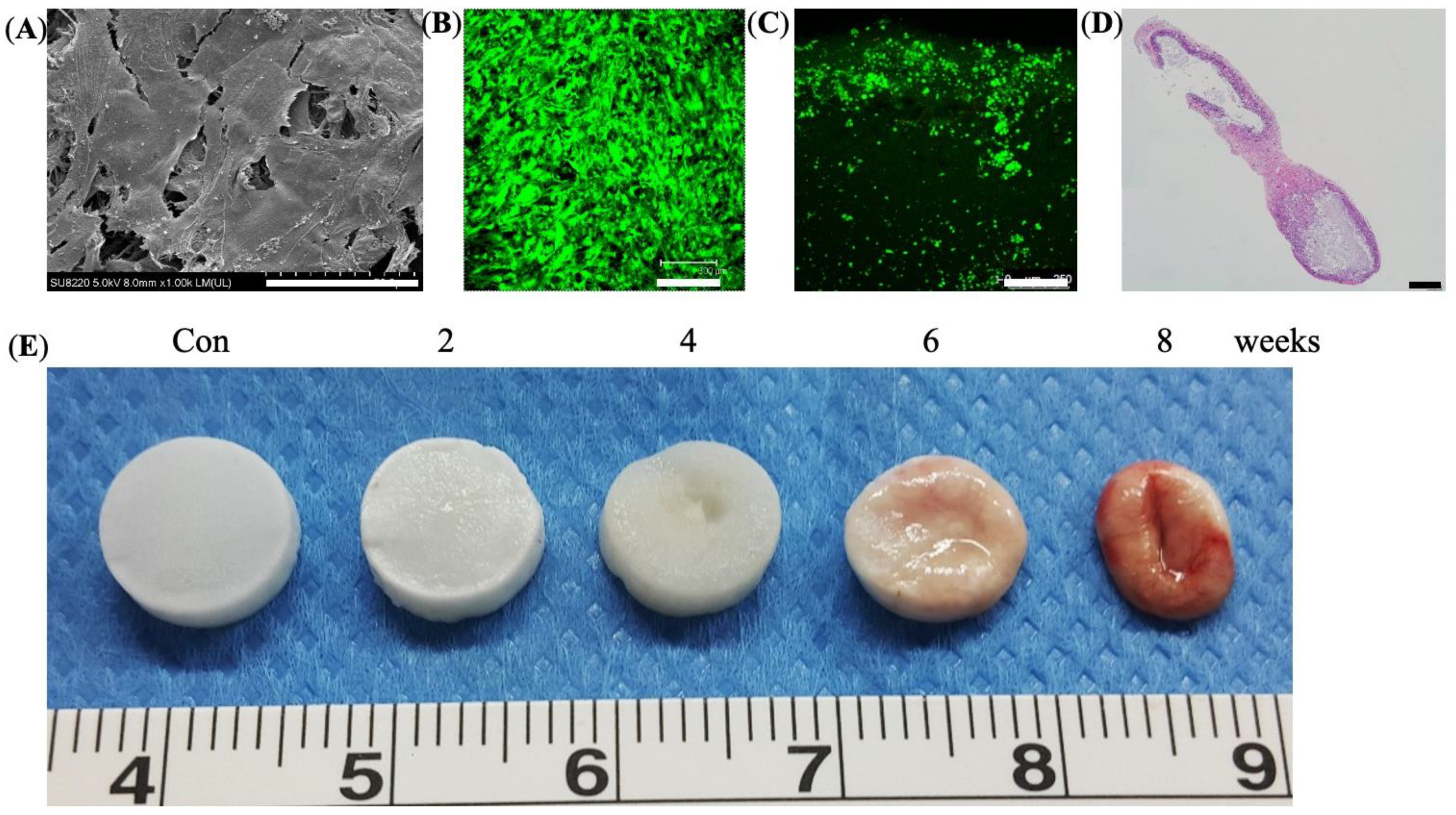
2.7. Live Dead Cell Viability Assays
2.8. Scanning Electron Microscope (SEM)
2.9. Subcutaneous Implantation and Degradation Profiles
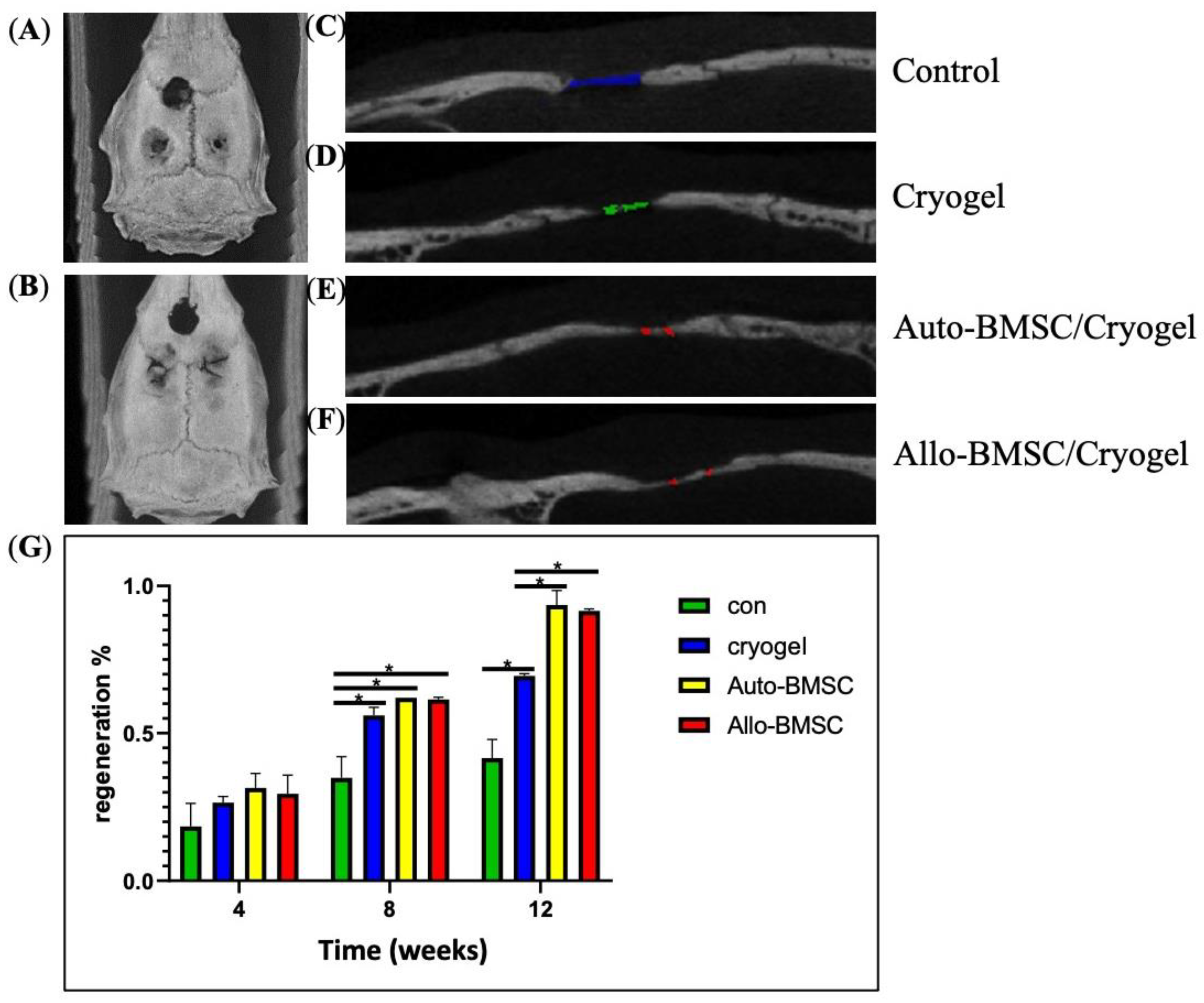
2.10. Transplantation of Allogeneic BMSCs onto Cranium Bone Defect
2.11. Micro-CT Imaging
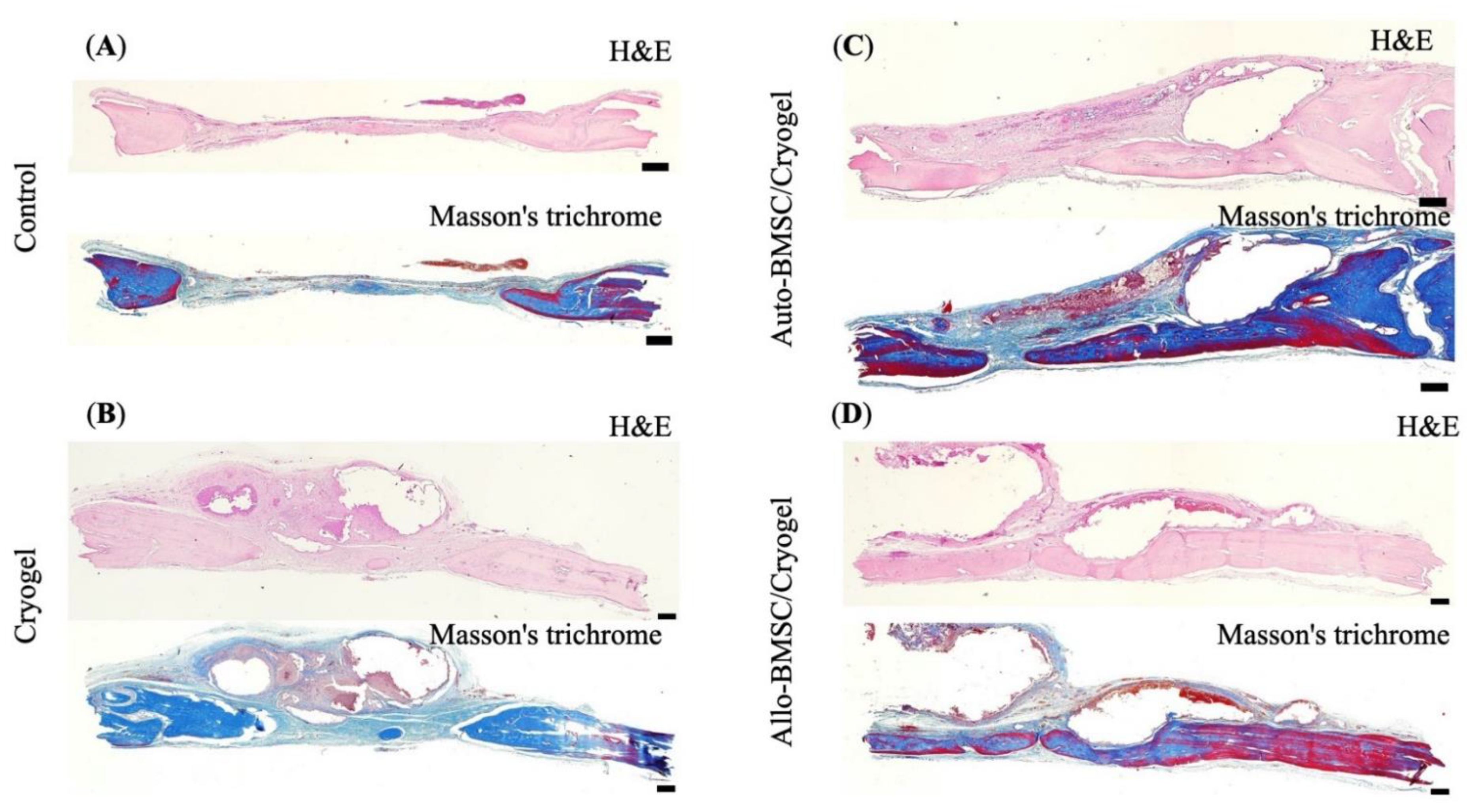
2.12. Histological Examinations
2.13. Immunohistochemistry Stain
2.14. Statistical Evaluation
3. Results
3.1. Characteristic and Osteogenic Potential of BMSC
3.2. Mixed Lymphocyte Reaction (MLR) Assay
3.3. In Vitro Culture and In Vivo Degradation of Cryogels
3.4. Animal Study of Bone Regeneration
3.4.1. Micro-Computed Tomography (Micro-CT)
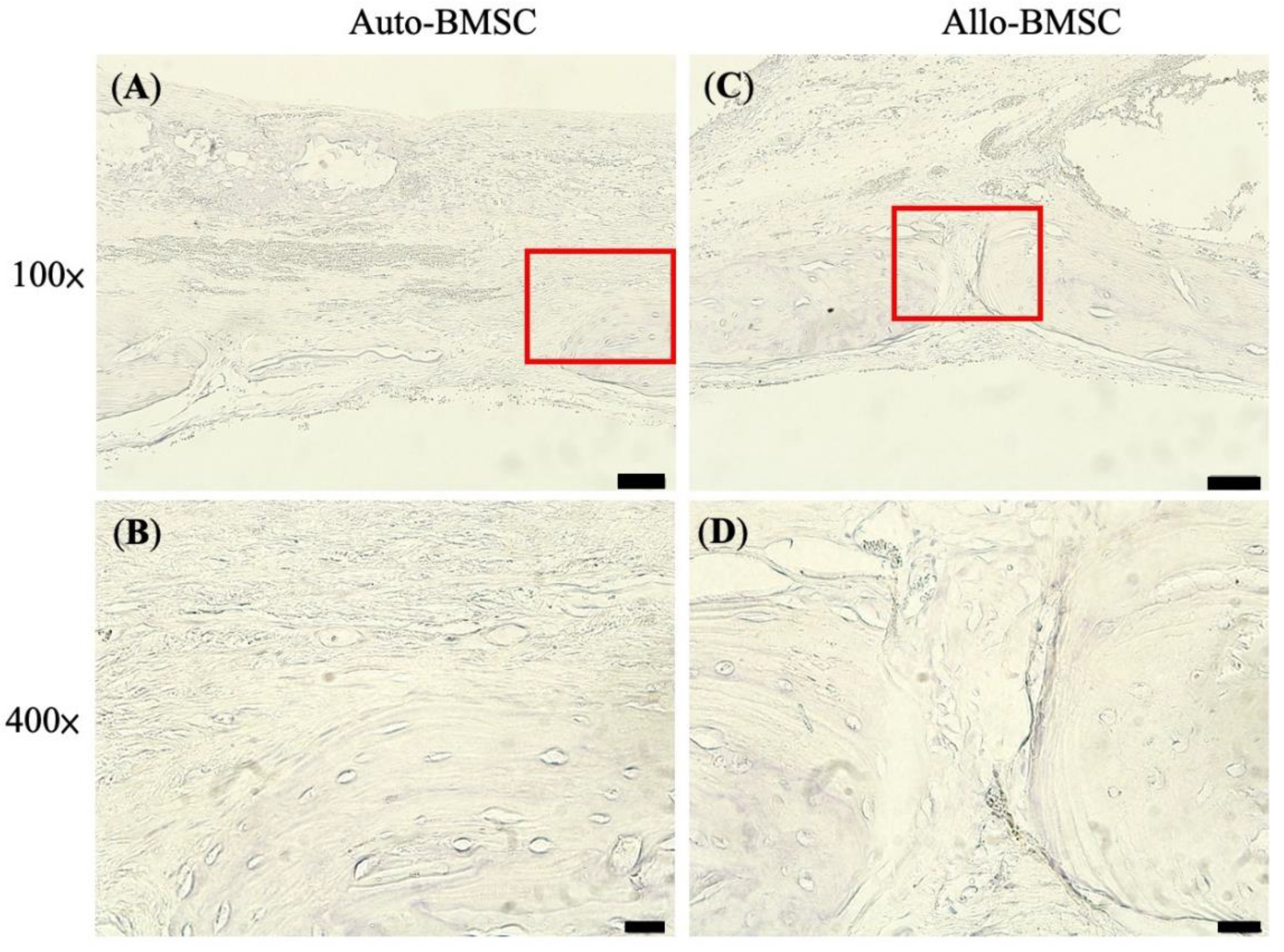
3.4.2. Histology and Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, J.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Stem cells, tissue engineering and periodontal regeneration. Aust. Dent. J. 2014, 59 (Suppl. S1), 117–130. [Google Scholar] [CrossRef] [PubMed]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.-Z.; Lee, J.H. Mesenchymal Stem Cell Therapy for Bone Regeneration. Clin. Orthop. Surg. 2018, 10, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chan, Y.-H.; Hsieh, S.-C.; Lew, W.-Z.; Feng, S.-W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int. J. Mol. Sci. 2019, 20, 5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, H.-T.; Chen, C.-T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J. Stem Cells 2014, 6, 288–295. [Google Scholar] [CrossRef]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of Large Bone Defects with the Use of Autologous Bone Marrow Stromal Cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef]
- Warnke, P.; Springer, I.; Wiltfang, J.; Acil, Y.; Eufinger, H.; Wehmöller, M.; Russo, P.; Bolte, H.; Sherry, E.; Behrens, E.; et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet 2004, 364, 766–770. [Google Scholar] [CrossRef]
- Jung, H.-G.; Ahn, E.-K.; Lee, J.-H.; Kim, Y.-H.; Leem, S.-H.; Heo, J.; Kim, H. Effects of harvesting sites and ages on adipose tissue-derived stem cells in rat. Tissue Eng. Regen. Med. 2014, 11, 137–142. [Google Scholar] [CrossRef]
- Cianfarani, F.; Toietta, G.; Di Rocco, G.; Cesareo, E.; Zambruno, G.; Odorisio, T. Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen. 2013, 21, 545–553. [Google Scholar] [CrossRef]
- Arinzeh, T.L.; Peter, S.J.; Archambault, M.P.; Bos, C.V.D.; Gordon, S.; Kraus, K.; Smith, A.; Kadiyala, S. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J. Bone Jt. Surg. Am. Vol. 2003, 85, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Q.; Fu, X.; Wu, X.; Gu, C.; Bi, J.; Xie, F.; Kang, N.; Liu, X.; Yan, L.; et al. Influence of Immunogenicity of Allogeneic Bone Marrow Mesenchymal Stem Cells on Bone Tissue Engineering. Cell Transplant. 2016, 25, 229–242. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Xu, J.; Zou, Q.; Jiang, D. Immunological Study of Allogeneic Mesenchymal Stem Cells during Bone Formation. J. Int. Med. Res. 2009, 37, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- De Kok, I.J.; Peter, S.J.; Archambault, M.; Bos, C.V.D.; Kadiyala, S.; Aukhil, L.; Cooper, L.F. Investigation of allogeneic mesenchyrnal stem cell-based alveolar bone formation: Preliminary findings. Clin. Oral Implant. Res. 2003, 14, 481–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, Z.; Zhang, F.; Wang, Z.; He, W.; Dong, S.-W.; Xu, J.; Dai, F. Improved Osteogenesis by HVEM-Expressing Allogenic Bone Marrow-Derived Mesenchymal Stem Cells in an Immune Activation Condition and Mouse Femoral Defect Model. Tissue Eng. Part A 2018, 24, 1167–1178. [Google Scholar] [CrossRef]
- Coathup, M.J.; Kalia, P.; Konan, S.; Mirza, K.; Blunn, G.W. A comparison of allogeneic and autologous mesenchymal stromal cells and osteoprogenitor cells in augmenting bone formation around massive bone tumor prostheses. J. Biomed. Mater. Res. Part A 2012, 101, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Tasso, R.; Augello, A.; Boccardo, S.; Salvi, S.; Caridà, M.; Postiglione, F.; Fais, F.; Truini, M.; Cancedda, R.; Pennesi, G. Recruitment of a Host’s Osteoprogenitor Cells Using Exogenous Mesenchymal Stem Cells Seeded on Porous Ceramic. Tissue Eng. Part A 2009, 15, 2203–2212. [Google Scholar] [CrossRef]
- Chatterjea, A.; LaPointe, V.; Alblas, J.; Chatterjea, S.; van Blitterswijk, C.; de Boer, J. Suppression of the immune system as a critical step for bone formation from allogeneic osteoprogenitors implanted in rats. J. Cell. Mol. Med. 2013, 18, 134–142. [Google Scholar] [CrossRef]
- Falacho, R.; Palma, P.; Marques, J.; Figueiredo, M.; Caramelo, F.; Dias, I.; Viegas, C.; Guerra, F. Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model. Molecules 2021, 26, 1339. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, X.; Wang, D.; Lu, W.; Ou, Y.; Han, Y.; Zhou, K.; Liu, H.; Fen, W.; Peng, L.; et al. Experimental study on the conduction function of nano-hydroxyapatite artificial bone. Micro Nano Lett. 2010, 5, 19–27. [Google Scholar] [CrossRef]
- Mao, S.-H.; Chen, C.-H.; Chen, C.-T. Osteogenic potential of induced pluripotent stem cells from human adipose-derived stem cells. Stem Cell Res. Ther. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shalumon, K.T.; Kuo, C.-Y.; Wong, C.-B.; Chien, Y.-M.; Chen, H.-A.; Chen, J.-P. Gelatin/Nanohyroxyapatite Cryogel Embedded Poly(lactic-co-glycolic Acid)/Nanohydroxyapatite Microsphere Hybrid Scaffolds for Simultaneous Bone Regeneration and Load-Bearing. Polymers 2018, 10, 620. [Google Scholar] [CrossRef] [Green Version]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobiesiak, M.; Sivasubramaniyan, K.; Hermann, C.; Tan, C.; Örgel, M.; Treml, S.; Cerabona, F.; de Zwart, P.; Ochs, U.; Müller, C.A.; et al. The Mesenchymal Stem Cell Antigen MSCA-1 is Identical to Tissue Non-specific Alkaline Phosphatase. Stem Cells Dev. 2010, 19, 669–677. [Google Scholar] [CrossRef]
- Krieger, N.R.; Yin, D.P.; Fathman, C.G. CD4+ but not CD8+ cells are essential for allorejection. J. Exp. Med. 1996, 184, 2013–2018. [Google Scholar] [CrossRef]
- Yamada, J.; Kurimoto, I.; Streilein, J.W. Role of CD4+ T cells in immunobiology of orthotopic corneal transplants in mice. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2614–2621. [Google Scholar]
- Rapp, A.E.; Bindl, R.; Erbacher, A.; Kruchen, A.; Rojewski, M.; Schrezenmeier, H.; Müller, I.; Ignatius, A. Autologous Mesenchymal Stroma Cells Are Superior to Allogeneic Ones in Bone Defect Regeneration. Int. J. Mol. Sci. 2018, 19, 2526. [Google Scholar] [CrossRef]
- Akahane, M.; Ohgushi, H.; Yoshikawa, T.; Sempuku, T.; Tamai, S.; Tabata, S.; Dohi, Y. Osteogenic Phenotype Expression of Allogeneic Rat Marrow Cells in Porous Hydroxyapatite Ceramics. J. Bone Miner. Res. 1999, 14, 561–568. [Google Scholar] [CrossRef]
- Schmidt, A.; Zhang, X.-M.; Joshi, R.N.; Iqbal, S.; Wahlund, C.; Gabrielsson, S.; Harris, R.A.; Tegnér, J. Human macrophages induce CD4+Foxp3+ regulatory T cells via binding and re-release of TGF-β. Immunol. Cell Biol. 2016, 94, 747–762. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Yoon, D.S.; Kim, H.O.; Lee, J.W. Characterization of Different Subpopulations from Bone Marrow-Derived Mesenchymal Stromal Cells by Alkaline Phosphatase Expression. Stem Cells Dev. 2012, 21, 2958–2968. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.C.; Salgado, C.L.; Sahu, A.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J. Preparation and characterization of collagen-nanohydroxyapatite biocomposite scaffolds by cryogelation method for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2013, 101A, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Tsung, L.H.; Chang, K.-H.; Chen, J.-P. Osteogenesis of adipose-derived stem cells on three-dimensional, macroporous gelatin–hyaluronic acid cryogels. Biomed. Eng. Appl. Basis Commun. 2011, 23, 127–133. [Google Scholar] [CrossRef]
- Liao, H.-T.; Shalumon, K.T.; Chang, K.-H.; Sheu, C.; Chen, J.-P. Investigation of synergistic effects of inductive and conductive factors in gelatin-based cryogels for bone tissue engineering. J. Mater. Chem. B 2016, 4, 1827–1841. [Google Scholar] [CrossRef]
- Chang, K.-H.; Liao, H.-T.; Chen, J.-P. Preparation and characterization of gelatin/hyaluronic acid cryogels for adipose tissue engineering: In vitro and in vivo studies. Acta Biomater. 2013, 9, 9012–9026. [Google Scholar] [CrossRef]
- Chen, C.-H.; Kuo, C.-Y.; Wang, Y.-J.; Chen, J.-P. Dual Function of Glucosamine in Gelatin/Hyaluronic Acid Cryogel to Modulate Scaffold Mechanical Properties and to Maintain Chondrogenic Phenotype for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2016, 17, 1957. [Google Scholar] [CrossRef]
- English, K.; Mahon, B.P. Allogeneic mesenchymal stem cells: Agents of immune modulation. J. Cell. Biochem. 2011, 112, 1963–1968. [Google Scholar] [CrossRef] [Green Version]
- Griffin, M.; Ritter, T.; Mahon, B.P. Immunological Aspects of Allogeneic Mesenchymal Stem Cell Therapies. Hum. Gene Ther. 2010, 21, 1641–1655. [Google Scholar] [CrossRef]
- Kotobuki, N.; Katsube, Y.; Katou, Y.; Tadokoro, M.; Hirose, M.; Ohgushi, H. In Vivo Survival and Osteogenic Differentiation of Allogeneic Rat Bone Marrow Mesenchymal Stem Cells (MSCs). Cell Transplant. 2008, 17, 705–712. [Google Scholar] [CrossRef]
- Wu, D.; Chang, X.; Tian, J.; Kang, L.; Wu, Y.; Liu, J.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: Release of exosomal miR-1260a improves osteogenesis and angiogenesis. J. Nanobiotechnol. 2021, 19, 209. [Google Scholar] [CrossRef]
- Lu, G.-D.; Cheng, P.; Liu, T.; Wang, Z. BMSC-Derived Exosomal miR-29a Promotes Angiogenesis and Osteogenesis. Front. Cell Dev. Biol. 2020, 8, 608521. [Google Scholar] [CrossRef]
- Zhao, S.-J.; Kong, F.-Q.; Jie, J.; Li, Q.; Liu, H.; Xu, A.-D.; Yang, Y.-Q.; Jiang, B.; Wang, D.-D.; Zhou, Z.-Q.; et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3β/β-catenin pathway. Theranostics 2020, 10, 17–35. [Google Scholar] [CrossRef]

| Long Bone | ||||||
|---|---|---|---|---|---|---|
| Reference | Animal Model | Cells | Scaffold | Immunosuppressants | In Vitro Results | In Vivo Results |
| Arinzeh, T.L. et al., 2003 [10] | Canine Femoral diaphysis mid-portion 21mm defect. | Allogeneic BMSC Autologous BMSC | HA-TCP | n/a | MLR: Significant T cell proliferation | 16 Weeks Allogeneic BMSC (49%) = Autologous BMSC (42%) > HA-TCP (24%) |
| Guo, S.Q. et al., 2009 [12] | Pig Middle tibia shaft 20mm Segmental defects | Allogeneic BMSC Autologous BMSC | β-TCP | n/a | MLR: SI not significant increase | ++ 16 Weeks Allogeneic BMSC (75%) = Autologous BMSC (75%) > β-TCP (42%) > blank (7%) |
| Coathup, M.J. et al., 2012 [15] | Ovine Tibial bone defect 50mm Prosthesis inserted | Allogeneic BMSC Autologous BMSC OPC | HA | n/a | MLR: Significant T cell proliferation | 6 months Autologous BMSC (149.5 mm2) > OPC (121.1 mm2) > Control (87.5 mm2) > Allogeneic BMSC (0 mm2) |
| Rong, Z. et al., 2017 [14] | Mouse Femur 1mm defect | Allogeneic BMSC Allogeneic HVEM-expressing BMSC | DBM | HVEM transfection | Allogeneic HVEM-expressing BMSC inhibit IL-17 secretion | 8 weeks Allogeneic HVEM-expressing BMSCs (73%) > Allogeneic BMSC (39%) > DBM (15%) |
| Rapp A.E. et al., 2018 [26] | Humanized Mouse Femur1mm defect | Allogeneic BMSC Autologous BMSC | Collagen type-I gel | n/a | n/a | 35 days Autologous BMSC (37%) > Allogeneic BMSC (16%) |
| Craniofacial bone | ||||||
| De Kok, I.J. et al., 2003 [13] | Beagle dog Bilateral mandible alveolar bone 6.5 × 20 mm | Allogeneic BMSC Autologous BMSC | HA-TCP | n/a | MLR: Significant T cell proliferation | 9 weeks Allogeneic BMSC (85%) > Autologous BMSC (83%) |
| Wu, J. et al., 2016 [11] | Beagle dog Mandibular body 30mm defect | Allogeneic BMSC Autologous BMSC | β-TCP | n/a | MLR: SI significant increase | 24 weeks Auto-bone (82%) > Allogeneic BMSC (44%) > Autologous BMSC (39%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.-F.; Mao, S.-H.; Shyu, V.B.-H.; Chen, C.-H.; Chen, C.-T. Allogeneic Bone-Marrow Mesenchymal Stem Cell with Moldable Cryogel for Craniofacial Bone Regeneration. J. Pers. Med. 2021, 11, 1326. https://doi.org/10.3390/jpm11121326
Chu C-F, Mao S-H, Shyu VB-H, Chen C-H, Chen C-T. Allogeneic Bone-Marrow Mesenchymal Stem Cell with Moldable Cryogel for Craniofacial Bone Regeneration. Journal of Personalized Medicine. 2021; 11(12):1326. https://doi.org/10.3390/jpm11121326
Chicago/Turabian StyleChu, Cheng-Feng, Shih-Hsuan Mao, Victor Bong-Hang Shyu, Chih-Hao Chen, and Chien-Tzung Chen. 2021. "Allogeneic Bone-Marrow Mesenchymal Stem Cell with Moldable Cryogel for Craniofacial Bone Regeneration" Journal of Personalized Medicine 11, no. 12: 1326. https://doi.org/10.3390/jpm11121326
APA StyleChu, C. -F., Mao, S. -H., Shyu, V. B. -H., Chen, C. -H., & Chen, C. -T. (2021). Allogeneic Bone-Marrow Mesenchymal Stem Cell with Moldable Cryogel for Craniofacial Bone Regeneration. Journal of Personalized Medicine, 11(12), 1326. https://doi.org/10.3390/jpm11121326






