Seasonal Variation and Severity of Acute Abdomen in Japan: A Nine-Year Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Study Population
2.3. Data Collection
2.4. Statistical Analysis
3. Results
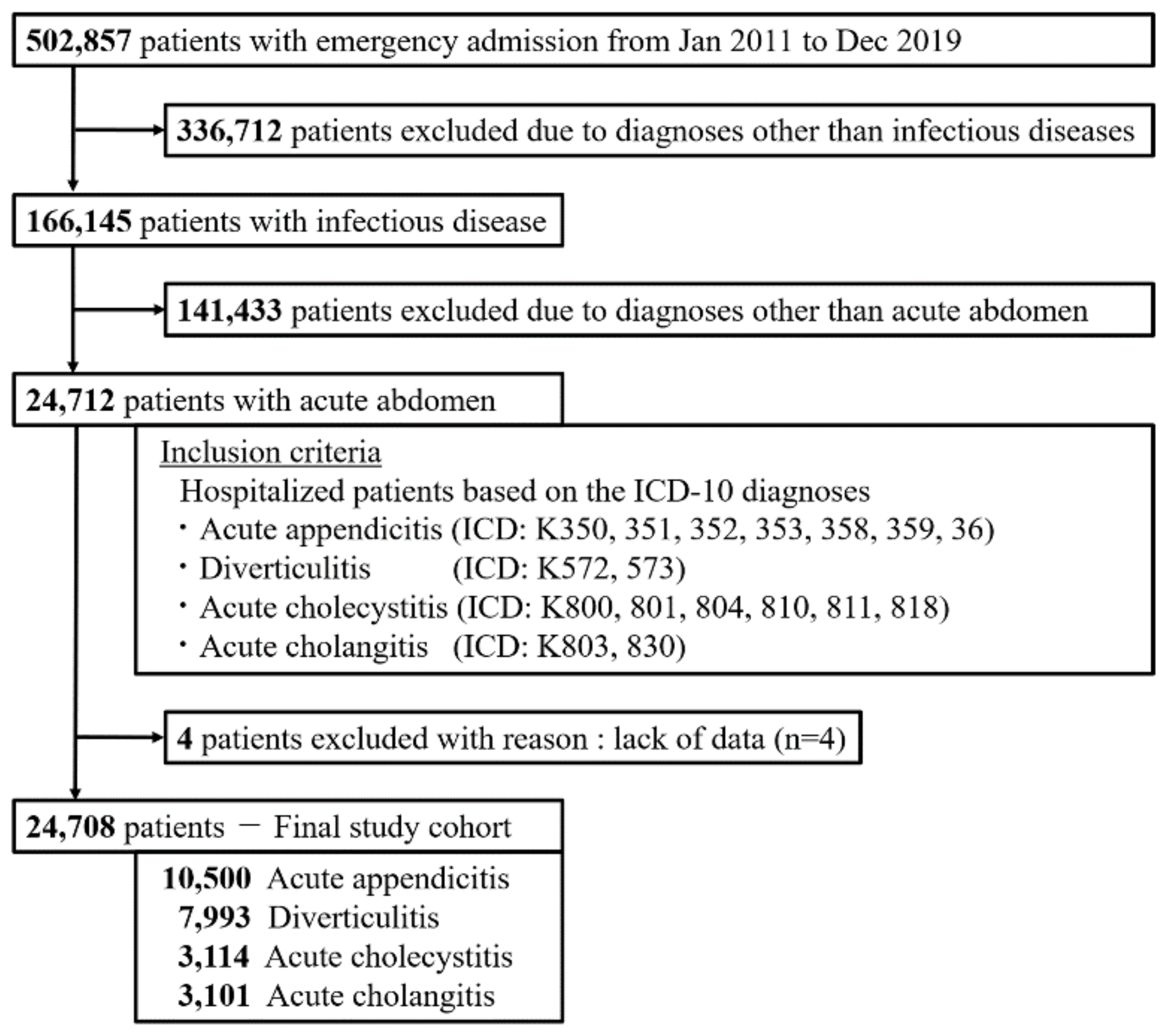
3.1. Study Population
3.2. Monthly and Seasonal Variations in Admissions
3.3. Seasonal Severity of Each Disease
4. Discussion
4.1. Principal Findings
4.2. Assumed Mechanisms Causing Seasonal Variations of Acute Abdomen
4.3. Disease Severity across Seasons
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hecker, A.; Reichert, M.; Reuß, C.J.; Schmoch, T.; Riedel, J.G.; Schneck, E.; Padberg, W.; Weigand, M.A.; Hecker, M. Intra-abdominal sepsis: New definitions and current clinical standards. Langenbeck’s Arch. Surg. 2019, 404, 257–271. [Google Scholar] [CrossRef]
- Ihnát, P.; Peteja, M.; Vávra, P.; Tulinský, L.; Zonča, P. Current Standards of Care in the Management of Patients with Abdominal Sepsis. Rozhl. Chir. 2015, 94, 234–237. [Google Scholar] [PubMed]
- Buckius, M.T.; McGrath, B.; Monk, J.; Grim, R.; Bell, T.; Ahuja, V. Changing Epidemiology of Acute Appendicitis in the United States: Study Period 1993–2008. J. Surg. Res. 2012, 175, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Sköldberg, F.; Granlund, J.; Discacciati, A.; Hjern, F.; Schmidt, P.T.; Olén, O. Incidence and lifetime risk of hospitalization and surgery for diverticular disease. Br. J. Surg. 2019, 106, 930–939. [Google Scholar] [CrossRef]
- Urbach, D.R.; Stukel, T.A. Rate of Elective Cholecystectomy and the Incidence of Severe Gallstone Disease. Can. Med. Assoc. J. 2005, 172, 1015–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöß, C.; Nitsche, U.; Neumann, P.-A.; Kehl, V.; Wilhelm, D.; Busse, R.; Friess, H.; Nimptsch, U. Acute appendicitis: Trends in surgical treatment—A population-based study of over 800 000 patients. Dtsch. Aerzteblatt Online 2021, 118, 244–249. [Google Scholar] [CrossRef]
- Emile, S.H.; Elfeki, H.; Sakr, A.; Shalaby, M. Management of acute uncomplicated diverticulitis without antibiotics: A systematic review, meta-analysis, and meta-regression of predictors of treatment failure. Tech. Coloproctol. 2018, 22, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Ceresoli, M.; Zucchi, A.; Pisano, M.; Allegri, A.; Bertoli, P.; Coccolini, F.; Falcone, C.; Manfredi, R.; Montori, G.; Nita, G.; et al. Epidemiology of acute cholecystitis and its treatment in Bergamo District, Northern Italy. Minerva Chir. 2015, 71, 106–113. [Google Scholar]
- Gomi, H.; Takada, T.; Hwang, T.-L.; Akazawa, K.; Mori, R.; Endo, I.; Miura, F.; Kiriyama, S.; Matsunaga, N.; Itoi, T.; et al. Updated comprehensive epidemiology, microbiology, and outcomes among patients with acute cholangitis. J. Hepato-Biliary-Pancreat. Sci. 2017, 24, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, A.; Dyson, K.; Bernard, S.; Smith, K. Seasonal Variation in Out-of-Hospital Cardiac Arrest in Victoria 2008–2017: Winter Peak. Prehosp. Emerg. Care 2020, 24, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, L.; Just, J.; Humbert, M.; Leroyer, C.; Epaud, R. Seasonality in Asthma: Impact and Treatments. Presse Med. 2016, 45, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Zangbar, B.; Rhee, P.; Pandit, V.; Hsu, C.-H.; Khalil, M.; Okeefe, T.; Neumayer, L.; Joseph, B. Seasonal Variation in Emergency General Surgery. Ann. Surg. 2016, 263, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.E.; Bickler, S.W.; Chang, D.C.; Talamini, M.A. Examining a Common Disease with Unknown Etiology: Trends in Epidemiology and Surgical Management of Appendicitis in California, 1995–2009. World J. Surg. 2012, 36, 2787–2794. [Google Scholar] [CrossRef]
- Ricciardi, R.; Roberts, P.L.; Read, T.E.; Marcello, P.W.; Hall, J.F.; Schoetz, D.J.; Foley, E.F. Cyclical Increase in Diverticulitis During the Summer Months. Arch. Surg. 2011, 146, 319–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.S.; Shahzad, N.; Arshad, S.; Shariff, A.H. Seasonal Variation in Acute Cholecystitis: An Analysis of Cholecystectomies Spanning Three Decades. J. Surg. Res. 2020, 246, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data from 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardsen, I.; Schöb, D.; Ulmer, T.F.; Steinau, G.; Neumann, U.P.; Klink, C.D.; Lambertz, A. Etiology of Appendicitis in Children: The Role of Bacterial and Viral Pathogens. J. Investig. Surg. 2016, 29, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Ilves, I.; Fagerström, A.; Herzig, K.H.; Juvonen, P.; Miettinen, P.; Paajanen, H. Seasonal Variations of Acute Appendicitis and Nonspecific Abdominal Pain in Finland. World J. Gastroenterol. 2014, 20, 4037–4042. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kojimahara, N.; Kiyohara, K.; Endo, M.; Yamaguchi, N.; Sato, Y.; Hatanaka, M.; Itagaki, R.; Yoshida, K.; Shimodaira, Y.; et al. Association between climatic elements and acute appendicitis in Japan. J. Surg. Res. 2017, 211, 64–69. [Google Scholar] [CrossRef]
- Onozuka, D.; Hashizume, M.; Hagihara, A. Effects of weather variability on infectious gastroenteritis. Epidemiol. Infect. 2010, 138, 236–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, A.; Sánchez, A.; Rey, A.; Fajardo, C. Recovery of Aerobic and Anaerobic Bacteria from Patients with Acute Appendicitis Using Blood Culture Bottles. Biomedica 2019, 39, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Gouma, D.J.; Garden, O.J.; Büchler, M.W.; Windsor, J.A.; Mayumi, T.; Yoshida, M.; et al. TG13 current terminology, etiology, and epidemiology of acute cholangitis and cholecystitis. J. Hepato-Biliary-Pancreat. Sci. 2013, 20, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Van Erpecum, K.J.; Henegouwen, G.P.V.B. Intestinal aspects of cholesterol gallstone formation. Dig. Liver Dis. 2003, 35, 8–11. [Google Scholar] [CrossRef]
- Gomi, H.; Solomkin, J.; Schlossberg, D.; Okamoto, K.; Takada, T.; Strasberg, S.M.; Ukai, T.; Endo, I.; Iwashita, Y.; Hibi, T.; et al. Tokyo Guidelines 2018: Antimicrobial therapy for acute cholangitis and cholecystitis. J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 3–16. [Google Scholar] [CrossRef]
- Eber, M.R.; Shardell, M.; Schweizer, M.L.; Laxminarayan, R.; Perencevich, E.N. Seasonal and Temperature-Associated Increases in Gram-Negative Bacterial Bloodstream Infections among Hospitalized Patients. PLoS ONE 2011, 6, e25298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danai, P.A.; Sinha, S.; Moss, M.; Haber, M.J.; Martin, G.S. Seasonal variation in the epidemiology of sepsis. Crit. Care Med. 2007, 35, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Mourtzoukou, E.G.; Falagas, M.E. Exposure to cold and respiratory tract infections. Int. J. Tuberc. Lung Dis. 2007, 11, 938–943. [Google Scholar] [PubMed]



| All Patients (n = 24,708) | Acute Appendicitis (n = 10,500) | Diverticulitis (n = 7993) | Acute Cholecystitis (n = 3114) | Acute Cholangitis (n = 3101) | |
|---|---|---|---|---|---|
| Age, yr | 60 (37–77) | 37 (19–57) | 65 (48–78) | 76 (65–84) | 79 (70–85) |
| <18, n (%) | 2457 (9.9%) | 2408 (22.9%) | 34 (0.4%) | 6 (0.2%) | 9 (0.3%) |
| 18–64, n (%) | 11,206 (45.4%) | 6160 (58.7%) | 3831 (47.9%) | 747 (24.0%) | 468 (15.1%) |
| ≥65, n (%) | 11,045 (44.7%) | 1932 (18.4%) | 4128 (51.6%) | 2361 (75.8%) | 2624 (84.6%) |
| Sex, male, (%) | 14,107 (57.1%) | 5868 (55.9%) | 4698 (58.8%) | 1811 (58.2%) | 1730 (55.8%) |
| Charlson comorbidity index | 2 (0–5) | 1 (0–3) | 3 (1–6) | 4 (2–8) | 6 (3–10) |
| SOFA score | 1 (0–2) | 0 (0–1) | 0 (0–1) | 1 (0–3) | 2 (1–3) |
| Sepsis-3, n (%) | 6224 (25.2%) | 1726 (16.4%) | 1090 (13.6%) | 1462 (46.9%) | 1946 (62.8%) |
| SOFA score for sepsis-3 patients | 3 (2–4) | 2 (2–3) | 2 (2–3) | 3 (2–4) | 3 (2–4) |
| DIC, n (%) | 732 (3.0%) | 154 (1.5%) | 196 (2.5%) | 173 (5.6%) | 209 (6.7%) |
| ICU admission, n (%) | 463 (1.9%) | 132 (1.3%) | 165 (2.1%) | 124 (4.0%) | 42 (1.4%) |
| Emergency surgery with general anesthesia n (%) | 6132 (24.8%) | 3989 (38.0%) | 675 (8.4%) | 911 (29.3%) | 557 (18.0%) |
| Catecholamine use, n (%) | 744 (3.0%) | 226 (2.2%) | 103 (1.3%) | 123 (3.9%) | 292 (9.4%) |
| All Patients (n = 24,708) | Acute Appendicitis (n = 10,500) | Diverticulitis (n = 7993) | Acute Cholecystitis (n = 3114) | Acute Cholangitis (n = 3101) | |
|---|---|---|---|---|---|
| Mortality, n (%) | 254 (1.0%) | 12 (0.1%) | 40 (0.5%) | 95 (3.1%) | 107 (3.5%) |
| <18 years, n (%) | 0/2457 (0%) | 0/2408 (0%) | 0/34 (0%) | 0/6 (0%) | 0/9 (0%) |
| 18–64 years, n (%) | 11/11,206 (0.1%) | 0/6160 (0%) | 4/3831 (0.1%) | 2/747 (0.3%) | 5/468 (1.1%) |
| ≥65 years, n (%) | 243/11,045 (2.2%) | 12/1932 (0.6%) | 36/4128 (0.9%) | 93/2361 (3.9%) | 102/2624 (3.9%) |
| Length of stay, d | 7 (4–11) | 5 (3–8) | 7 (5–10) | 12 (7–20) | 10 (6–15) |
| Acute Appendicitis | Diverticulitis | Acute Cholecystitis | Acute Cholangitis | |||||
|---|---|---|---|---|---|---|---|---|
| Season | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value | Odds Ratio (95% CI) | p Value |
| Spring | 1.20 (1.14–1.27) | <0.0001 | 1.18 (1.10–1.26) | <0.0001 | 1.08 (0.98–1.20) | 0.1285 | 1.08 (0.98–1.20) | 0.137 |
| Summer | 1.35 (1.28–1.43) | <0.0001 | 1.23 (1.16–1.31) | <0.0001 | 1.23 (1.11–1.36) | <0.0001 | 1.23 (1.12–1.36) | <0.0001 |
| Fall | 1.20 (1.13–1.27) | <0.0001 | 1.16 (1.08–1.23) | <0.0001 | 1.12 (1.01–1.24) | 0.031 | 1.16 (1.05–1.28) | 0.0048 |
| Winter | Reference | Reference | Reference | Reference | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimoto, H.; Yamakawa, K.; Umemura, Y.; Fujii, K.; Nakamura, E.; Taniguchi, K.; Tanaka, K.; Takasu, A.; Uchiyama, K. Seasonal Variation and Severity of Acute Abdomen in Japan: A Nine-Year Retrospective Analysis. J. Pers. Med. 2021, 11, 1346. https://doi.org/10.3390/jpm11121346
Yoshimoto H, Yamakawa K, Umemura Y, Fujii K, Nakamura E, Taniguchi K, Tanaka K, Takasu A, Uchiyama K. Seasonal Variation and Severity of Acute Abdomen in Japan: A Nine-Year Retrospective Analysis. Journal of Personalized Medicine. 2021; 11(12):1346. https://doi.org/10.3390/jpm11121346
Chicago/Turabian StyleYoshimoto, Hidero, Kazuma Yamakawa, Yutaka Umemura, Kensuke Fujii, Eriko Nakamura, Kohei Taniguchi, Keitaro Tanaka, Akira Takasu, and Kazuhisa Uchiyama. 2021. "Seasonal Variation and Severity of Acute Abdomen in Japan: A Nine-Year Retrospective Analysis" Journal of Personalized Medicine 11, no. 12: 1346. https://doi.org/10.3390/jpm11121346
APA StyleYoshimoto, H., Yamakawa, K., Umemura, Y., Fujii, K., Nakamura, E., Taniguchi, K., Tanaka, K., Takasu, A., & Uchiyama, K. (2021). Seasonal Variation and Severity of Acute Abdomen in Japan: A Nine-Year Retrospective Analysis. Journal of Personalized Medicine, 11(12), 1346. https://doi.org/10.3390/jpm11121346







