Abstract
The use of nanomedicine for antitumor therapy has been extensively investigated for a long time. Enhanced permeability and retention (EPR) effect-mediated drug delivery is currently regarded as an effective way to bring drugs to tumors, especially macromolecular drugs and drug-loaded pharmaceutical nanocarriers. However, a disordered vessel network, and occluded or embolized tumor blood vessels seriously limit the EPR effect. To augment the EPR effect and improve curative effects, in this review, we focused on the perspective of tumor blood vessels, and analyzed the relationship among abnormal angiogenesis, abnormal vascular structure, irregular blood flow, extensive permeability of tumor vessels, and the EPR effect. In this commentary, nanoparticles including liposomes, micelles, and polymers extravasate through the tumor vasculature, which are based on modulating tumor vessels, to increase the EPR effect, thereby increasing their therapeutic effect.
1. Introduction
Solid tumors are the major cause of death worldwide and their treatment remains a challenge [1,2,3]. Chemotherapy is one of the few treatment options available for metastasized tumors which cannot be removed surgically; however, the effectiveness of this therapeutic modality is not yet satisfactory [4]. This problem mainly stems from the lack of tumor selectivity by these agents; hence, the occurrence of severe adverse effects limits the usage of chemotherapy [5]. Nanomedicines have been designed to guide drugs more precisely to tumor cells and away from sites of toxicity. These agents have numerous theoretical advantages over low-molecular-weight drugs, including high drug loading, specific targeting, and the ability to protect the payload from degradation and release the drug in a controlled or sustained manner [6]. Theoretically, nanomedicines with larger particle size leak more slowly from blood vessels compared with most chemotherapy drugs. Fortunately, vascular leakage is a major feature of the vasculature of solid tumors. Specifically, tumor neovasculature has larger lumens and wider fenestrations (200 nm to 1.2 μm in diameter) due to its lack of a smooth muscle layer and pericytes [7]. When injected intravenously, nanomedicines ranging in size from 10 to 500 nm tend to circulate for a long time and can preferentially access the tumor tissue through the leaky tumor vasculature; subsequently, they are retained in the tumor bed due to reduced lymphatic drainage [8,9,10,11,12]. This pathophysiological phenomenon based on abnormal tumor angiogenesis to increase the delivery of nanomedicines in tumors is known as “the enhanced permeability and retention” (EPR) effect [10,11,12,13]. Matsumura and Maeda first reported the EPR effect in 1986 [11]. Follow-up studies rigorously verified that the EPR effect can be observed using macromolecules with an apparent molecular size >45 kDa (the threshold for renal clearance) and a longer plasma half-life. In recent years, Ding et al. conducted real-time research on human kidney tumors using X-ray computed tomography to confirm the existence of the EPR effect in humans. The results showed that the significant EPR effect can be found in >87% of human kidney tumors [14]. However, low-molecular-weight contrast agents do not stay in the tumor and can be washed out in a minute from tumor, which greatly differs from macromolecular drug retention in tumors. Therefore, Maeda et al. reported a more distinct method to prove the EPR effect in human by conjugating lipiodol with a macromolecular nanodrug [15]. This method lasts longer than X-ray computed tomography, and it can be used to further explore the significant difference between the EPR effect of macromolecular drugs and low-molecular-weight counterparts.
Nanodrug delivery is based on the accumulation of drugs in tumors due to the EPR effect, and the subsequent release of the therapeutic payload [11,16]. However, the EPR effect is inadequate in tumors; this inadequacy can be attributed to the high interstitial fluid pressure (IFP), the dense extracellular matrix (ECM), and the occluded or embolized tumor blood vessels [12,17,18]. Moreover, the prolonged circulation of the drug increases the ability of extravasation into the tumor through the EPR effect. Clinically, it has been demonstrated that the function of long-circulating liposomes, for example, doxorubicin (DOX)-loaded polyethylene glycol (PEG)ylated liposomes (Doxil), reduces opsonization and premature clearance, increases the blood circulation time, and potentially enhances drug accumulation in the tumor [19]. However, when the EPR effect is insufficient, the drug may extravasate and bring more toxicity into normal tissues. Thus, there is an urgent need to identify the physiological barriers that affect the EPR effect of tumors. The aim of such research would be the development of methods to enhance tumor penetration and retention, thereby improving tumor targeting and the therapeutic effect. In this review, we analyzed the barriers to drug delivery, focusing on the influence of tumor vasculature on the EPR effect. Moreover, we discussed the method utilized for the regulation of tumor blood vessels through the nanodrug delivery system to enhance the EPR effect [20,21,22,23,24].
2. Abnormal Vascular Functions Affect the Tumor EPR Effect
To satisfy the overgrowth of tumor cells, solid tumors need to induce and maintain a dedicated tumor blood supply, which is termed neovascularization. Under inflammatory or hypoxic tumor conditions, cells such as vascular endothelial cells release vascular permeability mediators, resulting in more enhanced tumor vascular permeability than in normal tissue, which can be demonstrated by angiography [25]. However, due to their short half-life and the rapid dilution in the bloodstream, these mediators mainly affect tumor vessels, but not normal tissue blood vessels. In such regions, macromolecules ranging from 10 to 500 nm (e.g., macromolecular anticancer agent, albumin, immunoglobulin, micelles, liposomes, and protein–polymer conjugates) can selectively leak out from the vascular bed and accumulate inside the interstitial space. However, in solid tumors, the EPR effect exhibits great heterogeneity. Tumors show different EPR effects regardless of their types and sizes, patients, or their developmental stages. Tumors with high blood vessel density (e.g., hepatocellular carcinoma) show a strong EPR effect, whereas others with low vascular density (e.g., pancreatic cancer) show a weak EPR effect [5]. Therefore, accurate monitoring and evaluation of the EPR effects in different tumors is essential for the development of personalized EPR-mediated plans for the treatment of tumors.
In principle, due to the widespread presence of EPR in tumors, nanomedicines based on the EPR effect show great promise for improving the efficacy of systemic anticancer drug therapy. However, their full anticancer potential has been hindered because of biological and pathophysiological barriers [26]. Obviously, the vascular system of tumors, which exhibit different vessel density, maturity, perfusion, and pore cutoff size, could be considered one of the main factors that affect the EPR effect [27]. In this review, we summarize the three main approaches through which abnormal tumor blood vessels affect the EPR effect and the related vascular mediators (Table 1).

Table 1.
Relationship between tumor vascular-related mediators and three typical vascular characteristics.
2.1. Abnormal Angiogenesis
Angiogenesis is essential for the continuous growth and development of solid tumors. Tumor vessels provide oxygen and nutrients and remove waste products, supply a favorable niche for cancer stem cells, and serve as a conduit for tumor cell metastatic spread and immune cell infiltration. Unlike normal blood vessels, tumor blood vessels with abnormal structure and function impede the delivery of adequate and effective oxygen, as well as therapeutic drugs to cancer cells [88,89]. In cancer progression, the overexpression of proangiogenic factors drives the pathological angiogenesis. An imbalance between local proangiogenic and antiangiogenic factors may lead to the proliferation, migration, and new vessel formation of endothelial cells (EC). Furthermore, pericyte coverage of EC is often absent in the tumor vasculature. Compared with normal tissue with an organized microvasculature with regular branching order, the vascular organization of tumor tissue is disorganized and lacks the conventional hierarchy. Abnormal angiogenesis may lead to structural and functional abnormalities of the vascular system, which are often characterized by tortuous, unorganized, and excessive leakage [90,91]. This feature contributes to the vascular permeability of fluids and the escape of metastatic cancer cells [92,93]. Furthermore, the solid pressure generated by the proliferation of cancer cells compresses the blood and lymphatic vessels in the tumor, further impairing blood and lymphatic flows. These abnormal vascular structures collectively lead to an abnormal tumor microenvironment (TME), characterized by high IFP, hypoxia, and acidosis [88,94,95]. A physiological consequence of these vascular abnormalities is heterogeneity of tumor blood flow, which can interfere with the EPR effect and the uniform distribution of drugs within the tumor.
Tumor cells can promote blood vessel sprouting by releasing angiogenic molecules that bind to their respective receptors in adjacent cells or by paracrine signals [96,97]. Vascular endothelial growth factor (VEGF) appears to play the most critical role in physiological and pathological angiogenesis among all the known angiogenic molecules. It is overexpressed in the majority of solid tumors [28,29] and can promote the survival and proliferation of ECs, increase the display of adhesion molecules on these cells, and increase vascular permeability. By downregulating VEGF signaling in solid tumors, the vasculature may return to a more “normal” state, accompanied by decreased IFP, increased tumor oxygenation, and improved drug permeability in these tumors [98].
In addition to VEGF, other factors and proteins can also promote the abnormal formation of tumor blood vessels. Thus far, 28 proangiogenic factors/genes have been found to mediate tumor angiogenesis [76,77], including the fibroblast growth factor (FGF), hypoxia-inducible factor (HIF), platelet-derived growth factor-B (PDGF-B), tumor necrosis factor-α (TNF-α), chemokines, integrins, and transforming growth factor-β (TGF-β), as well as their receptors [76,99,100,101,102,103]. Acidic and basic FGF (FGF1 and FGF2) have the ability to induce angiogenesis [39]. FGFs stimulate the proliferation and migration of ECs, as well as the production of collagenase and plasminogen activator (PDGF), which stimulate angiogenesis and are related to the aging process of the tumor vasculature in vivo [42,43]. TGF-β possesses dual pro- and antiangiogenic properties. At low levels, TGF-β participates in the switch of angiogenesis by upregulating angiogenic factors and proteinases. At high levels, it can inhibit EC growth, stimulate the differentiation and recruitment of smooth muscle cells, and promote the reorganization of the basement membrane [52]. Moreover, as effective angiogenic factors, chemokines can induce the migration and proliferation of ECs, and they have pro- or antiangiogenic activities [104]. As an angiogenic factor, HIF cooperates with TNF inhibitors to initiate angiogenesis under hypoxic conditions [48,49,50,51]. It activates the signaling pathway and upregulates the expression of VEGF. Growth factors generated by this pathway activate the mitogen-activated protein kinase and protein kinase B signaling pathways, leading to increased levels of HIF-1 protein, thereby promoting tumor angiogenesis. Adhesion molecules (e.g., α6β1 and α6β4 integrins) mediate VEGF-induced angiogenesis, which regulates the adhesion of ECs to the ECM, thereby promoting the migration and survival of tumor vasculature. Other integrins (e.g., αvβ3, αvβ5, and α5β1) also mediate angiogenesis [63,64].
2.2. Irregular Blood Flow
Compared with normal vessels, newly formed tumor vessels are irregular or inconsistent [87]. It has been reported that tumor vessels are insensitive to angiotensin receptor type 2 (AGTR2). In addition, there is intermittent flow (only one flow in 15–20 min) and reverse flow of blood at the tumor site [105,106]. Moreover, blood often flows in the opposite direction. Irregular blood flow in the tumor is usually caused by irregular vascular structure. Unlike normal tissues, angiogenic factors in tumors at the late stage of vascular maturation will continue to be activated, leading to vascular abnormalities, which are characterized by irregular vascular structure and spatiotemporal heterogeneity [107]. Tumor vessels with irregular structure are characterized by a curved vascular shape, filling of the EC septum, and damage of the basement membrane. These effects lead to distortion of the vascular morphology and high permeability of the vascular EC space [31,108,109,110]. The distortion of blood vessels increases the geometric resistance of blood flow. The high permeability of blood vessels increases the hematocrit of tumor blood, thus increasing the blood viscosity [111]. In addition, the phenomenon of rapid proliferation of tumor cells in a finite space and excessive deposition of ECM can lead to large solid stress between adjacent cells and matrix components. The continuous accumulation of solid stress can lead to the compression of tumor blood vessels and the reduction of cross-sectional area and pressure difference in the direction of blood vessels [112]. The increase in vascular resistance and blood viscosity and the compression of accumulated solid stress significantly increases the resistance to blood perfusion. The increased resistance of tumor vessels to blood perfusion results in a low blood perfusion rate and a slow blood flow rate [113]. The change in blood flow velocity on the transport of nanoparticles through blood vessels has been investigated. A computer simulation explained the effect of blood flow velocity on the transport of nanoparticles. The results showed that the pressure at the vessel wall and the pressure gradient between the vascular wall and interstitial tissue increase in turn with the increase of fluid velocity in the vascular domain. Moreover, the trans-vascular transport efficiency of nanoparticles initially increases and subsequently decreases [114]. In addition, driven by the difference in pressure along the vascular direction, blood perfusion has the characteristics of convection–diffusion. Convection–diffusion differs between tumor blocks and depends on the local pressure gradient and flow resistance due to the heterogeneity of tumor blood vessels [115].
In addition to an irregular structure, the abnormal blood vessels of tumors also exhibit spatiotemporal heterogeneity [116,117]. This heterogeneity indicates the differing distribution of tumor vessels in various parts of the tumor or during the proliferation period. This is mainly indicated by the fact that the distribution of vessels in the periphery of the tumor is usually very rich, while their extension into the interior of the tumor gradually decreases. Therefore, this uneven distribution complicates the delivery of nanodrugs to the tumor center, which seriously hinders the penetration and extravascular transport of such agents. Of note, the high heterogeneity of tumor vessels in experimental mice and humans reduces the antitumor effects of some nanomedicines [26,118].
2.3. Extensive Vascular Permeability
Increased vascular permeability is widely found in endothelium discontinuous tumor vessels such as neovessels and immature vessels, as well as in other pathological tissues with disturbed vascular function. Compared with normal blood vessels, macromolecular drugs can reach the tumor stroma through the leaky vessel wall with large pores without hindrance [12]. However, excessive vascular leakage can cause plasma escape and hemoconcentration. This results in flow stasis and high IFP, which greatly hinder the extravasation of drugs and their movement to the tumor parenchyma. Furthermore, deposited clots of fibrin transiently promote the formation of blood vessels and ECM and prevent the penetration of antitumor therapeutic agents. The vascular media affecting the tumor vascular permeability are summarized below.
Bradykinin (BK) is of great importance in elevating the permeability of inflammatory sites and tumor tissues, thereby maintaining tumor growth [79,81]. Overexpression of BK receptors in solid tumors has been observed, resulting in defective vascular architecture with large intracellular gaps [119]. Kinin can activate EC-derived nitric oxide (NO) synthase, leading to increased levels of NO, a well-established and effective endothelium-derived vascular modulator [85,120,121]. NO is of great significance in vascular permeability, cell proliferation and extravasation (EPR effect), blood vessel dilation, and elevation of blood flow [83,84]. For example, NO generated from l-arginine under the action of NO synthase induces tumor vascular permeability. It has been demonstrated that the inhibition of NO generation can decrease vascular permeability, thereby weakening the EPR effect. This further confirms that NO is inextricably linked to vascular permeability in solid tumors [84,85]. Prostaglandins E1 and I2 are commonly involved in inflammation and cancer, exert similar effects to those of NO, and can enhance extravasation and EPR effects [83,86]. In summary, vascular permeability in tumors is often directly or indirectly related to kinins.
In addition, it has been shown that several vascular mediators, such as vascular permeability factor (VPF), which is important in tumor angiogenesis, TNF-α, and others elevate the vascular permeability of tumors [31]. EC survival and vascular permeability are closely related to the level of VPF/VEGF, as increasing this level can lead to upregulation of the corresponding receptors on ECs. [34,35]. TNF-α, a multifunctional proinflammatory cytokine with vascular permeabilizing effects [22], can enhance vascular leakiness via disrupting the EC adherence junction vascular endothelial cadherin [36]. TNF-α can increase the sensitivity to nanoparticles through serving as a vascular disrupting agent (VDA). At low levels, TNF-α may promote angiogenesis; however, at higher concentrations, it destroys the tumor vessels and increases the accumulation of drug in tumors [122].
3. Nanoparticles for Enhancing the Tumor EPR Effect
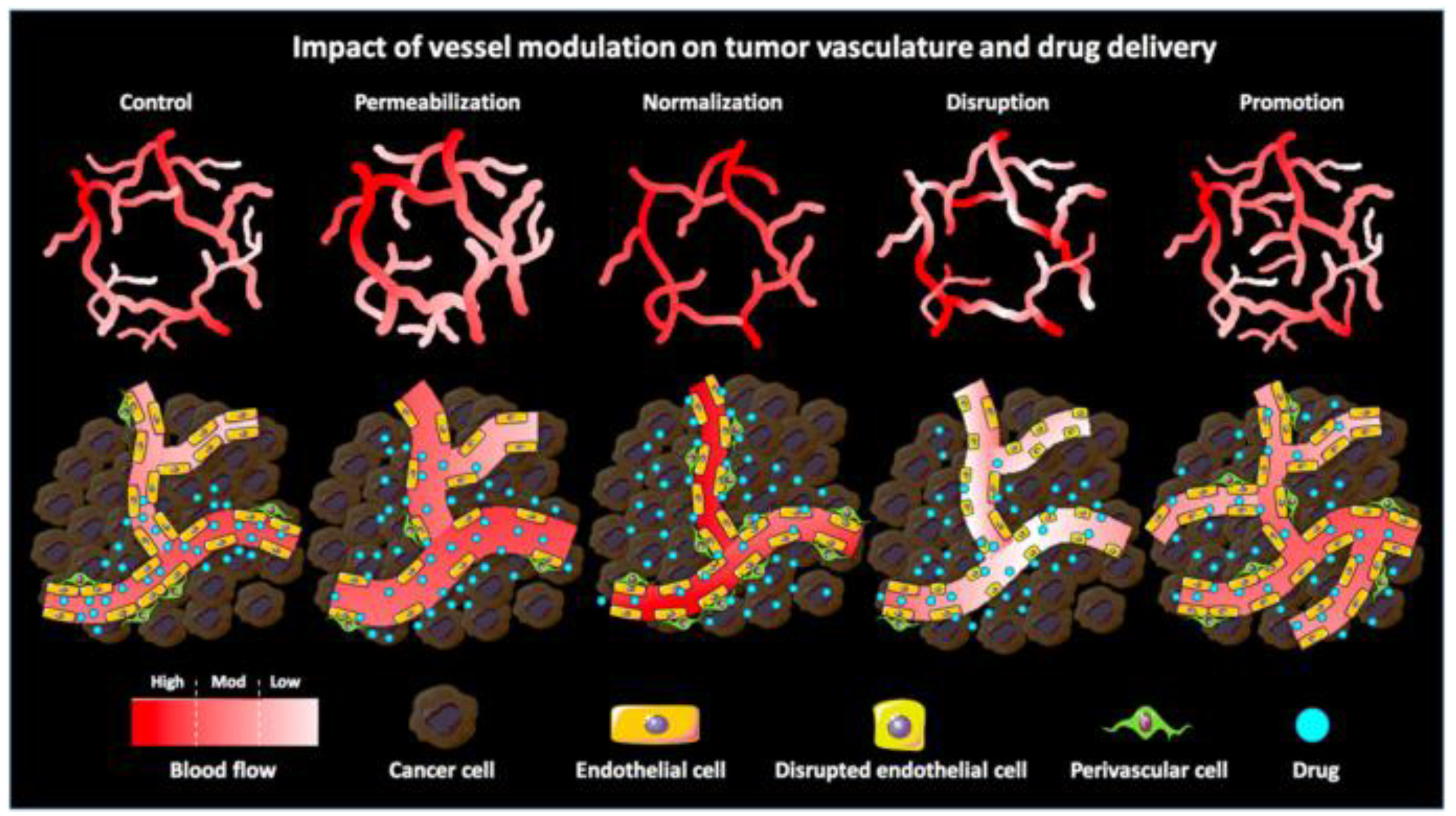
The EPR effect is an effective way for nanoparticles to passively target tumor cells. As opposed to passive drug targeting, nanoparticles based on the use of targeting ligands are termed “active drug targeting”. Actively targeted nanomedicines have failed to demonstrate benefit at the clinical level. This failure can be attributed to the fact that nanomedicines may face an insufficient endothelial vascular gap and a number of physiological barriers, such as high cellular density within solid malignancies and high IFP. Consequently, actively targeted nanoparticles have difficulties in identifying target cells due to the inadequate EPR effect. Therefore, enhancing the EPR effect through the use of nanoparticles can provide a better platform for subsequent treatment by elevating blood pressure, or conjugating with antibodies or EPR enhancers such as NO-generating agents. Several techniques have been employed to enhance the EPR effect, including the inhibition of angiogenesis, upregulation of tumor blood perfusion, and disruption of vascular or enhancement of vessel penetration to modulate the tumor vasculature [15,79,109,123,124]. Moreover, Ojha et al. described several pharmacological strategies for vascular regulation (Figure 1). Combined with nanoparticles, these strategies can enhance the EPR effect and improve treatment (Table 2).
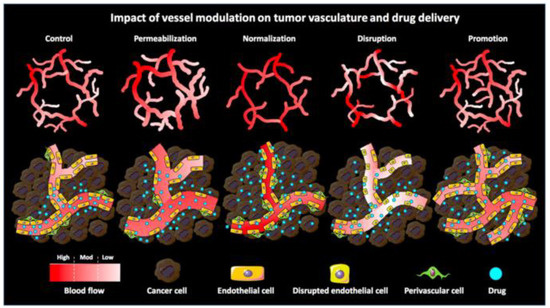
Figure 1.
Schematic illustration of the impact of pharmacological vascular regulation strategies on tumor vasculature and tumor-targeted drug delivery. Vascular permeability enlarges the gap between ECs by vasodilating and increasing the gap between ECs and perivascular cells. Vascular normalization promotes vascular maturation and improves vascular perfusion, thereby restoring the morphology and function of tumor vasculature to a certain extent. Vascular rupture enhances vascular permeability by disrupting the endothelial lining while reducing perfusion (especially in immature vessels). Vascular facilitation increases relative blood volume in tumors by increasing vascular density and distribution. Reproduced from Ojha [20].

Table 2.
Nanoparticles for enhancing the tumor enhanced permeability and retention (EPR) effect.
3.1. Antiangiogenesis
VEGF, FGF and their receptors, matrix metalloproteinases (MMPs), tubulin, and integrins are closely related to tumor survival, migration, metastasis, and angiogenesis [49,142,143]. It has been reported that drugs targeting these factors can inhibit tumor angiogenesis, thereby increasing blood perfusion and reducing the IFP [21,98,144]. Antiangiogenic agents, to some extent, can restore the pressure gradient between the vascular wall and tumor interstitium. Subsequently, they decrease the blood flow stasis to allow more nanoparticles to penetrate the blood vessels and reach the interstitial tissue [29,98,145]. Hence, antiangiogenesis improves the delivery of the therapeutic entities via maintaining the integrity of the EPR effect and reducing the IFP. Numerous different types of nanoparticles have been extensively investigated to facilitate the delivery of antiangiogenic agents [62,125,127].
Several studies have shown the potential effectiveness of soluble VEGF receptors on inhibiting pathological tumor angiogenesis. Nanoparticles are able to carry VEGF inhibitors to vascular EC. These inhibitors block pathological angiogenesis and promote tumor cell apoptosis, thereby inhibiting tumor growth and metastasis. Although nanoparticles are potentially applicable to antiangiogenesis, better delivery carriers that can improve the targeting activity are urgently sought. The arginylglycylaspartic acid (RGD) peptide can specifically bind to the integrin receptor of tumor vascular ECs with high affinity [146,147]. Grafting RGD onto nanoparticles may improve their active targeting ability and increase the drug transfection efficiency under conditions of sufficient EPR. However, Storm et al. stated that the potential of RGD-conjugate tumor targeting should not be overestimated due to the RGD receptors being widely distributed on blood vessels, which can induce the less tumor selectivity [148,149].
Some RNA interference (RNAi) strategies that require entry into tumor cells to function, such as small interfering RNA (siRNA) and short hairpin RNA (shRNA), are ideal for tumor-specific VEGF inhibition. The strategy of silencing VEGF by RNAi has achieved satisfactory results in some solid tumor models [150,151,152,153,154]. The angiogenesis of VEGF is mediated by binding to two endothelium-specific receptor tyrosine kinases with high affinity, namely, FLT1 (VEGFR1) and FLK1/KDR (VEGFR2). The use of homologous tyrosine kinase receptor soluble FLT1 (sFLT1) gene therapy has illustrated that the transduced sFLT1 protein can bind to VEGF and inhibit its activity, and this binding is similarly characterized by high affinity. Kim et al. reported an angiogenic EC-targeted polymeric gene vehicle, polyetherimide-g-polyethylene glycol (PEG)–RGD, which contained sFLT1 protein and siRNA [125,126]. These nanoparticles can effectively transfer therapeutic genes to angiogenic ECs, but not to nonangiogenic cells, and effectively inhibit the proliferation of VEGF-responsive ECs by the delivered genes. Kanazawa et al. prepared the amphiphilic and cationic triblock copolymer as an siRNA carrier to efficiently deliver small interfering VEGF into tumor tissues and significantly inhibit tumor growth because of the suppression of VEGF secretion from tumor tissues [155].
Some other vascular mediators are also involved in tumor angiogenesis. Targeting these mediators can also effectively inhibit abnormal tumor angiogenesis. Endostatin, a peptide cleaved from the carboxy terminus of collagen XVIII, suppresses the cell cycle and expression of antiapoptosis genes in proliferating ECs, thereby suppressing angiogenesis. To assess the endostatin gene therapy, Oga et al. prepared polyvinylpyrrolidone–pentostatin nanoparticles which exhibit a strong antiangiogenic effect and effective inhibition of metastatic growth in the brain [62]. Moreover, the combined use of sFLT1 with endostatin could be an effective antiangiogenic approach to the treatment of unresectable hepatocellular carcinoma [156]. Pigment epithelium-derived factor is a type of glycoprotein that plays a universally acknowledged role in the inhibition of angiogenesis via downregulation of VEGF [65]. The cyclic RGD–PEG–polyetherimide exhibited increased gene transfection efficiency in human umbilical vein ECs via binding to αvβ3, and significantly inhibited tumor growth and angiogenesis [157]. The binding of activated NF-κB to DNA can promote angiogenesis in addition to its role in facilitating cell proliferation, regulating apoptosis, facilitating angiogenesis, and stimulating invasion and metastasis [66]. Xiao et al. inhibited the growth and metastasis of breast cancer through delivering p65 shRNA into cells with a bioreducible polymer to block the signaling of NF-κB [158]. The proangiogenic effects of thyroid hormone on ECs and vascular smooth cells are initiated from the cell surface receptor for the hormone on the extracellular domain of integrin αvβ3 [67]. Tetraiodothyroacetic acid (tetrac) is a deamination product of l-thyroxine that blocks thyroid hormone binding with the integrin receptor [159]. Therefore, tetrac combined with liposomes and poly(lactide-co-glycolic acid) nanoparticles can achieve tetrac targeting of cell membrane integrin αvβ3 receptors and significantly inhibit angiogenesis [127,128,129,130]. MMPs participate in the process of angiogenesis in tissue reconstruction and neovascular growth through their proteolytic effect. Moreover, they release angiogenic factors residing in the matrix. Therefore, MMP inhibitors decrease angiogenesis and the migration of tumor cells, leading to slower progression of transplanted tumors [68]. Indeed, the antitumor efficacy of angiostatin and tissue inhibitor of metalloproteinases (TIMPs) has been demonstrated in various types of solid tumors [160,161]. Dendrimers containing plasmids of angiostatin and TIMP-2 showed high antitumor and antiangiogenic activity [162]. Nevertheless, antiangiogenic drugs also reduce the gap between tumor vascular ECs. Hence, the size of nanoparticles has to be strictly controlled if antiangiogenic drugs are employed to enhance the EPR effect [114].
3.2. Upregulated Tumor Blood Perfusion
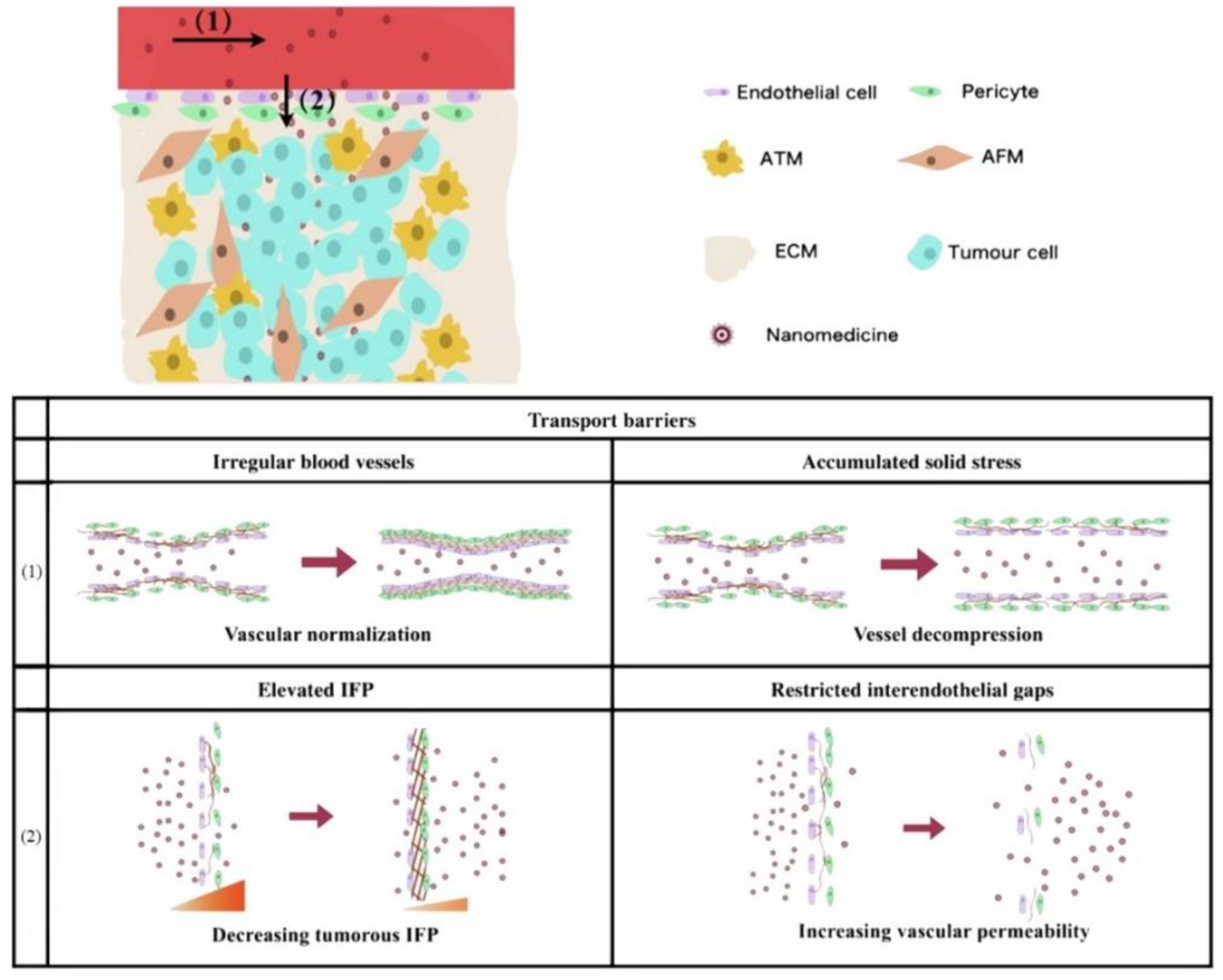
The main obstacle of blood perfusion in intravascular transport is due to irregular vascular structure and accumulated solid stress. Therefore, in accordance with the above two points, the blood perfusion of tumor vessels can be upregulated by vascular normalization and decompression, respectively (Figure 2). Yang et al. concluded that the former can use angiogenesis inhibitors to improve blood perfusion, so as to reduce the transport resistance of nanoparticles [115,163]. The latter can effectively reduce the solid stress through ablation of cells or the ECM, thus increasing the diameter of blood vessels to promote intravascular transport. Reduced blood flow directly limits the perfusion of nanoparticles into the tumor site [164]. In addition, the proliferating cancer cells in the center of the tumor tissue will form excessive pressure and compress the blood vessels and lymphatic vessels, leading to vascular collapse [88,94,95]. This results in an abundance and scarcity of functional blood vessels and lymphatic vessels in the periphery and center of the tumor, respectively [165]. This uneven distribution of blood vessels further worsens the relatively weak penetration ability of nanoparticles.
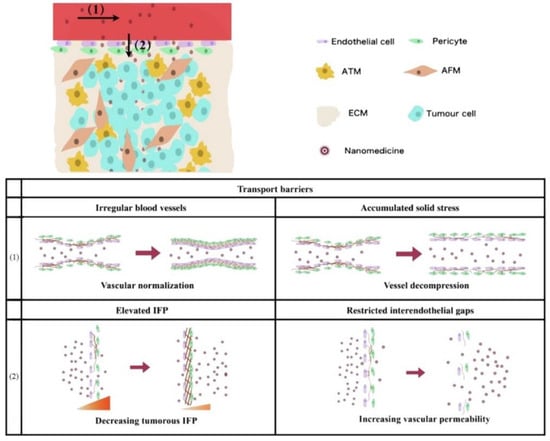
Figure 2.
Schematic representation of vascular-related problems and countermeasures faced in the process of nanodrug penetration. (1) Intravascular transport: vascular irregularity and cumulative solid stress are resolved by normalization and decompression, respectively. (2) Trans-vascular transport: elevated interstitial fluid pressure (IFP) and limited endothelial space are improved by decreasing intertumoral IFP and increasing vascular permeability, respectively. Adapted from Yang et al. [115].
Vascular promotion is a vascular regulation strategy that addresses the issue of poor accumulation and distribution of drugs in tumors via increasing the vascular density and upregulating blood perfusion. Induction of angiogenesis appears to promote tumor growth. However, moderate induction of angiogenesis or vascular promotion may also contribute to better enrichment and distribution of anticancer drugs and improve their anticancer efficacy in some tumor models [20]. Among the recently developed strategies, the use of vasodilator-encapsulated nanoparticles for tumor angiectasis has been investigated as a potential option for promoting the extravasation of nanoparticles in tumors. Some vasodilator formulation nanoparticles have been employed, including angiotensin inhibitor, antihypertensive agents, gaseous vascular mediator-generating vasodilators, and ECM degradation agents.
The change of angiotensin I to angiotensin II mediated via carboxypeptidase can be inhibited by angiotensin-converting enzyme inhibitors (ACEI). AGTR2 is an effective agent in enhancing blood flow and promoting vascular permeability in tumors due to its vasoconstrictive function in healthy tissues, as well as increasing the systemic blood pressure. It has been shown that the perfusion of tumor vessels is gradually shifted from poor to good after slow systemic administration of AGTR2 [87]. An increase in BK levels leads to the activation of endothelial NO synthase. ACEIs (e.g., captopril) inhibit the degradation of BK, thereby increasing its local concentration in tumor tissues. Captopril, an ACEI, acts by downregulating the expression of AGTR2, thereby dilating blood vessels and lowering blood pressure. A combination of captopril with paclitaxel-loaded nanoparticles has been employed to simultaneously ameliorate tumor perfusion and expand EC gaps, thus enhancing nanodrug delivery to cancer cells [132]. Meanwhile, losartan is an angiotensin II receptor antagonist that increases nanodrug delivery through two mechanisms [166]. Losartan can lower solid stress that compresses blood vessels, thus improving vessel perfusion and drug delivery. However, it also increases the intratumoral penetration of the intraperitoneally or intravenously injected nanoparticles into the tumors by decreasing the ECM [167].
In addition to AGTR2, other drugs may also expand blood vessels. Hydralazine (HDZ), a drug applied to hypertension and heart failure therapy, has been used as a tumor vasodilator to modulate the TME. It is thought that HDZ functions by dilating blood vessels. Therefore, Chen et al. prepared HDZ-encapsulated liposomes which can expand tumor vessels and strengthen tumor permeability. These liposomes also ameliorated the accumulation and permeation of nanoparticles inside the tumor. Compared with free HDZ, intravenous injection of these liposomes in desmoplastic tumor-bearing mice prolonged the blood circulation time of HDZ. Moreover, its vasodilation effect increased the penetration and accumulation of nanoparticles into tumors mediated by the EPR effect to some extent [131]. Of note, in vivo and in vitro studies have shown that HDZ exerts certain antiangiogenesis effects [168]. Therefore, such nanomedicines have great potential in upregulating tumor blood perfusion. Sildenafil, a conventional medicine utilized for pulmonary hypertension therapy, can be utilized for developing effective and tumor-selective angiectasis approaches. Sildenafil can be encapsulated into the hydrophobic core of a cisplatin-incorporated polymeric micelle to form a nanoparticle with a hydrophobic center and a dense PEG shell. This polymeric micelle is effective in dilating tumor vessels and boosting the accumulation of cisplatin–sildenafil coloaded nanoparticles in tumors [133].
Endogenous signal molecule endogenous carbon monoxide (CO) and heme oxygenase (HO) play an important role in regulating vascular tension and inducing angiogenesis [69,70]. Fang et al. clearly demonstrated that vascular permeability and blood flow were significantly increased after using CO donors or HO-1 inducers (PEGylated heme) [72]. They designed two CO generators with tumor selectivity. The first was the CO external donor tricarbonyldichlororuthenium (II) dimer nanomicelle, which can slowly enhance the release characteristics and selective accumulation of tumors mediated by the EPR effect [71]. The second was the HO-1 inducer (PEGylated hemin), which can be selectively enriched in tumors after injection and produce CO by inducing HO-1 expression in tumors [72,73]. In solid tumor models, both nanodrugs exhibited higher selectivity for CO production in tumor tissues versus normal tissues, which resulted in augmented tumor blood flow recovery [72].
Platelets preserve tumor vessel integrity and prevent nanomedicines from diffusing into solid tumors. Previous findings have shown that the specific depletion of tumor-associated platelets may be a potent approach for disrupting vascular barriers and enhancing the extravasation of nanoparticles from tumor vessels [169,170,171]. Nie et al. showed that drug delivery can be facilitated via functionalizing nanoparticles, thereby locally depleting tumor-associated platelets that normally restore the leaky vessels [172]. They developed a polymer lipid peptide nanoparticle core consisting of a charged amphiphilic polymer where the positively charged region adsorbs the antibody R300. The platelet-specific R300 antibody can bind to platelets, leading to their micro-aggregation and subsequent removal by macrophages, and further increasing the intratumoral accumulation and retention of drugs.
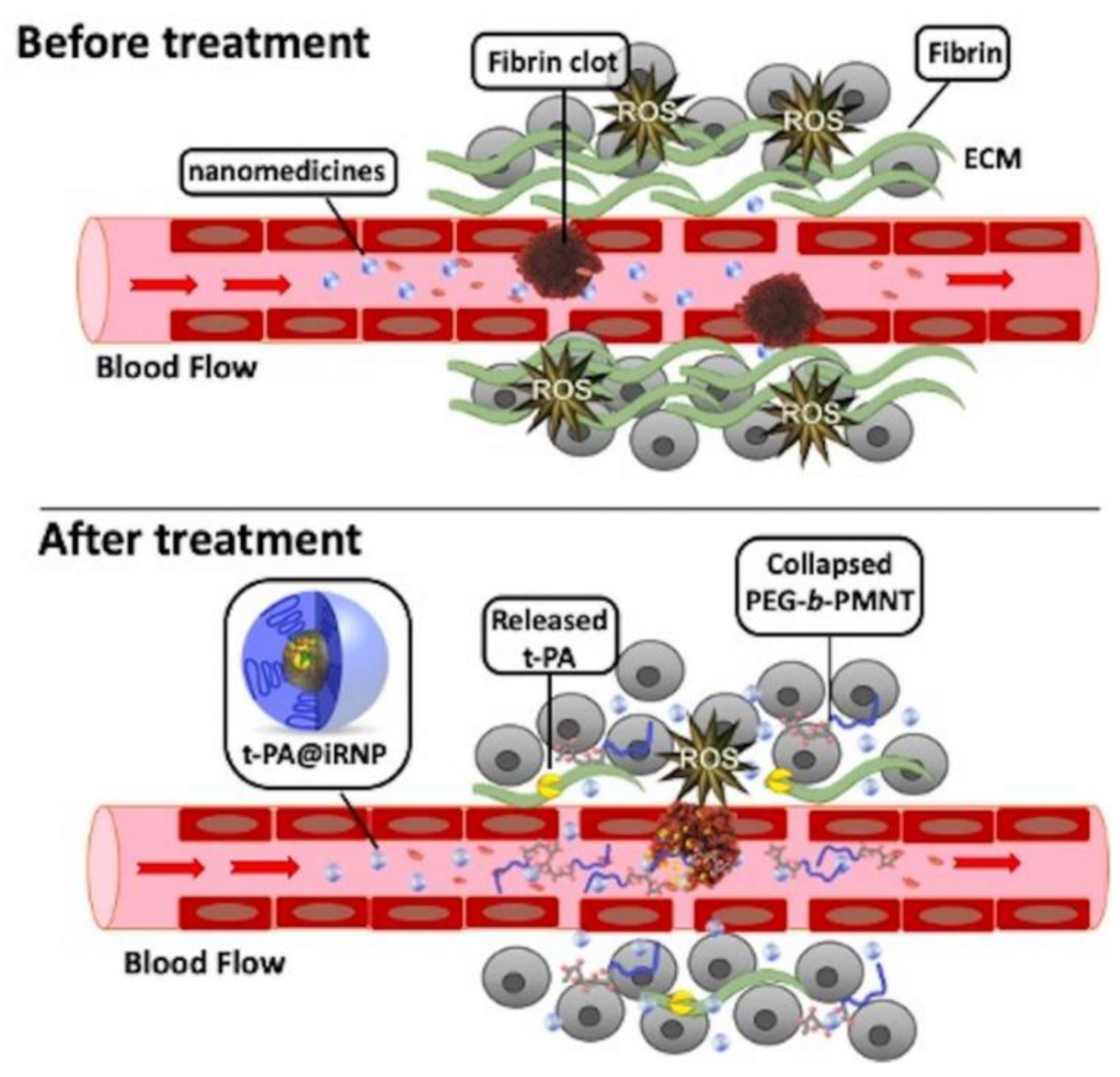
Excessive constituents of ECM, such as collagen, fibrin, laminin, elastin, and aggregated platelet in the TME, deposit in the tumor vessels [173]. This hinders the blood supply, impairing the delivery of drugs to the tumor site and reducing efficacy. However, degradation of ECM components by enzymatic treatment (e.g., collagenase) can improve the vascular properties and upregulate blood perfusion at tumor sites [174,175]. Tissue plasminogen activator (t-PA) binds to drug carriers to degrade fibrin. Mei et al. developed t-PA-assembled redox-active nanoparticles (T-PA@iRNP) by degrading fibrin to reduce the pressure on tumor blood vessels, thereby increasing the perfusion of blood and nanomedicines in tumors (Figure 3). When applied to colon cancer models, T-PA@iRNP degradation of deposited fibrin enhances the infiltration of iRNP and immune cells into tumor tissues through an increase in blood flow. This enhances the EPR effect and consequently amplifies the inhibitory effect on tumor growth [134]. Zhang et al. encapsulated DOX and near-infrared spectroscopy-activated losartan in hollow mesoporous Prussian blue nanoparticles to degrade the ECM. The results showed that losartan-containing nanoparticles can enhance the penetration of DOX, and exhibit a good tumor inhibition effect under the synergistic action of photothermal therapy/chemotherapy [135].
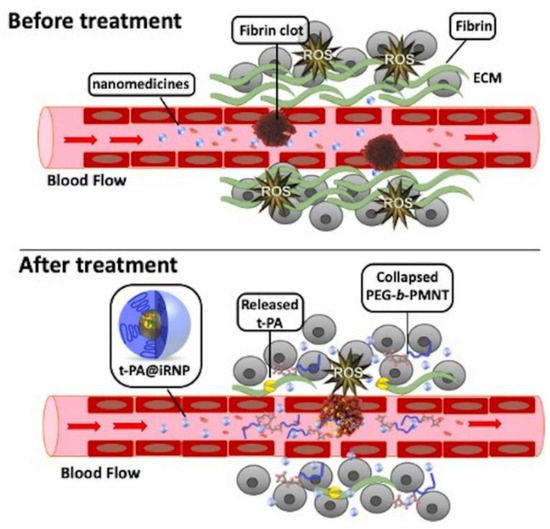
Figure 3.
Schematic representation of transmission and action in the microenvironment of large amounts of fibrin around colon tumors and their tissues. Compared with free tissue plasminogen activator (t-PA), using t-PA-assembled redox-active nanoparticles (T-PA@iRNP) can effectively relieve the compressed tumor vessels and upregulate blood perfusion, and improve the poor distribution of nanodrugs in tumors. Reproduced from Mei [134].
3.3. Enhanced Vessel Penetration
The gap in tumor vascular ECs is one of the important bases of the EPR effect. However, in some tumors with poor permeability, the size and rate limitation of large-scale nanodrugs by the vascular endothelial space cannot achieve the purpose of trans-vascular transport [115]. Therefore, augmenting the permeability of tumor vessels and even destroying the vascular system can effectively promote extravasation [176,177]. Nanodrugs with a size <10 nm can effectively permeate inside tumors via trans- and extravascular transport. However, the rapid clearance by the kidneys is a problem, resulting in an insufficient EPR effect. Nanodrugs, with size ranging 50–200 nm, can realize long-time circulation and passively target the tumor site by intravascular transport. However, due to the existence of various barriers, they often have difficulty in reaching the core of the tumor. Therefore, nanodrugs of variable size can be used to simultaneously achieve long-time circulation, good passive targeting, and high permeability [178,179,180,181,182,183].
Integrating VDAs in nanomedicines is a promising therapy for meliorating vascular permeability and the EPR effect. Several VDAs have been evaluated; for example, combretastatin A4 phosphate (CA4P) is a tubulin-binding agent which induces vessel disruption by suppressing tubulin polymerization. Furthermore, flavonoid acetic acid-based agent 5,6-dimethylxanthenone-4-acetic acid (DMXAA) increases the levels of NO and serotonin, resulting in weak endothelial function. Sengupta et al. introduced poly(lactide-co-glycolic acid) nanoparticles conjugated to DOX, which were trapped in a phospholipid block-copolymer membrane containing CA4P [136]. The nanoparticles were designed to first release CA4P, which initially induces vessel disruption, thereby creating a niche for the release of DOX. This approach was linked to significant tumor inhibition and improvement in overall survival. VDAs and other physiological agents are commonly used to enhance vascular permeability and, thus, promote the extravasation of nanoparticles. Zhang et al. developed a bioinspired nanodesign, which combined vasculature-destructive DMXAA and hypoxia-activated tirapazamine with a mesoporous silica nanoparticle core, as well as a hidden platelet membrane shell [137]. The platelet membrane can be continuously “recruited” by the tumors with characteristics of artificial blood vessel destruction. The results indicated that disruption of the tumor vasculature caused by DMXAA and the platelet membrane-mediated targeting of the intratumoral disrupted vasculature were beneficial to each other and strengthened mutually. Studies have shown that the EPR effect of nanoparticles is induced by rupture of blood vessels, which is closely related to tumor density and the speed of blood flow [176]. As mentioned above, the tumor vasculature consists of only a single layer of ECs with a missing or incomplete basement membrane [184]. Furthermore, the vasculature is closely related to the blood supply of tumor cells [185,186]. Destruction of the vasculature can significantly improve the EPR effect and, if the vasculature is inadequate, the tumor tissue will undergo programmed death [187,188,189].
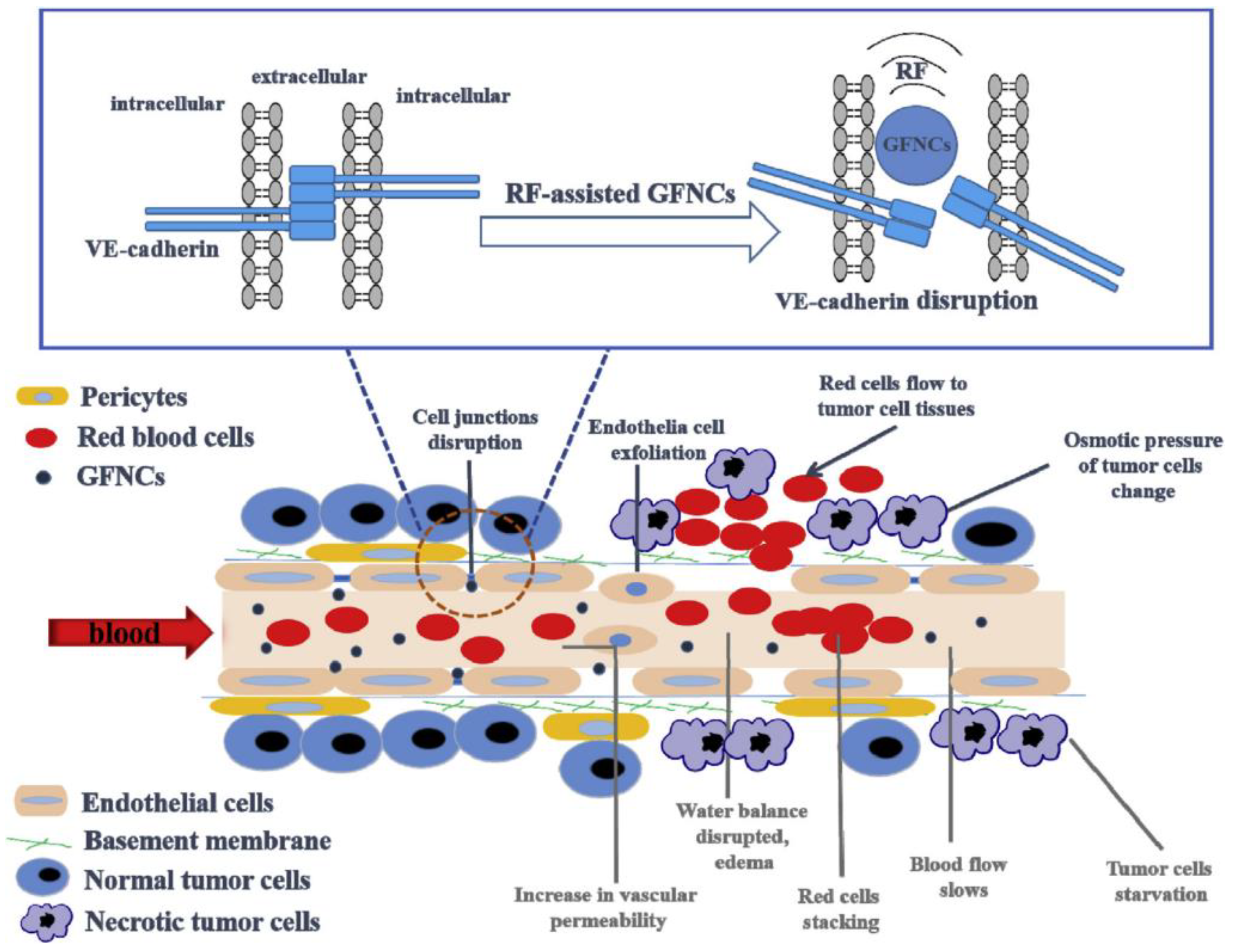
The development of strategies for interacting with ECs or destroying vascular EC connections is another effective approach to improving vascular permeability. Inspired by this, Palomba et al. transferred the purified leukocyte membrane onto nanoporous silicon particles to produce a type of leukolike vector (LLV) [138]. Multiple receptors on LLV can interact with ECs and reduce the vascular barrier function. The investigators also demonstrated that the leukocyte plasma membrane on the surface of LLV can effectively interact with the overexpressed intercellular adhesion molecule-1 (ICAM-1) in the tumor vasculature, activate the endothelial receptor ICAM-1 pathway, and boost vascular permeability through the phosphorylation of vascular endothelial cadherin. Li et al. found that phase-induced size expansion through radiofrequency-assisted gadofullerene nanocrystals (GFNCs) can destroy abnormal tumor vasculature. Biocompatible GFNCs with a nanoparticle size were designed to penetrate the leaking tumor blood vessel. With the assistance of radiofrequency, the phase transition occurs when GFNCs spill over the tumor vessels. In addition, the abrupt and drastic changes in nanoparticle structure caused by phase transition directly disrupt the abnormal tumor blood vessels (Figure 4). Treatment with this method can cause rapid ischemia, necrosis, and atrophy of tumor tissues, while significantly reducing the toxic and side effects of other antivascular treatments [139,190].

Figure 4.
Schematic diagram of the mechanism of tumor vascular rupture after radiofrequency-assisted gadofullerene nanocrystal (GFNC) treatment. GFNC particles injected intravenously into tumor-bearing mice penetrate the vulnerability of tumor vascular ECs. When radiofrequency irradiation is applied, the sudden volume expansion of GFNCs can lead to the destruction of vascular endothelial cadherin at the junction of endothelial adhesion bodies of tumor vessels, thereby increasing vascular permeability and realizing the destruction of tumor vessels. Reproduced from Li and Zhen et al. [139,190].
In addition to chemotherapy, some physical therapies can also significantly enhance blood vessel penetration and improve the effects of antitumor treatment. Ionizing irradiation can increase vascular leakiness by inducing EC apoptosis and enhancing the expression of VEGF and FGF [191]. Liang et al. designed a radioisotope therapy by encapsulating the radioisotope iodine-131 (131I)-labeled bovine serum albumin (BSA) in liposomes. 131I-BSA-liposomes were intravenously injected into 4T1 tumor-bearing mice. Compared with untreated mice, those treated with 131I -BSA-liposomes showed high retention in the tumor site, demonstrating enhanced tumor vascular permeability and improved EPR effect [140]. Koukourakis et al. underlined the value of combining radiotherapy with drug delivery systems based on nanomedicines [192]. Patients were treated with radiolabeled PEGylated liposomal DOX, and achieved an overall remission rate >70%. This is an effective anticancer treatment modality for inducing hyperthermia in tumors. This generally leads to an increase in blood flow and vascular permeability in tumors, thus promoting drug and oxygen supply to tumors [193]. Hyperthermia can be applied to increase the EPR effect, particularly in nonleaky tumors with low baseline levels of nanomedicine accumulation [194]. Temperature-sensitive liposomes have developed into an ideal nanocarrier for coadministration with hyperthermia, enabling triggered drug release locally at the heated tumor site. Several studies have demonstrated that drug delivery and intratumoral distribution can be ameliorated through combining temperature-sensitive liposomes with modest hyperthermia. It was found that the human ovarian carcinoma tumor model was rather impermeable to liposomes with a size of 100 nm at room temperature. However, as the temperature increased, the release of liposomes was significantly elevated [195]. Manzoor et al. established temperature-sensitive liposomes containing DOX, which can enhance blood vessel penetration and liposome accumulation [141].
4. Conclusions and Future Perspectives
The EPR effect, which involves the pathophysiological mediators and unique anatomical architecture of tumor tissues, is becoming a promising avenue for targeted anti-tumor therapy. Thus, the tumor-selective delivery of anticancer nanomedicines based on the EPR effect is becoming possible. However, the EPR effect can be highly heterogeneous. Specifically, in the complex tumor environment, it is difficult for nanoparticles to diffuse into vascular areas of the tumor due to high IFP, abnormal ECM, and massive interaction sites in the tumor. Hence, in the last couple of years, on the basis of the EPR effect, scientists have investigated other mechanisms of nanoparticle entry into solid tumors [196]. Recently, Sindhwani et al. proposed that most of the tumor vasculature is continuous and does not have sufficient EC gaps to explain the accumulation of nanoparticles in tumors. Moreover, they stated that most nanoparticles can reach the interior of the tumor via active trans-endothelial pathways rather than passive transport via gaps [197]. Although they found that the trans-endothelial pathways play a significant role in the accumulation of nanoparticles in tumor sites, their experimental method had certain limitations. Firstly, they only utilized PEGylated gold nanoparticles as simulated nanoparticles to examine the accumulation in the tumor, and could not cover the accumulation of other nanoparticles in tumors. Secondly, they used a Zombie mouse model to distinguish the contribution of the passive gap from active trans-endothelial transport. This model could deactivate active mechanisms and retain the passive way that fixed the mouse by transcardiac perfusion and relied on a peristaltic pump to retain a physiologically relevant flow rate. This could not simulate the blood vessels and blood flow under normal physiological conditions. Lastly, the blood driven by the peristaltic pump only circulated for a short period of time (15 and 60 min). In summary, trans-endothelial pathways may be a reason for the accumulation of nanoparticles in tumor sites; nevertheless, the EPR effect remains the basis of nanodrug delivery to tumors. Furthermore, nanoparticles which can improve tumor vessel penetration, reduce IFP, and degrade the ECM can be applied to enhance the EPR effect [26].
Herein, we summarized the mechanism of abnormal vascular functions, such as tumor angiogenesis, irregular blood flow, and extensive vascular permeability, as well as their influence on the EPR effect. In addition, we analyzed some nanoparticles developed to facilitate the EPR effect in tumors in response to the above factors. In terms of antiangiogenesis, gene therapy nanomedicines targeting angiogenic growth factors and their receptors are the most widely studied, and offer another approach to directly inhibiting tumor angiogenesis early in the process. Its diverse nanocarrier form provides a rich selection for delivery to different types of tumors. For irregular blood flow caused by abnormal vascular morphology and structure, blood perfusion can be effectively upregulated by slight vascular facilitation, vasodilation, or removal of excessive ECM in the TME. Nanoparticles encapsulated with different types of drugs exhibit the diversity and universality of nanocarriers, providing more possibilities for the selection of nanodrugs. However, EPR effect-based drug delivery strategies continue to be characterized by numerous problems and limitations. For example, enhancing the EPR effect may help maintain nutrient and oxygen transport, thereby accelerating tumor growth. Therefore, when designing such nanoparticles, it is particularly important to properly balance the relationship between tumor killing or inhibition and tumor growth promotion caused by the EPR effect.
Author Contributions
Conceptualization, L.H. and Y.C.; writing, review, and editing, Y.C., D.H., and L.S.; preparation of tables and figures, D.H. and L.S.; revision, Y.C., L.H., and L.S.; funding acquisition, L.H. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work of L.H. was funded by the Carolina Center for Cancer Nanotechnology Excellence, United States (NIH grant CA198999). Y.C. is supported by the Natural Science Foundation of Shanghai (19ZR1472500).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACEI | angiotensin-converting enzyme inhibitor |
| AGTR2 | angiotensin receptor type 2 |
| BK | bradykinin |
| BSA | bovine serum albumin |
| CA4P | combretastatin A4 phosphate |
| CO | carbon monoxide |
| DMXAA | 5,6-dimethylxanthenone-4-acetic acid |
| DOX | doxorubicin |
| EC | endothelial cell |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| EPR | enhanced permeability and retention |
| Et-A/B | endothelin-A/B |
| FGF | fibroblast growth factor |
| FLK1/KDR | vascular endothelial growth factor receptor 2 |
| FLT1 | vascular endothelial growth factor receptor 1 |
| GFNC | gadofullerene nanocrystal |
| HDZ | hydralazine |
| HGF | hepatocyte growth factor |
| HIF | hypoxia-inducible factor |
| HO | heme oxygenase |
| 131I | iodine-131 |
| ICAM-1 | intercellular adhesion molecule-1 |
| IFP | interstitial fluid pressure |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| LLV | leukolike vector |
| MMP | matrix metalloproteinase |
| NO | nitric oxide |
| NF-κB | nuclear factor kappa-B |
| PDGF | platelet-derived growth factor |
| PDGFR | platelet-derived growth factor receptor |
| PEG | polyethylene glycol |
| PLGF | placenta growth factor |
| RGD | arginylglycylaspartic acid |
| RNAi | RNA interference |
| SDF-1 | stromal cell-derived factor 1 |
| sFLT1 | soluble vascular endothelial growth factor receptor 1 |
| shRNA | short hairpin RNA |
| siRNA | small interfering RNA |
| tetrac | tetraiodothyroacetic acid |
| TGF-β | transforming growth factor-β |
| TIMP | tissue inhibitor of metalloproteinase |
| TME | tumor microenvironment |
| TNF-α | tumor necrosis factor-α |
| t-PA | tissue plasminogen activator |
| VDA | vascular disrupting agent |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| VPF | vascular permeability factor |
References
- Sudhakar, A. History of Cancer, Ancient and Modern Treatment Methods. J. Cancer Sci. Ther. 2009, 1, 1–4. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1252–1276. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K.; Maheshwari, R.; Soni, N.; Tekade, M.; Chougule, M.B. Chapter 1 -Nanotechnology for the Development of Nanomedicine. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Academic Press: Cambridge, MA, USA, 2017; pp. 3–61. [Google Scholar] [CrossRef]
- Nagy, J.A.; Chang, S.H.; Dvorak, A.M.; Dvorak, H.F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 2009, 100, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef]
- Greish, K. Enhanced permeability and retention effect for selective targeting of anticancer nanomedicine: Are we there yet? Drug Discov. Today Technol. 2012, 9, 71–174. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl. Cancer Inst. 1990, 82, 4–6. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, Y.; Yang, W.; Niu, P.; Li, X.; Chen, Y.; Li, Z.; Liu, Y.; An, Y.; Liu, Y.; et al. Investigating the EPR effect of nanomedicines in human renal tumors via ex vivo perfusion strategy. Nano Today 2020, 35. [Google Scholar] [CrossRef]
- Nagamitsu, A.; Greish, K.; Maeda, H. Elevating blood pressure as a strategy to increase tumor-targeted delivery of macromolecular drug SMANCS: Cases of advanced solid tumors. Jpn J. Clin. Oncol. 2009, 39, 756–766. [Google Scholar] [CrossRef]
- Satchi-Fainaro, R.; Duncan, R.; Barnes, C.M. Polymer Therapeutics for Cancer: Current Status and Future Challenges. In Polymer Therapeutics II; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–65. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Danquah, M.K.; Zhang, X.A.; Mahato, R.I. Extravasation of polymeric nanomedicines across tumor vasculature. Adv. Drug Deliv. Rev. 2011, 63, 623–639. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil(R)—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.T.W.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017, 119, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Stylianopoulos, T.; Martin, J.D.; Popovic, Z.; Chen, O.; Kamoun, W.S.; Bawendi, M.G.; Fukumura, D.; Jain, R.K. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012, 7, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Carroll, F.; Illingworth, S.; Green, N.; Cawood, R.; Bachtarzi, H.; Subr, V.; Fisher, K.D.; Seymour, L.W. Tumour necrosis factor-alpha increases extravasation of virus particles into tumour tissue by activating the Rho A/Rho kinase pathway. J. Control. Release 2011, 156, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.T.; Gerstner, E.R.; Emblem, K.E.; Duda, D.G.; Kalpathy-Cramer, J.; Snuderl, M.; Ancukiewicz, M.; Polaskova, P.; Pinho, M.C.; Jennings, D.; et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl. Acad. Sci. USA 2013, 110, 19059–19064. [Google Scholar] [CrossRef] [PubMed]
- Satterlee, A.B.; Rojas, J.D.; Dayton, P.A.; Huang, L. Enhancing Nanoparticle Accumulation and Retention in Desmoplastic Tumors via Vascular Disruption for Internal Radiation Therapy. Theranostics 2017, 7, 253–269. [Google Scholar] [CrossRef]
- Ganten, D.; Ruckpaul, K. Tumor Angiogenesis. In Encyclopedic Reference of Genomics and Proteomics in Molecular Medicine; Springer: Berlin/Heidelberg, Germany, 2006; p. 1930. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Koukouraki, S.; Giatromanolaki, A.; Archimandritis, S.C.; Skarlatos, J.; Beroukas, K.; Bizakis, J.G.; Retalis, G.; Karkavitsas, N.; Helidonis, E.S. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J. Clin. Oncol. 1999, 17, 3512–3521. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.T.; Boucher, Y.; Kozin, S.V.; Winkler, F.; Hicklin, D.J.; Jain, R.K. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004, 64, 3731–3736. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit. Rev. Oncol. Hematol. 2007, 62, 179–213. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Fang, J.; Inutsuka, T.; Kitamoto, Y. Vascular permeability enhancement in solid tumor: Various factors, mechanisms involved and its implications. Int. Immunopharmacol. 2003, 3, 319–328. [Google Scholar] [CrossRef]
- Li, B.; Vincent, A.; Cates, J.; Brantley-Sieders, D.M.; Polk, D.B.; Young, P.P. Low Levels of Tumor Necrosis Factor α Increase Tumor Growth by Inducing an Endothelial Phenotype of Monocytes Recruited to the Tumor Site. Cancer Res. 2009, 69, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, A.; Garcia-Cardena, G.; Madri, J.A.; Sessa, W.C. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J. Clin. Investig. 1997, 100, 3131–3139. [Google Scholar] [CrossRef]
- Murohara, T.; Horowitz, J.R.; Silver, M.; Tsurumi, Y.; Chen, D.; Sullivan, A.; Isner, J.M. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation 1998, 97, 99–107. [Google Scholar] [CrossRef]
- Friedl, J.; Puhlmann, M.; Bartlett, D.L.; Libutti, S.K.; Turner, E.N.; Gnant, M.F.; Alexander, H.R. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: Relationship between the procoagulant and permeability effects of TNF. Blood 2002, 100, 1334–1339. [Google Scholar] [CrossRef]
- Compagni, A.; Wilgenbus, P.; Impagnatiello, M.-A.; Cotten, M.; Christofori, G. Fibroblast Growth Factors Are Required for Efficient Tumor Angiogenesis. Cancer Res. 2000, 60, 7163–7169. [Google Scholar]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, G.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: An autocrine mechanism contributing to angiogenesis. J. Cell Biol. 1998, 141, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Millauer, B.; Wizigmann-Voos, S.; Schnurch, H.; Martinez, R.; Moller, N.P.; Risau, W.; Ullrich, A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72, 835–846. [Google Scholar] [CrossRef]
- Laschke, M.W.; Elitzsch, A.; Vollmar, B.; Vajkoczy, P.; Menger, M.D. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet-derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Hum. Reprod 2006, 21, 262–268. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.P.; Pei, Z.H.; Ren, J. Making up or breaking up: The tortuous role of platelet-derived growth factor in vascular ageing. Clin. Exp. Pharmacol. Physiol. 2009, 36, 739–747. [Google Scholar] [CrossRef]
- Taylor, A.P.; Rodriguez, M.; Adams, K.; Goldenberg, D.M.; Blumenthal, R.D. Altered tumor vessel maturation and proliferation in placenta growth factor-producing tumors: Potential relationship to post-therapy tumor angiogenesis and recurrence. Int. J. Cancer 2003, 105, 158–164. [Google Scholar] [CrossRef]
- Ellis, L.M. Epidermal growth factor receptor in tumor angiogenesis. Hematol. Oncol. Clin. N. Am. 2004, 18, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Tamatani, T.; Hattori, K.; Iyer, A.; Tamatani, K.; Oyasu, R. Hepatocyte growth factor is an invasion/migration factor of rat urothelial carcinoma cells in vitro. Carcinogenesis 1999, 20, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Lu, K.V.; Petritsch, C.; Liu, P.; Ganss, R.; Passegue, E.; Song, H.; Vandenberg, S.; Johnson, R.S.; Werb, Z.; et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 2008, 13, 206–220. [Google Scholar] [CrossRef]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, I.; Phang, B.H.; Othman, R.; Tan, S.Y.; Vijayaraghavan, A.; Goh, L.K.; Martin-Lopez, M.; Marques, M.M.; Li, C.W.; Wang de, Y.; et al. Hypoxia-inducible TAp73 supports tumorigenesis by regulating the angiogenic transcriptome. Nat. Cell Biol. 2015, 17, 511–523. [Google Scholar] [CrossRef]
- Palucka, A.K.; Coussens, L.M. The Basis of Oncoimmunology. Cell 2016, 164, 1233–1247. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Cook, B.D.; Terushkin, V.; Pintucci, G.; Mignatti, P. Transforming growth factor-beta 1 (TGF-β1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J. Cell Physiol. 2009, 219, 449–458. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Chae, B.-C.; Kim, H.-A.; Seo, G.-Y.; Seo, D.-W.; Chun, G.-T.; Kim, N.-S.; Yie, S.-W.; Byeon, W.-H.; Eom, S.-H.; et al. Mechanisms underlying TGF-β1-induced expression of VEGF and Flk-1 in mouse macrophages and their implications for angiogenesis. J. Leukoc Biol. 2007, 81, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Stoeltzing, O.; Liu, W.; McCarty, M.F.; Jung, Y.D.; Reinmuth, N.; Ellis, L.M. Interleukin-1β Regulates Angiopoietin-1 Expression in Human Endothelial Cells. Cancer Res. 2004, 64, 3186–3190. [Google Scholar] [CrossRef] [PubMed]
- Dentelli, P.; Sorbo, L.D.; Rosso, A.; Molinar, A.; Garbarino, G.; Camussi, G.; Pegoraro, L.; Brizzi, M.F. Human IL-3 Stimulates Endothelial Cell Motility and Promotes In Vivo New Vessel Formation. J. Immunol. 1999, 163, 2151–2159. [Google Scholar]
- Loeffler, S.; Fayard, B.; Weis, J.; Weissenberger, J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int. J. Cancer 2005, 115, 202–213. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Roy Choudhury, S.; Karmakar, S.; Banik, N.L.; Ray, S.K. Targeting Angiogenesis for Controlling Neuroblastoma. J. Oncol. 2012, 2012, 782020. [Google Scholar] [CrossRef] [PubMed]
- Oehler, M.K.; Hague, S.; Rees, M.C.P.; Bicknell, R. Adrenomedullin promotes formation of xenografted endometrial tumors by stimulation of autocrine growth and angiogenesis. Oncogene 2002, 21, 2815–2821. [Google Scholar] [CrossRef][Green Version]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Oga, M.; Takenaga, K.; Sato, Y.; Nakajima, H.; Koshikawa, N.; Osato, K.; Sakiyama, S. Inhibition of metastatic brain tumor growth by intramuscular administration of the endostatin gene. Int. J. Oncol. 2003, 23, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, Y.; Hu, B.; Jarzynka, M.J.; Guo, P.; Elishaev, E.; Bar-Joseph, I.; Cheng, S.Y. Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res. 2007, 67, 4254–4263. [Google Scholar] [CrossRef]
- Srivastava, K.; Hu, J.; Korn, C.; Savant, S.; Teichert, M.; Kapel, S.S.; Jugold, M.; Besemfelder, E.; Thomas, M.; Pasparakis, M.; et al. Postsurgical Adjuvant Tumor Therapy by Combining Anti-Angiopoietin-2 and Metronomic Chemotherapy Limits Metastatic Growth. Cancer Cell 2014, 26, 880–895. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.D.; Yang, X.; Shao, C.K.; Xu, Z.M.; Cheng, R.; Cai, W.B.; Ma, J.X.; Yang, Z.H.; Gao, G.Q. Pigment epithelium-derived factor inhibits angiogenesis and growth of gastric carcinoma by down-regulation of VEGF. Oncol. Rep. 2011, 26, 681–686. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, J.; Rychahou, P.G.; Qiu, S.M.; Evers, B.M.; Zhou, B.P.H. Stabilization of Snail by NF-kappa B Is Required for Inflammation-Induced Cell Migration and Invasion. Cancer Cell 2009, 15, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.B.; Mousa, S.A.; O′Connor, L.; Mohamed, S.; Lin, H.Y.; Cao, H.J.; Davis, P.J. Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ. Res. 2004, 94, 1500–1506. [Google Scholar] [CrossRef]
- Itoh, T.; Tanioka, M.; Yoshida, H.; Yoshioka, T.; Nishimoto, H.; Itohara, S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998, 58, 1048–1051. [Google Scholar]
- Motterlini, R.; Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.Z.; Fang, J.; Liao, L.; Nakamura, H.; Maeda, H. Styrene-maleic acid copolymer-encapsulated CORM2, a water-soluble carbon monoxide (CO) donor with a constant CO-releasing property, exhibits therapeutic potential for inflammatory bowel disease. J. Control. Release 2014, 187, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Qin, H.; Nakamura, H.; Tsukigawa, K.; Shin, T.; Maeda, H. Carbon monoxide, generated by heme oxygenase-1, mediates the enhanced permeability and retention effect in solid tumors. Cancer Sci. 2012, 103, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Qin, H.B.; Seki, T.; Nakamura, H.; Tsukigawa, K.; Shin, T.; Maeda, H. Therapeutic Potential of Pegylated Hemin for Reactive Oxygen Species-Related Diseases via Induction of Heme Oxygenase-1: Results from a Rat Hepatic Ischemia/Reperfusion Injury Model. J. Pharmacol. Exp. Ther. 2011, 339, 779–789. [Google Scholar] [CrossRef]
- Moroianu, J.; Riordan, J.F. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc. Natl. Acad. Sci. USA 1994, 91, 1677–1681. [Google Scholar] [CrossRef]
- Eilken, H.M.; Adams, R.H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010, 22, 617–625. [Google Scholar] [CrossRef]
- Rolny, C.; Mazzone, M.; Tugues, S.; Laoui, D.; Johansson, I.; Coulon, C.; Squadrito, M.L.; Segura, I.; Li, X.J.; Knevels, E.; et al. HRG Inhibits Tumor Growth and Metastasis by Inducing Macrophage Polarization and Vessel Normalization through Downregulation of PIGF. Cancer Cell 2011, 19, 31–44. [Google Scholar] [CrossRef]
- Facciabene, A.; Peng, X.H.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L.; et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T-reg cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef]
- Yu, H.S.; Lin, T.H.; Tang, C.H. Involvement of intercellular adhesion molecule-1 up-regulation in bradykinin promotes cell motility in human prostate cancers. Int. J. Mol. Sci. 2013, 14, 13329–13345. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.; Greif, D.; Michel, T. Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporin A. J. Biol. Chem. 2002, 277, 29669–29673. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kimura, M.; Yamamoto, T.; Maeda, H. Involvement of the kinin-generating cascade in enhanced vascular permeability in tumor tissue. Jpn J. Cancer Res. 1988, 79, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsumura, Y.; Kato, H. Purification and identification of [hydroxyprolyl3]bradykinin in ascitic fluid from a patient with gastric cancer. J. Biol. Chem. 1988, 263, 16051–16054. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maruo, K.; Kimura, M.; Yamamoto, T.; Konno, T.; Maeda, H. Kinin-generating cascade in advanced cancer patients and in vitro study. Jpn J. Cancer Res. 1991, 82, 732–741. [Google Scholar] [CrossRef]
- Wu, J.; Akaike, T.; Maeda, H. Modulation of enhanced vascular permeability in tumors by a bradykinin antagonist, a cyclooxygenase inhibitor, and a nitric oxide scavenger. Cancer Res. 1998, 58, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Akaike, T.; Hayashida, K.; Okamoto, T.; Okuyama, A.; Maeda, H. Enhanced vascular permeability in solid tumor involving peroxynitrite and matrix metalloproteinases. Jpn J. Cancer Res. 2001, 92, 439–451. [Google Scholar] [CrossRef]
- Maeda, H.; Noguchi, Y.; Sato, K.; Akaike, T. Enhanced vascular permeability in solid tumor is mediated by nitric oxide and inhibited by both new nitric oxide scavenger and nitric oxide synthase inhibitor. Jpn J. Cancer Res. 1994, 85, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Akaike, T.; Wu, J.; Fang, J.; Sawa, T.; Ogawa, M.; Beppu, T.; Maeda, H. Modulation of tumor-selective vascular blood flow and extravasation by the stable prostaglandin 12 analogue beraprost sodium. J. Drug Target. 2003, 11, 45–52. [Google Scholar] [CrossRef]
- Hori, K.; Suzuki, M.; Tanda, S.; Saito, S.; Shinozaki, M.; Zhang, Q.H. Fluctuations in tumor blood flow under normotension and the effect of angiotensin II-induced hypertension. Jpn. J. Cancer Res. 1991, 82, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Omidi, Y.; Barar, J. Targeting tumor microenvironment: Crossing tumor interstitial fluid by multifunctional nanomedicines. Bioimpacts 2014, 4, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Tredan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef]
- Nagy, J.A.; Vasile, E.; Feng, D.; Sundberg, C.; Brown, L.F.; Detmar, M.J.; Lawitts, J.A.; Benjamin, L.; Tan, X.L.; Manseau, E.J.; et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J. Exp. Med. 2002, 196, 1497–1506. [Google Scholar] [CrossRef]
- Huang, Y.H.; Yuan, J.P.; Righi, E.; Duda, D.G.; Fukumura, D.; Poznanasky, M.C.; Jain, R.K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef]
- Cooke, V.G.; LeBleu, V.S.; Keskin, D.; Khan, Z.; O′Conne, J.T.; Teng, Y.; Duncan, M.B.; Xie, L.; Maeda, G.; Vong, S.; et al. Pericyte Depletion Results in Hypoxia-Associated Epithelial-to-Mesenchymal Transition and Metastasis Mediated by Met Signaling Pathway. Cancer Cell 2012, 21, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Nam, J.O.; Jean, C.; Lawson, C.; Walsh, C.T.; Goka, E.; Lim, S.T.; Tomar, A.; Tancioni, I.; Uryu, S.; et al. VEGF-Induced Vascular Permeability Is Mediated by FAK. Dev. Cell 2012, 22, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Padera, T.P.; Stoll, B.R.; Tooredman, J.B.; Capen, D.; di Tomaso, E.; Jain, R.K. Pathology: Cancer cells compress intratumour vessels. Nature 2004, 427, 695. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, H.; Heikamp, E.; Turley, H.; Dragovic, R.; Thomas, P.; Oon, C.E.; Leek, R.; Edelmann, M.; Kessler, B.; Sainson, R.C.; et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood 2010, 116, 2385–2394. [Google Scholar] [CrossRef]
- Oon, C.E.; Bridges, E.; Sheldon, H.; Sainson, R.C.A.; Jubb, A.; Turley, H.; Leek, R.; Buffa, F.; Harris, A.L.; Li, J.L. Role of Delta-like 4 in Jagged1-induced tumour angiogenesis and tumour growth. Oncotarget 2017, 8, 40115–40131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, C.G.; Heijn, M.; di Tomaso, E.; Griffon-Etienne, G.; Ancukiewicz, M.; Koike, C.; Park, K.R.; Ferrara, N.; Jain, R.K.; Suit, H.D.; et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000, 60, 5565–5570. [Google Scholar]
- Luttun, A.; Tjwa, M.; Carmeliet, P. Placental growth factor (PIGF) and its receptor Flt-1 (VEGFR-1)—Novel therapeutic targets for angiogenic disorders. Ann. N. Y. Acad. Sci. 2002, 979, 80–93. [Google Scholar] [CrossRef]
- Shahneh, F.Z.; Baradaran, B.; Zamani, F.; Aghebati-Maleki, L. Tumor angiogenesis and anti-angiogenic therapies. Hum. Antibodies 2013, 22, 15–19. [Google Scholar] [CrossRef]
- Ribatti, D. The discovery of angiogenic growth factors: The contribution of Italian scientists. Vasc. Cell 2014, 6, 8–14. [Google Scholar] [CrossRef]
- Peterson, T.; Kirkpatrick, N.; Huang, Y.H.; Farrar, C.; Marjit, K.; Kloepper, J.; Datta, M.; Amoozgar, Z.; Seano, G.; Jung, K.; et al. Dual Inhibition of Ang-2 and Vegf Receptors Normalizes Tumor Vasculature and Prolongs Survival in Glioblastoma by Altering Macrophages. Proc. Natl. Acad. Sci. USA 2016, 113, 4470–4475. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Chow, M.T.; Luster, A.D. Chemokines in cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hori, K.; Abe, I.; Saito, S.; Sato, H. A new approach to cancer chemotherapy: Selective enhancement of tumor blood flow with angiotensin II. J. Natl. Cancer Inst. 1981, 67, 663–669. [Google Scholar] [CrossRef]
- Hori, K.; Saito, S.; Takahashi, H.; Sato, H.; Maeda, H.; Sato, Y. Tumor-selective blood flow decrease induced by an angiotensin converting enzyme inhibitor, temocapril hydrochloride. Jpn. J. Cancer Res. 2000, 91, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Stylianopoulos, T.; Boucher, Y.; Jain, R.K. Delivery of Molecular and Nanoscale Medicine to Tumors: Transport Barriers and Strategies. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 281–298. [Google Scholar] [CrossRef]
- Fang, J.; Sawa, T.; Maeda, H. Factors and mechanism of “EPR” effect and the enhanced antitumor effects of macromolecular drugs including SMANCS. Adv. Exp. Med. Biol. 2003, 519, 29–49. [Google Scholar] [CrossRef]
- Greish, K.; Fang, J.; Inutsuka, T.; Nagamitsu, A.; Maeda, H. Macromolecular therapeutics: Advantages and prospects with special emphasis on solid tumour targeting. Clin. Pharmacokinet 2003, 42, 1089–1105. [Google Scholar] [CrossRef] [PubMed]
- Daruwalla, J.; Greish, K.; Malcontenti-Wilson, C.; Muralidharan, V.; Iyer, A.; Maeda, H.; Christophi, C. Styrene maleic acid-pirarubicin disrupts tumor microcirculation and enhances the permeability of colorectal liver metastases. J. Vasc. Res. 2009, 46, 218–228. [Google Scholar] [CrossRef]
- Sun, C.; Jain, R.K.; Munn, L.L. Non-uniform plasma leakage affects local hematocrit and blood flow: Implications for inflammation and tumor perfusion. Ann. Biomed. Eng. 2007, 35, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Jain, R.K. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 18632–18637. [Google Scholar] [CrossRef]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, Y.; Fu, M.; Feng, Y.; Lin, G.; Kong, D.; Jiang, B. Simulation study of the effects of interstitial fluid pressure and blood flow velocity on transvascular transport of nanoparticles in tumor microenvironment. Comput. Methods Programs Biomed. 2020, 193, 105493. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tong, Z.; Sun, S.; Mao, Z. Enhancement of tumour penetration by nanomedicines through strategies based on transport processes and barriers. J. Control. Release 2020, 328, 28–44. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Secomb, T.W. Transport of drugs from blood vessels to tumour tissue. Nat. Rev. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Plendl, J.; Snyman, C.; Naidoo, S.; Sawant, S.; Mahabeer, R.; Bhoola, K.D. Expression of tissue kallikrein and kinin receptors in angiogenic microvascular endothelial cells. Biol. Chem. 2000, 381, 1103–1115. [Google Scholar] [CrossRef]
- Seki, T.; Fang, J.; Maeda, H. Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application. Cancer Sci. 2009, 100, 2426–2430. [Google Scholar] [CrossRef]
- Maeda, H.; Tsukigawa, K.; Fang, J. A Retrospective 30 Years After Discovery of the Enhanced Permeability and Retention Effect of Solid Tumors: Next-Generation Chemotherapeutics and Photodynamic Therapy—Problems, Solutions, and Prospects. Microcirculation 2016, 23, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Shenoi, M.M.; Iltis, I.; Choi, J.; Koonce, N.A.; Metzger, G.J.; Griffin, R.J.; Bischof, J.C. Nanoparticle delivered vascular disrupting agents (VDAs): Use of TNF-alpha conjugated gold nanoparticles for multimodal cancer therapy. Mol. Pharm. 2013, 10, 1683–1694. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Cho, J.Y.; Switzer, C.H. Chapter 2—The Chemical Properties of Nitric Oxide and Related Nitrogen Oxides. In Nitric Oxide; Ignarro, L.J., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 23–40. [Google Scholar] [CrossRef]
- Jordan, B.F.; Misson, P.; Demeure, R.; Baudelet, C.; Beghein, N.; Gallez, B. Changes in tumor oxygenation/perfusion induced by the no donor, isosorbide dinitrate, in comparison with carbogen: Monitoring by EPR and MRI. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 565–570. [Google Scholar] [CrossRef]
- Kim, W.J.; Yockman, J.W.; Jeong, J.H.; Christensen, L.V.; Lee, M.; Kim, Y.H.; Kim, S.W. Anti-angiogenic inhibition of tumor growth by systemic delivery of PEI-g-PEG-RGD/pCMV-sFlt-1 complexes in tumor-bearing mice. J. Control. Release 2006, 114, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, S.W.; Kim, W.J. PEI-g-PEG-RGD/small interference RNA polyplex-mediated silencing of vascular endothelial growth factor receptor and its potential as an anti-angiogenic tumor therapeutic strategy. Oligonucleotides 2011, 21, 101–107. [Google Scholar] [CrossRef]
- Bharali, D.J.; Yalcin, M.; Davis, P.J.; Mousa, S.A. Tetraiodothyroacetic acid-conjugated PLGA nanoparticles: A nanomedicine approach to treat drug-resistant breast cancer. Nanomedicine 2013, 8, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Rebbaa, A.; Chu, F.; Davis, F.B.; Davis, P.J.; Mousa, S.A. Novel function of the thyroid hormone analog tetraiodothyroacetic acid: A cancer chemosensitizing and anti-cancer agent. Angiogenesis 2008, 11, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; Bergh, J.J.; Dier, E.; Rebbaa, A.; O’Connor, L.J.; Yalcin, M.; Aljada, A.; Dyskin, E.; Davis, F.B.; Lin, H.Y.; et al. Tetraiodothyroacetic acid, a small molecule integrin ligand, blocks angiogenesis induced by vascular endothelial growth factor and basic fibroblast growth factor. Angiogenesis 2008, 11, 183–190. [Google Scholar] [CrossRef]
- Rajabi, M.; Sudha, T.; Darwish, N.H.E.; Davis, P.J.; Mousa, S.A. Synthesis of MR-49, a deiodinated analog of tetraiodothyroacetic acid (tetrac), as a novel pro-angiogenesis modulator. Bioorg. Med. Chem. Lett. 2016, 26, 4112–4116. [Google Scholar] [CrossRef]
- Chen, Y.; Song, W.; Shen, L.; Qiu, N.; Hu, M.; Liu, Y.; Liu, Q.; Huang, L. Vasodilator Hydralazine Promotes Nanoparticle Penetration in Advanced Desmoplastic Tumors. ACS Nano 2019, 13, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiang, T.; Tuo, Y.Y.; Jin, K.; Luo, Z.M.; Shi, W.; Mei, H.; Hu, Y.; Pang, Z.Q.; Jiang, X.G. Captopril improves tumor nanomedicine delivery by increasing tumor blood perfusion and enlarging endothelial gaps in tumor blood vessels. Cancer Lett. 2017, 410, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, Y.; Ding, X.Y.; Xiao, C.S.; Chen, X.S. Enhanced nanoparticle accumulation by tumor-acidity-activatable release of sildenafil to induce vasodilation. Biomater. Sci. 2020, 8, 3052–3062. [Google Scholar] [CrossRef] [PubMed]
- Mei, T.; Shashni, B.; Maeda, H.; Nagasaki, Y. Fibrinolytic tissue plasminogen activator installed redox-active nanoparticles (t-PA@iRNP) for cancer therapy. Biomaterials 2020, 259, 120290. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Gao, X.; Li, X.; Niu, X.; Yuan, Z.; Wang, W. Near-infrared-light induced nanoparticles with enhanced tumor tissue penetration and intelligent drug release. Acta Biomater. 2019, 90, 314–323. [Google Scholar] [CrossRef]
- Sengupta, S.; Eavarone, D.; Capila, I.; Zhao, G.; Watson, N.; Kiziltepe, T.; Sasisekharan, R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 2005, 436, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.K.; Ye, J.J.; Xia, Y.; Wang, Z.Y.; Li, C.X.; Wang, X.S.; Yu, W.Y.; Song, W.; Feng, J.; Zhang, X.Z. Platelet-Mimicking Biotaxis Targeting Vasculature-Disrupted Tumors for Cascade Amplification of Hypoxia-Sensitive Therapy. ACS Nano 2019, 13, 14230–14240. [Google Scholar] [CrossRef]
- Palomba, R.; Parodi, A.; Evangelopoulos, M.; Acciardo, S.; Corbo, C.; de Rosa, E.; Yazdi, I.K.; Scaria, S.; Molinaro, R.; Furman, N.E.T.; et al. Biomimetic carriers mimicking leukocyte plasma membrane to increase tumor vasculature permeability. Sci. Rep. 2016, 6, 34422. [Google Scholar] [CrossRef]
- Li, X.; Zhen, M.; Deng, R.; Yu, T.; Li, J.; Zhang, Y.; Zou, T.; Zhou, Y.; Lu, Z.; Guan, M.; et al. RF-assisted gadofullerene nanoparticles induces rapid tumor vascular disruption by down-expression of tumor vascular endothelial cadherin. Biomaterials 2018, 163, 142–153. [Google Scholar] [CrossRef]
- Liang, C.; Chao, Y.; Yi, X.; Xu, J.; Feng, L.; Zhao, Q.; Yang, K.; Liu, Z. Nanoparticle-mediated internal radioisotope therapy to locally increase the tumor vasculature permeability for synergistically improved cancer therapies. Biomaterials 2019, 197, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. Overcoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Rajabi, M.; Mousa, S.A. Chapter 3—Nanobiomaterials in cancer therapy. In Nanobiomaterials in Cancer Therapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Bucharest, Romania, 2016; pp. 57–89. [Google Scholar] [CrossRef]
- Yoncheva, K.; Momekov, G. Antiangiogenic anticancer strategy based on nanoparticulate systems. Expert Opin. Drug Deliv. 2011, 8, 1041–1056. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Huber, P.E.; Bischof, M.; Jenne, J.; Heiland, S.; Peschke, P.; Saffrich, R.; Gröne, H.J.; Debus, J.; Lipson, K.E.; Abdollahi, A. Trimodal cancer treatment: Beneficial effects of combined antiangiogenesis, radiation, and chemotherapy. Cancer Res. 2005, 65, 3643–3655. [Google Scholar] [CrossRef]
- Kok, R.J.; Schraa, A.J.; Bos, E.J.; Moorlag, H.E.; Asgeirsdottir, S.A.; Everts, M.; Meijer, D.K.; Molema, G. Preparation and functional evaluation of RGD-modified proteins as alpha(v)beta(3) integrin directed therapeutics. Bioconjug. Chem. 2002, 13, 128–135. [Google Scholar] [CrossRef]
- Hynes, R.O. A reevaluation of integrins as regulators of angiogenesis. Nat. Med. 2002, 8, 918–921. [Google Scholar] [CrossRef]
- Sakae, M.; Ito, T.; Yoshihara, C.; Iida-Tanaka, N.; Yanagie, H.; Eriguchi, M.; Koyama, Y. Highly efficient in vivo gene transfection by plasmid/PEI complexes coated by anionic PEG derivatives bearing carboxyl groups and RGD peptide. Biomed. Pharmacother. 2008, 62, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Pola, R.; Gremse, F.; Theek, B.; Ehling, J.; Moeckel, D.; Hermanns-Sachweh, B.; Pechar, M.; Ulbrich, K.; Hennink, W.E.; et al. Passive versus Active Tumor Targeting Using RGD- and NGR-Modified Polymeric Nanomedicines. Nano Lett. 2014, 14, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Dong, L.; Chen, J.J.; Gao, F.B.; Zhang, Z.P.; Chen, J.N.; Zhang, J.F. Low-molecular weight chitosan/vascular endothelial growth factor short hairpin RNA for the treatment of hepatocellular carcinoma. Life Sci. 2012, 91, 1207–1215. [Google Scholar] [CrossRef]
- Guo, J.F.; Ogier, J.R.; Desgranges, S.; Darcy, R.; O′Driscoll, C. Anisamide-targeted cyclodextrin nanoparticles for siRNA delivery to prostate tumours in mice. Biomaterials 2012, 33, 7775–7784. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Chang, C.W.; Lee, M.; Kim, S.W. Efficient siRNA delivery using water soluble lipopolymer for anti-anglogenic gene therapy. J. Control. Release 2007, 118, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gu, H.; Zhang, D.S.; Li, F.; Liu, T.; Xia, W. Highly effective inhibition of lung cancer growth and metastasis by systemic delivery of siRNA via multimodal mesoporous silica-based nanocarrier. Biomaterials 2014, 35, 10058–10069. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Liu, T.; Zhang, D.S.; Wang, Y.; Gu, H.; Di, W. Highly effective antiangiogenesis via magnetic mesoporous silica-based siRNA vehicle targeting the VEGF gene for orthotopic ovarian cancer therapy. Int. J. Nanomed. 2015, 10, 2579–2594. [Google Scholar] [CrossRef]
- Kanazawa, T.; Sugawara, K.; Tanaka, K.; Horiuchi, S.; Takashima, Y.; Okada, H. Suppression of tumor growth by systemic delivery of anti-VEGF siRNA with cell-penetrating peptide-modified MPEG-PCL nanomicelles. Eur. J. Pharm. Biopharm. 2012, 81, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Graepler, F.; Verbeek, B.; Graeter, T.; Smirnow, I.; Kong, H.L.; Schuppan, D.; Bauer, M.; Vonthein, R.; Gregor, M.; Lauer, U.M. Combined endostatin/sFlt-1 antiangiogenic gene therapy is highly effective in a rat model of HCC. Hepatology 2005, 41, 879–886. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.; Wang, W.W.; Yao, Y.C.; Fang, S.H.; Dai, Z.Y.; Hong, H.H.; Yang, X.; Shuai, X.T.; Gao, G.Q. Pigment epithelium-derived factor gene loaded in cRGD-PEG-PEI suppresses colorectal cancer growth by targeting endothelial cells. Int. J. Pharm. 2012, 438, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.S.; Duan, X.P.; Yin, Q.; Miao, Z.H.; Yu, H.J.; Chen, C.Y.; Zhang, Z.W.; Wang, J.; Li, Y.P. The inhibition of metastasis and growth of breast cancer by blocking the NF-kappa B signaling pathway using bioreducible PEI-based/p65 shRNA complex nanoparticles. Biomaterials 2013, 34, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Lin, H.Y.; Zhang, S.L.; Davis, F.B.; Davis, P.J. Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology 2004, 145, 3265–3272. [Google Scholar] [CrossRef]
- Indraccolo, S.; Minuzzo, S.; Gola, E.; Habeler, W.; Carrozzino, F.; Noonan, D.; Albini, A.; Santi, L.; Amadori, A.; Chieco-Bianchi, L. Generation of expression plasmids for angiostatin, endostatin and TIMP-2 for cancer gene therapy. Int. J. Biol. Markers 1999, 14, 251–256. [Google Scholar] [CrossRef]
- Li, H.; Lindenmeyer, F.; Grenet, C.; Opolon, P.; Menashi, S.; Soria, C.; Yeh, P.; Perricaudet, M.; Lu, H. AdTIMP-2 inhibits tumor growth, angiogenesis, and metastasis, and prolongs survival in mice. Hum. Gene Ther. 2001, 12, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Vincent, L.; Varet, J.; Pille, J.Y.; Bompais, H.; Opolon, P.; Maksimenko, A.; Malvy, C.; Mirshahi, M.; Lu, H.; Soria, J.P.V.C.; et al. Efficacy of dendrimer-mediated angiostatin and TIMP-2 gene delivery on inhibition of tumor growth and angiogenesis: In vitro and in vivo studies. Int. J. Cancer 2003, 105, 419–429. [Google Scholar] [CrossRef]
- Steins, A.; Klaassen, R.; Jacobs, I.; Schabel, M.C.; van Lier, M.G.J.T.B.; Ebbing, E.A.; Hectors, S.J.; Tas, S.W.; Maracle, C.X.; Punt, C.J.A.; et al. Rapid stromal remodeling by short-term VEGFR2 inhibition increases chemotherapy delivery in esophagogastric adenocarcinoma. Mol. Oncol. 2020, 14, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.X.; Adstamongkonkul, P.; et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013, 4, 2516–2528. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing Tumor Microenvironment to Treat Cancer: Bench to Bedside to Biomarkers. J. Clin. Oncol. 2013, 31, 2205–2210. [Google Scholar] [CrossRef]
- Farahani, A.; Azimi, S.; Tajaddodi, A.; Docoslis, A.; Tashakori, C. Screen-printed anion-exchange solid-phase extraction: A new strategy for point-of-care determination of angiotensin receptor blockers. Talanta 2021, 222, 121518. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Cao, J.H.; Melamed, A.; Worley, M.; Gockley, A.; Jones, D.; Nia, H.T.; Zhang, Y.L.; Stylianopoulos, T.; Kumar, A.S.; et al. Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc. Natl. Acad. Sci. USA 2019, 116, 2210–2219. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, Z.; Yin, X.; Tang, L.; Luo, H.; Li, H.; Zhang, Y.; Luo, W. In vitro and in vivo study of hydralazine, a potential anti-angiogenic agent. Eur. J. Pharmacol. 2016, 779, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Wagner, D.D. Targeting platelet function to improve drug delivery. Oncoimmunology 2012, 1, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Ho-Tin-Noe, B.; Schatzberg, D.; Yang, J.J.; Wagner, D.D. Increased efficacy of breast cancer chemotherapy in thrombocytopenic mice. Cancer Res. 2011, 71, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Di, C.; Li, S.; Yang, X.; Nie, G. Smart Nanotherapeutic Targeting of Tumor Vasculature. Acc. Chem. Res. 2019, 52, 2703–2712. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Wang, J.; Zhao, Y.; Ji, T.; Zhao, X.; Ding, Y.; Zhao, X.; Zhao, R.; Li, F.; et al. Nanoparticle-mediated local depletion of tumour-associated platelets disrupts vascular barriers and augments drug accumulation in tumours. Nat. Biomed. Eng. 2017, 1, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Netti, P.A.; Berk, D.A.; Swartz, M.A.; Grodzinsky, A.J.; Jain, R.K. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000, 60, 2497–2503. [Google Scholar]
- Eikenes, L.; Bruland, O.S.; Brekken, C.; Davies, C.D.L. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004, 64, 4768–4773. [Google Scholar] [CrossRef]
- McKee, T.D.; Grandi, P.; Mok, W.; Alexandrakis, G.; Insin, N.; Zimmer, J.P.; Bawendi, M.G.; Boucher, Y.; Breakefield, X.O.; Jain, R.K. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006, 66, 2509–2513. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Nichols, J.W.; Toh, K.; Nomoto, T.; Cabral, H.; Miura, Y.; Christie, R.J.; Yamada, N.; Ogura, T.; Kano, M.R.; et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 2016, 11, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yang, L.; Jin, J.; Yang, F.; Liu, D.; Hu, K.; Wang, Q.X.; Yue, Y.B.; Gu, N. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics 2020, 10, 462–483. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Q.; Liu, R.; Zhou, Y.; Gao, H.L. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Cent. Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.L.; Li, X.; Yao, M.J.; Niu, P.Y.; Yuan, X.C.; Li, K.; Chen, M.W.; Fu, Z.X.; Duan, X.L.; Liu, H.B.; et al. Programmable prodrug micelle with size-shrinkage and charge-reversal for chemotherapy-improved IDO immunotherapy. Biomaterials 2020, 241, 119901. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, Z.M.; Wu, C.C.; Zi, Y.X.; Chu, X.Y.; Liu, J.P.; Zhang, W.L. Size-adaptable and ligand (biotin)-sheddable nanocarriers equipped with avidin scavenging technology for deep tumor penetration and reduced toxicity. J. Control. Release 2020, 320, 142–158. [Google Scholar] [CrossRef]
- Wang, K.W.; Tu, Y.L.; Yao, W.; Zong, Q.Y.; Xiao, X.; Yang, R.M.; Jiang, X.Q.; Yuan, Y.Y. Size-Switchable Nanoparticles with Self-Destructive and Tumor Penetration Characteristics for Site-Specific Phototherapy of Cancer. ACS Appl. Mater. Interfaces 2020, 12, 6933–6943. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, T.C.; Zhao, M.Y.; Wang, W.X.; Sun, C.X.; Liu, L.; Li, Q.; Zhang, F.; Zhao, D.Y.; Li, X.M. Size and charge dual-transformable mesoporous nanoassemblies for enhanced drug delivery and tumor penetration. Chem. Sci. 2020, 11, 2819–2827. [Google Scholar] [CrossRef]
- Song, C.F.; Li, Y.G.; Li, T.L.; Yang, Y.M.; Huang, Z.C.; de la Fuente, J.M.; Ni, J.; Cui, D.X. Long-Circulating Drug-Dye-Based Micelles with Ultrahigh pH-Sensitivity for Deep Tumor Penetration and Superior Chemo-Photothermal Therapy. Adv. Funct. Mater. 2020, 30, 1462–1474. [Google Scholar] [CrossRef]
- Nagy, J.A.; Feng, D.; Vasile, E.; Wong, W.H.; Shih, S.C.; Dvorak, A.M.; Dvorak, H.F. Permeability properties of tumor surrogate blood vessels induced by VEGF-A. Lab. Investig. 2006, 86, 767–780. [Google Scholar] [CrossRef]
- Siemann, D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef]
- Yang, Y.L.; Zhang, Y.; Iwamoto, H.; Hosaka, K.; Seki, T.; Andersson, P.; Lim, S.; Fischer, C.; Nakamura, M.; Abe, M.; et al. Discontinuation of anti-VEGF cancer therapy promotes metastasis through a liver revascularization mechanism. Nat. Commun. 2016, 7, 12680. [Google Scholar] [CrossRef]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Kunjachan, S.; Detappe, A.; Kumar, R.; Ireland, T.; Cameron, L.; Biancur, D.E.; Motto-Ros, V.; Sancey, L.; Sridhar, S.; Makrigiorgos, G.M.; et al. Nanoparticle Mediated Tumor Vascular Disruption: A Novel Strategy in Radiation Therapy. Nano Lett. 2015, 15, 7488–7496. [Google Scholar] [CrossRef]
- Song, C.H.; Zhang, Y.J.; Li, C.Y.; Chen, G.C.; Kang, X.F.; Wang, Q.B. Enhanced Nanodrug Delivery to Solid Tumors Based on a Tumor Vasculature-Targeted Strategy. Adv. Funct. Mater. 2016, 26, 4192–4200. [Google Scholar] [CrossRef]
- Zhen, M.M.; Shu, C.Y.; Li, J.; Zhang, G.Q.; Wang, T.S.; Luo, Y.; Zou, T.J.; Deng, R.J.; Fang, F.; Lei, H.; et al. A highly efficient and tumor vascular-targeting therapeutic technique with size-expansible gadofullerene nanocrystals. Sci. China Mater. 2015, 58, 799–810. [Google Scholar] [CrossRef]
- Park, J.-S.; Su, Z.-Z.; Hinman, D.; Willoughby, K.; McKinstry, R.; Yacoub, A.; Duigou, G.; Young, C.; Grant, S.; Hagan, M.; et al. Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene 2001, 20, 3266–3280. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Koukouraki, S.; Giatromanolaki, A.; Kakolyris, S.; Georgoulias, V.; Velidaki, A.; Archimandritis, S.; Karkavitsas, N.N. High intratumoral accumulation of stealth liposomal doxorubicin in sarcomas--rationale for combination with radiotherapy. Acta Oncol. 2000, 39, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Burd, R.; Dziedzic, T.S.; Xu, Y.; Caligiuri, M.A.; Subjeck, J.R.; Repasky, E.A. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. J. Cell Physiol. 1998, 177, 137–147. [Google Scholar] [CrossRef]
- Lammers, T.; Peschke, P.; Kuhnlein, R.; Subr, V.; Ulbrich, K.; Debus, J.; Huber, P.; Hennink, W.; Storm, G. Effect of radiotherapy and hyperthermia on the tumor accumulation of HPMA copolymer-based drug delivery systems. J. Control. Release 2007, 117, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001, 61, 3027–3032. [Google Scholar] [PubMed]
- Islam, W.; Fang, J.; Imamura, T.; Etrych, T.; Subr, V.; Ulbrich, K.; Maeda, H. Augmentation of the Enhanced Permeability and Retention Effect with Nitric Oxide–Generating Agents Improves the Therapeutic Effects of Nanomedicines. Mol. Cancer Ther. 2018, 17, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.W.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).