PET/CT Imaging for Personalized Management of Infectious Diseases
Abstract
:1. Introduction
2. Current Common Applications of FDG-PET/CT
2.1. Bloodstream Infection of Unknown Origin
2.2. Fever of Unknown Origin
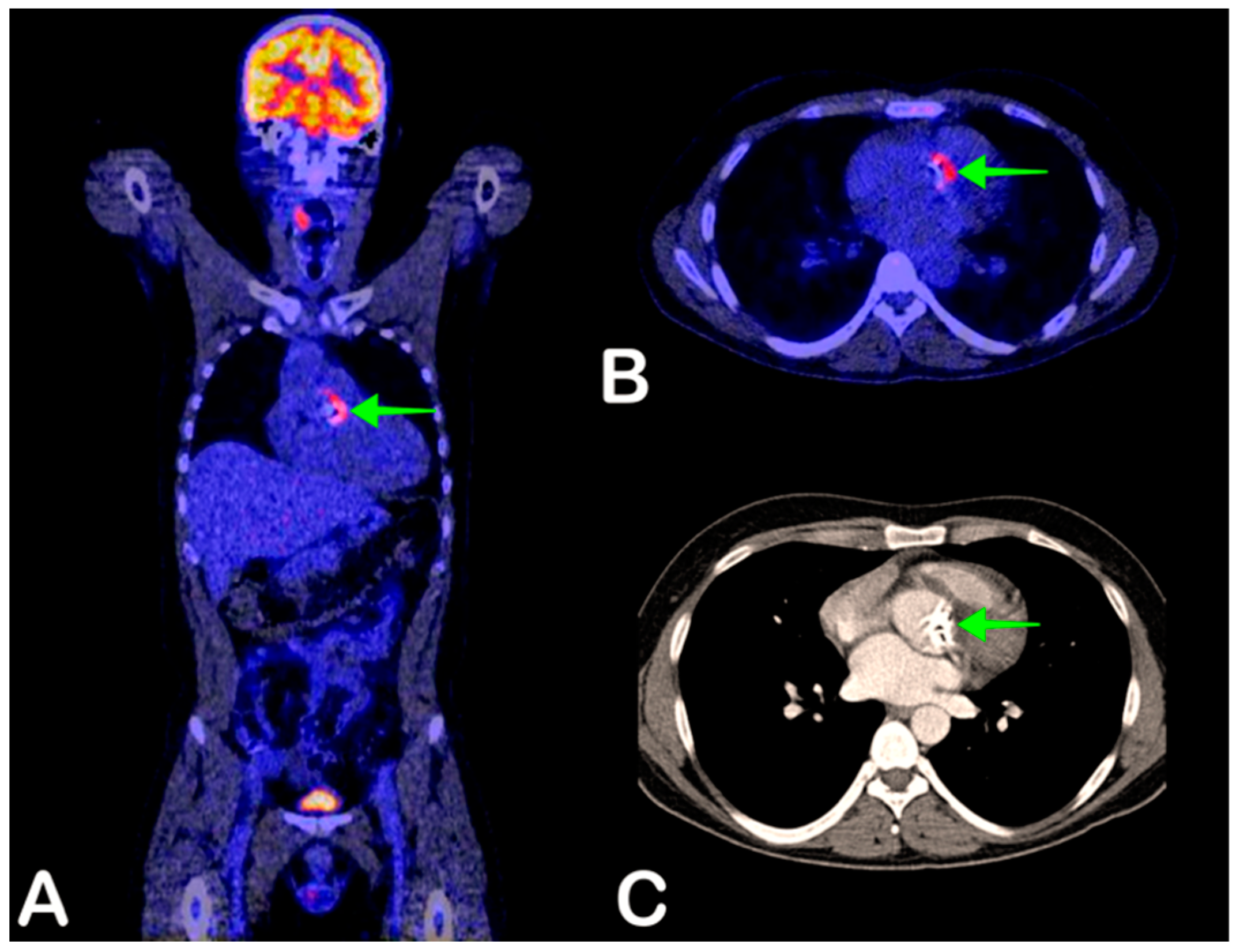
2.3. Infective Endocarditis
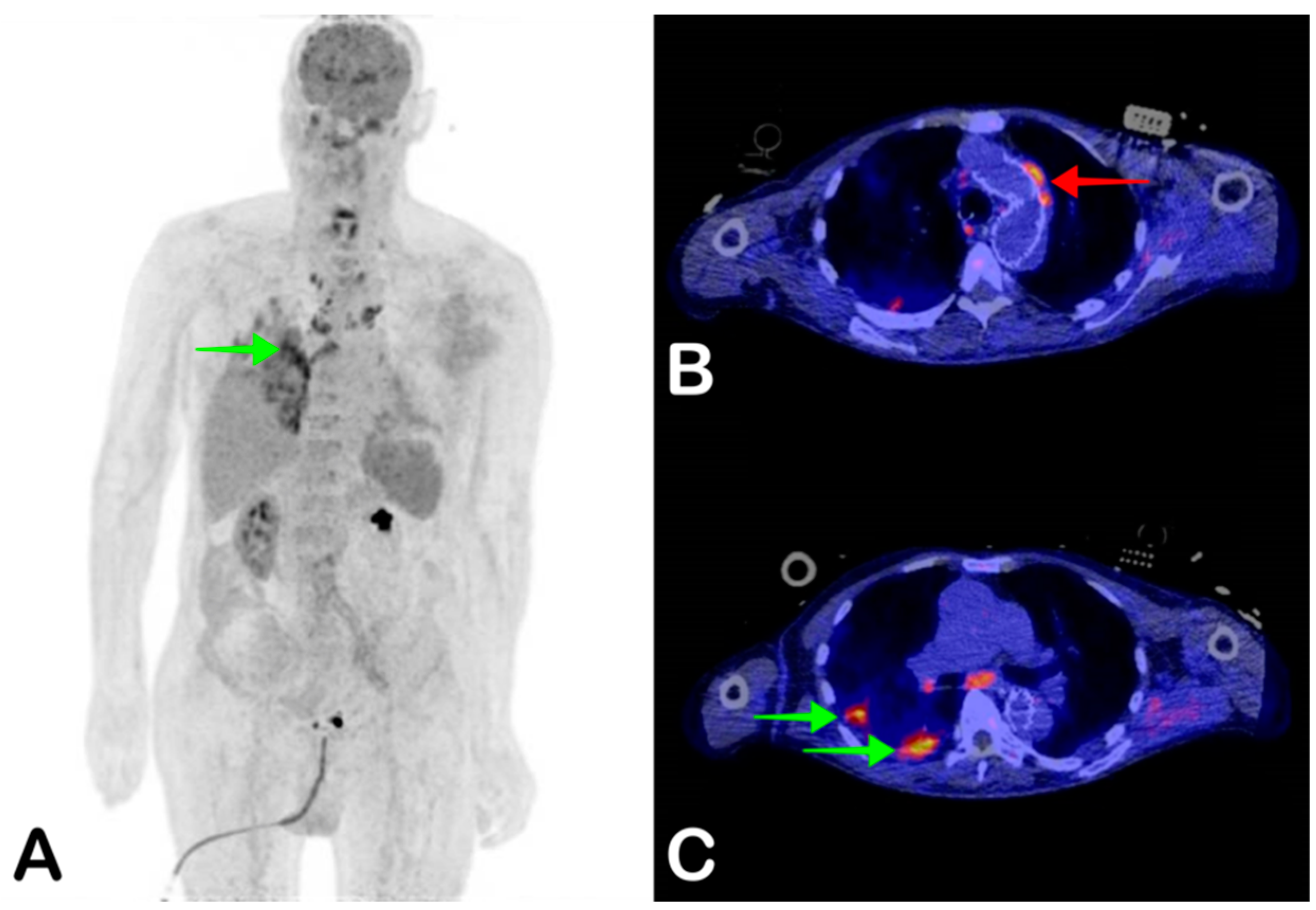
2.4. Vascular Graft Infection
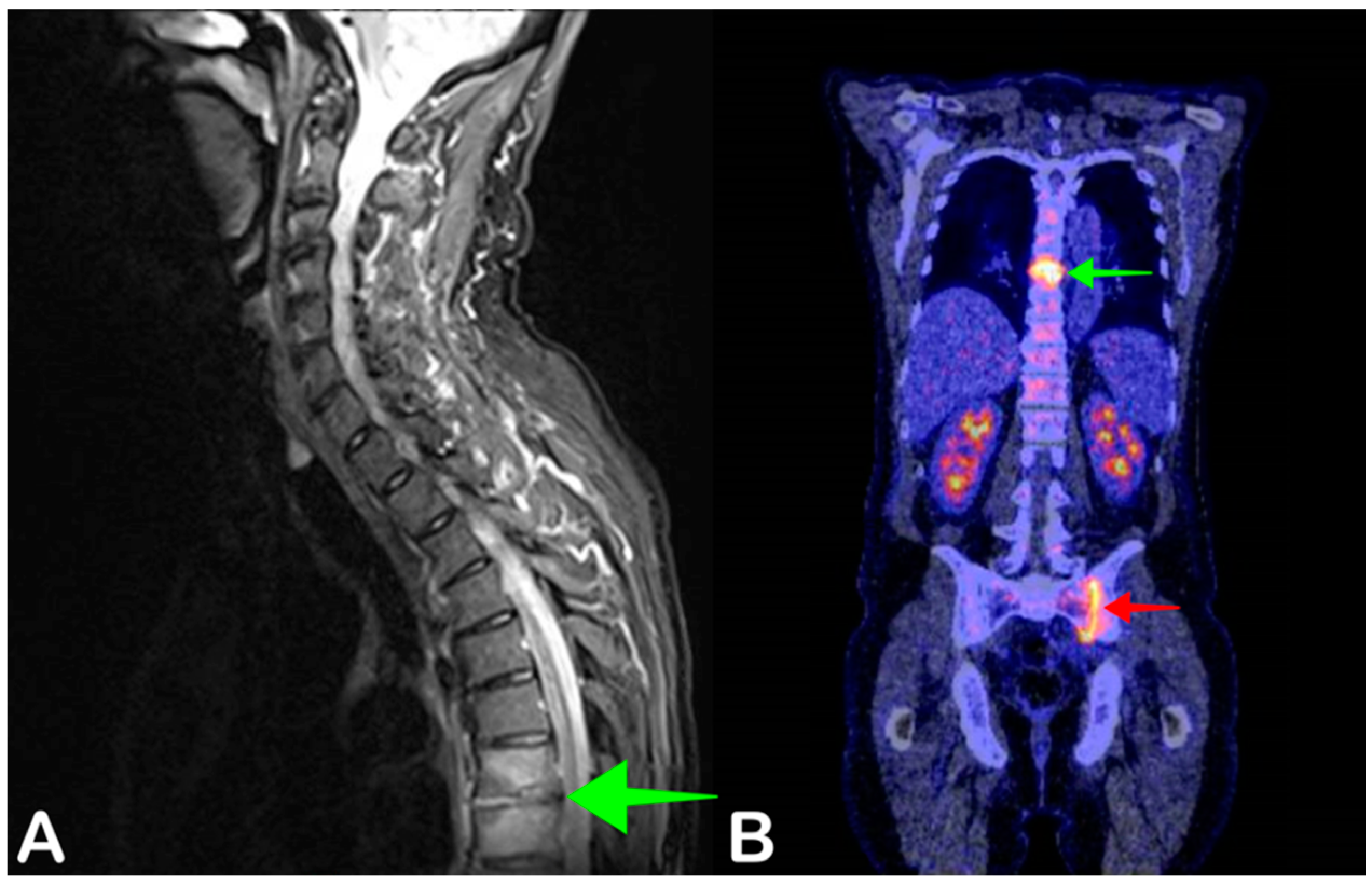
2.5. Spondylodiscitis
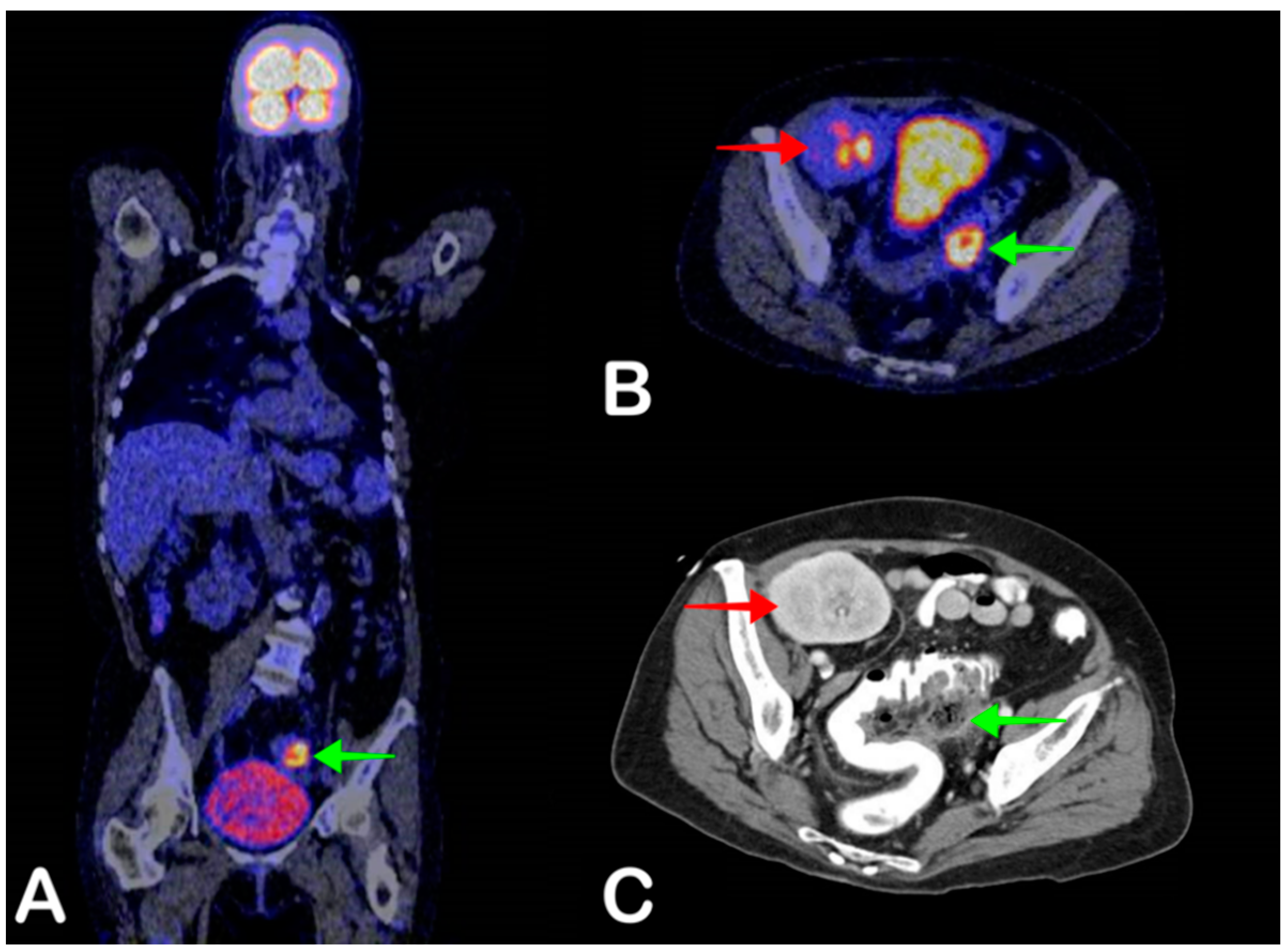
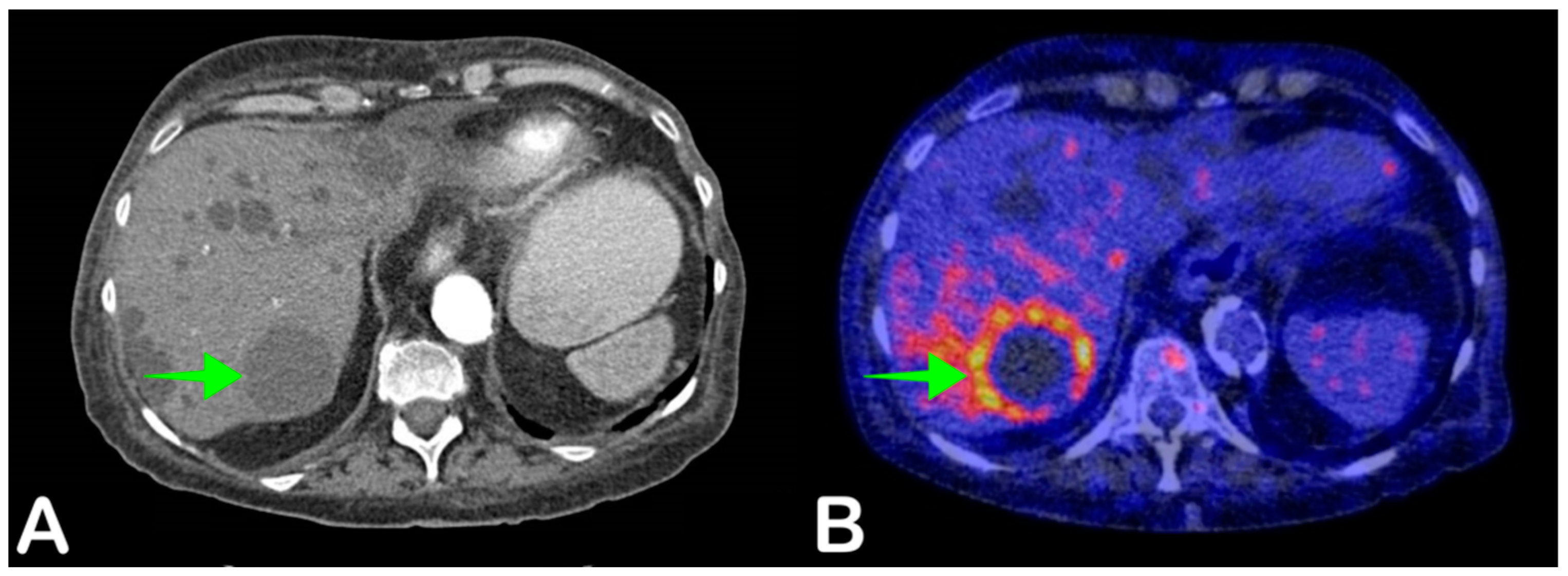
2.6. Cyst Infection
3. Limitations of FDG-PET/CT
4. White Blood Cell Cintigraphy
5. Future Applications of PET/CT
5.1. Total Body PET/CT
5.2. Infection-Specific Radiotracers
5.3. Personalized Duration of Antibiotic Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Bradley, S.F. Infections. Pract. Geriatr. 2007, 475–494. [Google Scholar] [CrossRef]
- Vanepps, J.S.; Younger, J.G. Implantable Device-Related Infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, F.J.; Bleeker-Rovers, C.P.; Sturm, P.D.; Krabbe, P.F.M.; Van Dijk, A.P.J.; Cuijpers, M.L.H.; Adang, E.M.M.; Wanten, G.J.A.; Kullberg, B.-J.; Oyen, W.J.G. 18F-FDG PET/CT for Detection of Metastatic Infection in Gram-Positive Bacteremia. J. Nucl. Med. 2010, 51, 1234–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidyanathan, S.; Patel, C.; Scarsbrook, A.; Chowdhury, F. FDG PET/CT in infection and inflammation—Current and emerging clinical applications. Clin. Radiol. 2015, 70, 787–800. [Google Scholar] [CrossRef]
- Fuchs, S.; Grössmann, N.; Ferch, M.; Busse, R.; Wild, C. Evidence-based indications for the planning of PET or PET/CT capacities are needed. Clin. Transl. Imaging 2019, 7, 65–81. [Google Scholar] [CrossRef] [Green Version]
- Jamar, F.; Buscombe, J.; Chiti, A.; Christian, P.E.; Delbeke, D.; Donohoe, K.J.; Israel, O.; Martin-Comin, J.; Signore, A. EANM/SNMMI Guideline for 18F-FDG Use in Inflammation and Infection. J. Nucl. Med. 2013, 54, 647–658. [Google Scholar] [CrossRef] [Green Version]
- Kung, B.T.; Seraj, S.M.; Zadeh, M.Z.; Rojulpote, C.; Kothekar, E.; Ayubcha, C.; Ng, K.S.; Ng, K.K.; Au-Yong, T.K.; Werner, T.J.; et al. An update on the role of 18F-FDG-PET/CT in major infectious and inflammatory diseases. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 255–273. [Google Scholar]
- Goto, M.; Al-Hasan, M.N. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin. Microbiol. Infect. 2013, 19, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Lagunes, L.; Encina, B.; Ramirez-Estrada, S. Current understanding in source control management in septic shock patients: A review. Ann. Transl. Med. 2016, 4, 330. [Google Scholar] [CrossRef] [Green Version]
- Treglia, G. Diagnostic Performance of 18F-FDG PET/CT in Infectious and Inflammatory Diseases according to Published Meta-Analyses. Contrast Media Mol. Imaging 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, J.-R.; Chen, K.-Y.; Lee, M.-H.; Huang, C.-T.; Wen, Y.-H.; Yen, T.-C. Potential Usefulness of FDG PET/CT in Patients with Sepsis of Unknown Origin. PLoS ONE 2013, 8, e66132. [Google Scholar] [CrossRef] [PubMed]
- Brøndserud, M.B.; Pedersen, C.; Rosenvinge, F.S.; Høilund-Carlsen, P.F.; Hess, S. Clinical value of FDG-PET/CT in bacteremia of unknown origin with catalase-negative gram-positive cocci or Staphylococcus aureus. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Berrevoets, M.A.; Kouijzer, I.J.; Aarntzen, E.H.; Janssen, M.J.; Wertheim, H.F.; Kullberg, B.-J.; Oyen, W.J.G.; Bleeker-Rovers, C.P.; De Geus-Oei, L.-F.; Oever, J.T. 18 F-FDG PET/CT Optimizes Treatment in Staphylococcus Aureus Bacteremia and Is Associated with Reduced Mortality. J. Nucl. Med. 2017, 58, 1504–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pijl, J.; Glaudemans, A.; Slart, R.; Yakar, D.; Wouthuyzen-Bakker, M.; Kwee, T. FDG-PET/CT for Detecting an Infection Focus in Patients with a Bloodstream Infection: Factors Affecting Diagnostic Yield. SSRN Electron. J. 2018, 44, 99–106. [Google Scholar] [CrossRef]
- Tabah, A.; Koulenti, D.; Laupland, K.B.; Misset, B.; Valles, J.; De Carvalho, F.B.; Paiva, J.A.; Çakar, N.; Ma, X.; Eggimann, P.; et al. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: The EUROBACT International Cohort Study. Intensiv. Care Med. 2012, 38, 1930–1945. [Google Scholar] [CrossRef] [Green Version]
- Hess, S.; Hansson, S.H.; Pedersen, K.T.; Basu, S.; Høilund-Carlsen, P.F. FDG-PET/CT in Infectious and Inflammatory Diseases. PET Clin. 2014, 9, 497–519. [Google Scholar] [CrossRef]
- Vos, F.J.; Bleeker-Rovers, C.P.; Kullberg, B.J.; Adang, E.M.; Oyen, W.J.G. Cost-Effectiveness of Routine 18F-FDG PET/CT in High-Risk Patients with Gram-Positive Bacteremia. J. Nucl. Med. 2011, 52, 1673–1678. [Google Scholar] [CrossRef] [Green Version]
- Holland, T.L.; Arnold, C.J.; Fowler, V.G. Clinical Management of Staphylococcus aureus Bacteremia. JAMA 2014, 312, 1330–1341. [Google Scholar] [CrossRef] [Green Version]
- Petersdorf, R.G.; Beeson, P.B. Fever of unexplained origin: Report on 100 cases. Medicine 1961, 40, 1–30. [Google Scholar] [CrossRef]
- Meller, J.; Sahlmann, C.-O.; Scheel, A.K. 18F-FDG PET and PET/CT in fever of unknown origin. J. Nucl. Med. 2007, 48, 35–45. [Google Scholar]
- Kouijzer, I.J.; Mulders-Manders, C.M.; Bleeker-Rovers, C.P.; Oyen, W.J. Fever of Unknown Origin: The Value of FDG-PET/CT. Semin. Nucl. Med. 2018, 48, 100–107. [Google Scholar] [CrossRef]
- Pijl, J.P.; Kwee, T.C.; Legger, G.; Peters, H.J.; Armbrust, W.; Schölvinck, E.; Glaudemans, A.W. Role of FDG-PET/CT in children with fever of unknown origin. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1596–1604. [Google Scholar] [CrossRef] [Green Version]
- Nakayo, E.B.; Vicente, A.G.; Castrejón, Á.M.S.; Narváez, J.M.; Rubio, M.T.; García, V.P.; García, J.C. Analysis of cost-effectiveness in the diagnosis of fever of unknown origin and the role of 18F-FDG PET–CT: A proposal of diagnostic algorithm. Rev. Esp. Med. Nucl. Imagen Mol. Engl. Ed. 2012, 31, 178–186. [Google Scholar] [CrossRef]
- Balink, H.; Tan, S.S.; Veeger, N.J.G.M.; Holleman, F.; Van Eck-Smit, B.L.F.; Bennink, R.J.; Verberne, H.J. 18F-FDG PET/CT in inflammation of unknown origin: A cost-effectiveness pilot-study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.R. Acute Infective Endocarditis. Infect. Dis. Clin. N. Am. 2009, 23, 643–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajani, R.; Klein, J.L. Infective endocarditis: A contemporary update. Clin. Med. 2020, 20, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Shakya, S.; Hussain, S.; Pettersson, G.; Griffin, B.; Gordon, S. Sensitivity and Specificity of Duke Criteria for Diagnosis of Definite Infective Endocarditis: A Cohort Study. Open Forum Infect. Dis. 2017, 4, 550. [Google Scholar] [CrossRef] [Green Version]
- Evangelista, A. Echocardiography in infective endocarditis. Hearth 2004, 90, 614–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Dilsizian, V. FDG PET/CT for the diagnosis and management of infective endocarditis: Expert consensus vs evidence-based practice. J. Nucl. Cardiol. 2018, 26, 313–315. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Glaudemans, A.W.J.M.; Touw, D.J.; Van Melle, J.P.; Willems, T.P.; Maass, A.H.; Natour, E.; Prakken, N.H.J.; Borra, R.J.H.; Van Geel, P.P.; et al. Diagnostic value of imaging in infective endocarditis: A systematic review. Lancet Infect. Dis. 2017, 17, e1–e14. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis. The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). G. Ital. Cardiol. Rome 2016, 17, 3075–3128. [Google Scholar]
- Erba, P.A.; Pizzi, M.N.; Roque, A.; Salaun, E.; Lancellotti, P.; Tornos, P.; Habib, G. Multimodality Imaging in Infective Endocarditis. Circulation 2019, 140, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Bleeker-Rovers, C.P.; Vos, F.J.; Wanten, G.J.A.; Van Der Meer, J.W.M.; Corstens, F.H.M.; Kullberg, B.-J.; Oyen, W.J.G. 18F-FDG PET in detecting metastatic infectious disease. J. Nucl. Med. 2005, 46, 2014–2019. [Google Scholar] [PubMed]
- Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Gheysens, O.; Lubberink, M.; Kero, T.; Dweck, M.R.; Habib, G.; Gaemperli, O.; Saraste, A.; Gimelli, A.; et al. Procedural recommendations of cardiac PET/CT imaging: Standardization in inflammatory-, infective-, infiltrative-, and innervation (4Is)-related cardiovascular diseases: A joint collaboration of the EACVI and the EANM. Eur. J. Nucl. Med. Mol. Imaging 2020, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Bower, T.C.; Creager, M.A.; Amin-Hanjani, S.; O’Gara, P.T.; Lockhart, P.B.; Darouiche, R.O.; Ramlawi, B.; Derdeyn, C.P.; Bolger, A.F.; et al. Vascular Graft Infections, Mycotic Aneurysms, and Endovascular Infections: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e412–e460. [Google Scholar] [CrossRef] [Green Version]
- Keidar, Z.; Nitecki, S. FDG-PET in Prosthetic Graft Infections. Semin. Nucl. Med. 2013, 43, 396–402. [Google Scholar] [CrossRef]
- Keidar, Z.; Engel, A.; Hoffman, A.; Israel, O.; Nitecki, S. Prosthetic Vascular Graft Infection: The Role of 18F-FDG PET/CT. J. Nucl. Med. 2007, 48, 1230–1236. [Google Scholar] [CrossRef] [Green Version]
- Saleem, B.R.; Pol, R.A.; Slart, R.H.J.A.; Reijnen, M.M.P.J.; Zeebregts, C.J. 18F-Fluorodeoxyglucose Positron Emission Tomography/CT Scanning in Diagnosing Vascular Prosthetic Graft Infection. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chakfé, N.; Diener, H.; Lejay, A.; Assadian, O.; Berard, X.; Caillon, J.; Fourneau, I.; Glaudemans, A.W.; Koncar, I.; Lindholt, J.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Vascular Graft and Endograft Infections. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 339–384. [Google Scholar] [CrossRef] [Green Version]
- Smids, C.; Kouijzer, I.J.E.; Vos, F.J.; Sprong, T.; Hosman, A.J.F.; De Rooy, J.W.J.; Aarntzen, E.H.J.G.; De Geus-Oei, L.-F.; Oyen, W.J.G.; Bleeker-Rovers, C.P. A comparison of the diagnostic value of MRI and 18F-FDG-PET/CT in suspected spondylodiscitis. Infection 2017, 45, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Gouliouris, T.; Aliyu, S.H.; Brown, N.M. Spondylodiscitis: Update on diagnosis and management. J. Antimicrob. Chemother. 2010, 65, iii11–iii24. [Google Scholar] [CrossRef] [Green Version]
- Altini, C.; Lavelli, V.; Niccoli-Asabella, A.; Sardaro, A.; Branca, A.; Santo, G.; Ferrari, C.; Rubini, G. Comparison of the Diagnostic Value of MRI and Whole Body 18F-FDG PET/CT in Diagnosis of Spondylodiscitis. J. Clin. Med. 2020, 9, 1581. [Google Scholar] [CrossRef]
- Torres, V.E.; Harris, P.C.; Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 2007, 369, 1287–1301. [Google Scholar] [CrossRef]
- Oh, J.; Shin, C.-I.; Kim, S.Y. Infected cyst in patients with autosomal dominant polycystic kidney disease: Analysis of computed tomographic and ultrasonographic imaging features. PLoS ONE 2018, 13, e0207880. [Google Scholar] [CrossRef]
- Lantinga, M.A.; Drenth, J.P.; Gevers, T.J.G. Diagnostic criteria in renal and hepatic cyst infection. Nephrol. Dial. Transpl. 2014, 30, 744–751. [Google Scholar] [CrossRef] [Green Version]
- Lantinga, M.A.; Casteleijn, N.F.; Geudens, A.; De Sévaux, R.G.; Van Assen, S.; Leliveld, A.M.; Gansevoort, R.T.; Drenth, J.P. Management of renal cyst infection in patients with autosomal dominant polycystic kidney disease: A systematic review. Nephrol. Dial. Transpl. 2016, 32, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallée, M.; Rafat, C.; Zahar, J.-R.; Paulmier, B.; Grünfeld, J.-P.; Knebelmann, B.; Fakhouri, F. Cyst Infections in Patients with Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 1183–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobot, M.; Ghez, C.; Gondouin, B.; Sallée, M.; Fournier, P.; Burtey, S.; Legris, T.; Dussol, B.; Berland, Y.; Souteyrand, P.; et al. Diagnostic performance of [18F] fluorodeoxyglucose positron emission tomography–computed tomography in cyst infection in patients with autosomal dominant polycystic kidney disease. Clin. Microbiol. Infect. 2016, 22, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouret, F.; Lhommel, R.; Beguin, C.; Devuyst, O.; Pirson, Y.; Hassoun, Z.; Kanaan, N. Positron-Emission Computed Tomography in Cyst Infection Diagnosis in Patients with Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Pijl, J.P.; Glaudemans, A.W.; Slart, R.H.; Kwee, T.C. 18F-FDG PET/CT in Autosomal Dominant Polycystic Kidney Disease Patients with Suspected Cyst Infection. J. Nucl. Med. 2018, 59, 1734–1741. [Google Scholar] [CrossRef] [Green Version]
- Garg, G.; Benchekroun, M.T.; Abraham, T. FDG-PET/CT in the Postoperative Period: Utility, Expected Findings, Complications, and Pitfalls. Semin. Nucl. Med. 2017, 47, 579–594. [Google Scholar] [CrossRef]
- Gelderman, S.J.; Jutte, P.C.; Boellaard, R.; Ploegmakers, J.J.W.; García, D.V.; Kampinga, G.A.; Glaudemans, A.W.J.M.; Wouthuyzen-Bakker, M. 18F-FDG-PET uptake in non-infected total hip prostheses. Acta Orthop. 2018, 89, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Kagna, O.; Kurash, M.; Ghanem-Zoubi, N.; Keidar, Z.; Israel, O. Does Antibiotic Treatment Affect the Diagnostic Accuracy of18F-FDG PET/CT Studies in Patients with Suspected Infectious Processes? J. Nucl. Med. 2017, 58, 1827–1830. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Kwee, T.C.; Saboury, B.; Garino, J.P.; Nelson, C.L.; Zhuang, H.; Parsons, M.; Chen, W.; Kumar, R.; Salavati, A.; et al. FDG PET for Diagnosing Infection in Hip and Knee Prostheses. Clin. Nucl. Med. 2014, 39, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Lauri, C.; Glaudemans, A.W.J.M.; Campagna, G.; Keidar, Z.; Muchnik Kurash, M.; Georga, S.; Arsos, G.; Noriega-Álvarez, E.; Argento, G.; Kwee, T.C.; et al. Comparison of White Blood Cell Scintigraphy, FDG PET/CT and MRI in Suspected Diabetic Foot Infection: Results of a Large Retrospective Multicenter Study. J. Clin. Med. 2020, 9, 1645. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, S.; Moskal, P.; Karp, J.S. State of the art in total body PET. EJNMMI Phys. 2020, 7, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Cherry, S.R.; Jones, T.; Karp, J.S.; Qi, J.; Moses, W.W.; Badawi, R.D. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J. Nucl. Med. 2018, 59, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Diederich, S.; Lenzen, H. Radiation exposure associated with imaging of the chest: Comparison of different radiographic and computed tomography techniques. Cancer 2000, 89, 2457–2460. [Google Scholar] [CrossRef]
- Auletta, S.; Varani, M.; Horvat, R.; Galli, F.; Signore, A.; Hess, S. PET Radiopharmaceuticals for Specific Bacteria Imaging: A Systematic Review. J. Clin. Med. 2019, 8, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welling, M.M.; Hensbergen, A.W.; Bunschoten, A.; Velders, A.H.; Roestenberg, M.; Van Leeuwen, F.W.B. An update on radiotracer development for molecular imaging of bacterial infections. Clin. Transl. Imaging 2019, 7, 105–124. [Google Scholar] [CrossRef] [Green Version]
- Gordon, O.; Ruiz-Bedoya, C.A.; Ordonez, A.A.; Tucker, E.W.; Jain, S.K. Molecular Imaging: A Novel Tool to Visualize Pathogenesis of Infections In Situ. mBio 2019, 10, e00317-19. [Google Scholar] [CrossRef] [Green Version]
- Pickett, J.E.; Thompson, J.M.; Sadowska, A.; Tkaczyk, C.; Sellman, B.R.; Minola, A.; Corti, D.; Lanzavecchia, A.; Miller, L.S.; Thorek, D.L. Molecularly specific detection of bacterial lipoteichoic acid for diagnosis of prosthetic joint infection of the bone. Bone Res. 2018, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, O.; Brunner, M.; Zeitlinger, M.; Ziegler, S.; Dobrozemsky, G.; Lackner, E.; Joukhadar, C.; Mitterhauser, M.; Wadsak, W.; Minar, E.; et al. In vitro and in vivo evaluation of [18F]ciprofloxacin for the imaging of bacterial infections with PET. Eur. J. Nucl. Med. Mol. Imaging 2004, 32, 143–150. [Google Scholar] [CrossRef]
- Sellmyer, M.A.; Lee, I.; Hou, C.; Weng, C.-C.; Li, S.; Lieberman, B.P.; Zeng, C.; Mankoff, D.A.; Mach, R.H. Bacterial Infection Imaging with [18F]Fluoropropyl-Trimethoprim. Proc. Natl. Acad. Sci. USA 2017, 114, 8372–8377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebenhan, T.; Sathekge, M.M.; Lengana, T.; Koole, M.; Gheysens, O.; Govender, T.; Zeevaart, J.R.; Lenagana, T. 68Ga-NOTA-Functionalized Ubiquicidin: Cytotoxicity, Biodistribution, Radiation Dosimetry, and First-in-Human PET/CT Imaging of Infections. J. Nucl. Med. 2017, 59, 334–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Ordonez, A.A.; Wang, H.; Li, Y.; Gogarty, K.R.; Weinstein, E.A.; Daryaee, F.; Merino, J.; Yoon, G.E.; Kalinda, A.S.; et al. Positron Emission Tomography Imaging with 2-[18F]F-p-Aminobenzoic Acid Detects Staphylococcus Aureus Infections and Monitors Drug Response. ACS Infect. Dis. 2018, 4, 1635–1644. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, E.A.; Ordonez, A.A.; Demarco, V.P.; Murawski, A.M.; Pokkali, S.; Macdonald, E.M.; Klunk, M.; Mease, R.C.; Pomper, M.G.; Jain, S.K. Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci. Transl. Med. 2014, 6, 259ra146. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.; Winter, G.; Baur, B.; Witulla, B.; Solbach, C.; Reske, S.; Lindén, M. Synthesis, characterization, and biodistribution of multiple 89Zr-labeled pore-expanded mesoporous silica nanoparticles for PET. Nanoscale 2014, 6, 4928–4935. [Google Scholar] [CrossRef]
- Rahmani, S.; Shahhoseini, S.; Mohamadi, R.; Vojdani, M. Synthesis, Quality Control and Stability Studies of 2-[18F]Fluoro-2-Deoxy-D-Glucose(18F-FDG) at Different Conditions of Temperature by Physicochemical and Microbiological Assays. Iran. J. Pharm. Res. IJPR 2017, 16, 602–610. [Google Scholar]
- Martiniova, L.; De Palatis, L.; Etchebehere, E.; Ravizzini, G. Gallium-68 in Medical Imaging. Curr. Radiopharm. 2016, 9, 187–207. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Weinstein, E.A.; Bambarger, L.E.; Saini, V.; Chang, Y.S.; Demarco, V.P.; Klunk, M.H.; Urbanowski, M.E.; Moulton, K.L.; Murawski, A.M.; et al. A Systematic Approach for Developing Bacteria-Specific Imaging Tracers. J. Nucl. Med. 2016, 58, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zheng, H.; Fodah, R.A.; Warawa, J.M.; Ng, C.K. Validation of 2-18F-Fluorodeoxysorbitol as a Potential Radiopharmaceutical for Imaging Bacterial Infection in the Lung. J. Nucl. Med. 2017, 59, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Moek, K.L.; Giesen, D.; Kok, I.C.; De Groot, D.J.A.; Jalving, M.; Fehrmann, R.S.; Hooge, M.N.L.-D.; Brouwers, A.H.; De Vries, E.G. Theranostics Using Antibodies and Antibody-Related Therapeutics. J. Nucl. Med. 2017, 58, 83S–90S. [Google Scholar] [CrossRef] [Green Version]
- Schoenmakers, J.W.A.; Heuker, M.; López-Álvarez, M.; Nagengast, W.B.; Van Dam, G.M.; Van Dijl, J.M.; Jutte, P.C.; Van Oosten, M. Image-guided in situ detection of bacterial biofilms in a human prosthetic knee infection model: A feasibility study for clinical diagnosis of prosthetic joint infections. Eur. J. Nucl. Med. Mol. Imaging 2020, 1–11. [Google Scholar] [CrossRef]
- Lau, J.S.Y.; Kiss, C.; Roberts, E.; Horne, K.; Korman, T.M.; Woolley, I.J. Surveillance of life-long antibiotics: A review of antibiotic prescribing practices in an Australian Healthcare Network. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, J.S.Y.; Korman, T.M.; Woolley, I. Life-long antimicrobial therapy: Where is the evidence? J. Antimicrob. Chemother. 2018, 73, 2601–2612. [Google Scholar] [CrossRef] [PubMed]
- Perng, P.; Marcus, C.; Subramaniam, R.M. (18)F-FDG PET/CT and Melanoma: Staging, Immune Modulation and Mutation-Targeted Therapy Assessment, and Prognosis. AJR Am. J. Roentgenol. 2015, 205, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Mayr, A.; Aigner, M.; Lass-Flörl, C. Anidulafungin for the treatment of invasive candidiasis. Clin. Microbiol. Infect. 2011, 17 (Suppl. 1), 1–12. [Google Scholar] [CrossRef] [Green Version]

| Tracer | Isotope (Half-Life) | Ligand | Target/Substrate | Comment |
|---|---|---|---|---|
| 89Zr-SAC55 | Zirkonium-89 (78.4 h) [69] | Monoclonal antibody | Staphylococcus aureus surface molecule lipoteichoic acid | Promising results from animal studies, currently no human studies available [63] |
| 18F-ciprofloxacin | Fluorine-18 (110 min) [70] | Ciprofloxacin | Bacterial DNA gyrase or topoisomerase IV | In-human studies did not indicate bacteria-specific binding [64] |
| 18F-fluoropropyl-trimethoprim | Fluorine-18 (110 min) [70] | Trimethoprim | Bacterial dihydrofolate reductase | No in-human studies available [65] |
| 68Ga-NOTA-UBI | Gallium-68 (68 min) [71] | Chelated ubiquicidin | Bacterial cytoplasmic membrane | Promising results in animal models, but very limited in-human experience [66] |
| 2-18F-F-p-Aminobenzoic Acid | Fluorine-18 (110 min) [70] | p-aminobenzoic acid | Dihydropteroate synthase | Promising results from animal studies, also targets different types of bacteria [72] |
| 2-18F-fluorodeoxysorbitol | Fluorine-18 (110 min) [70] | Sorbitol | Sorbitol-6-phosphate dehydrogenase | Promising results from animal studies, limited in-human experience. Sorbitol is not metabolized by Gram positive bacteria [73]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pijl, J.P.; Kwee, T.C.; Slart, R.H.J.A.; Glaudemans, A.W.J.M. PET/CT Imaging for Personalized Management of Infectious Diseases. J. Pers. Med. 2021, 11, 133. https://doi.org/10.3390/jpm11020133
Pijl JP, Kwee TC, Slart RHJA, Glaudemans AWJM. PET/CT Imaging for Personalized Management of Infectious Diseases. Journal of Personalized Medicine. 2021; 11(2):133. https://doi.org/10.3390/jpm11020133
Chicago/Turabian StylePijl, Jordy P., Thomas C. Kwee, Riemer H. J. A. Slart, and Andor W. J. M. Glaudemans. 2021. "PET/CT Imaging for Personalized Management of Infectious Diseases" Journal of Personalized Medicine 11, no. 2: 133. https://doi.org/10.3390/jpm11020133
APA StylePijl, J. P., Kwee, T. C., Slart, R. H. J. A., & Glaudemans, A. W. J. M. (2021). PET/CT Imaging for Personalized Management of Infectious Diseases. Journal of Personalized Medicine, 11(2), 133. https://doi.org/10.3390/jpm11020133









