Towards Personalised Contrast Injection: Artificial-Intelligence-Derived Body Composition and Liver Enhancement in Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Imaging Protocols
2.3. Contrast Material Injection and CT Protocols
2.4. Quantitative Image Analysis
2.5. Qualitative Image Analysis
2.6. Statistical Analysis
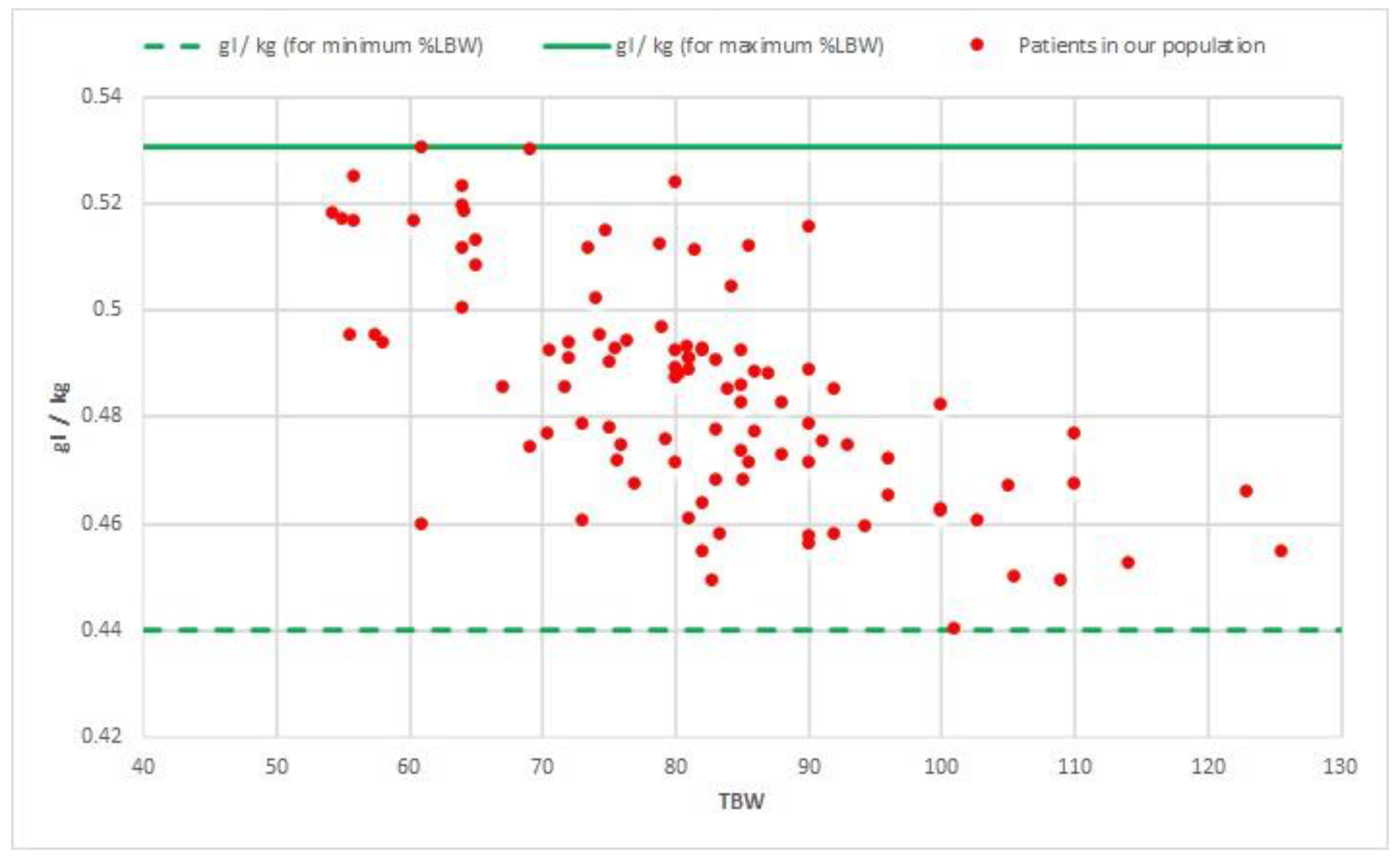
2.7. Simulation of Future Potential Clinical Applicability
3. Results
3.1. Baseline Characteristics
3.2. Quantitative Image Quality
3.3. Qualitative Image Quality
3.4. Regression Analysis
3.5. Simulation of Future Potential Clinical Applicability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tchelepi, H.; Ralls, P.W. Ultrasound of focal liver masses. Ultrasound Q. 2004, 20, 155–169. [Google Scholar] [CrossRef]
- Robinson, E.; Babb, J.; Chandarana, H.; Macari, M. Dual Source Dual Energy MDCT: Comparison of 80 kVp and Weighted Average 120 kVp Data for Conspicuity of Hypo-Vascular Liver Metastases. Investig. Radiol. 2010, 45, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.A.; Amitai, M.M.; Rappaport, D.C.; O’Malley, M.E.; Hanbidge, A.E.; Redston, M.; Lockwood, G.A.; Gallinger, S. Multi–Detector Row Helical CT in Preoperative Assessment of Small (≤1.5 cm) Liver Metastases: Is Thinner Collimation Better? Radiology 2002, 225, 137–142. [Google Scholar] [CrossRef]
- Stevens, W.R.; Johnson, C.D.; Stephens, D.H.; Batts, K.P. CT findings in hepatocellular carcinoma: Correlation of tumor characteristics with causative factors, tumor size, and histologic tumor grade. Radiology 1994, 191, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Gore, R.M.; Thakrar, K.H.; Wenzke, D.R.; Newmark, G.M.; Mehta, U.K.; Berlin, J.W. That liver lesion on MDCT in the oncology patient:is it important? Cancer Imaging 2012, 12, 373–384. [Google Scholar] [CrossRef]
- Brink, J.A.; Heiken, J.P.; Forman, H.P.; Sagel, S.S.; Molina, P.L.; Brown, P.C. Hepatic Spiral CT: Reduction of Dose of Intravenous Contrast Material. Radiology 1995, 197, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.N.; Rosenberg, M.; Vernuccio, F.; Ramirez-Giraldo, J.C.; Nelson, R.; Farjat, A.; Marin, D. Characterization of Small Incidental Indeterminate Hypoattenuating Hepatic Lesions: Added Value of Single-Phase Contrast-Enhanced Dual-Energy CT Material Attenuation Analysis. AJR Am. J. Roentgenol. 2018, 211, 571–579. [Google Scholar] [CrossRef]
- Heiken, J.P.; Brink, J.A.; McClennan, B.L.; Sagel, S.S.; Crowe, T.M.; Gaines, M.V. Dynamic incremental CT: Effect of volume and concentration of contrast material and patient weight on hepatic enhancement. Radiology 1995, 195, 353–357. [Google Scholar] [CrossRef]
- Bae, K.T. Intravenous Contrast Medium Administration and Scan Timing at CT: Considerations and Approaches. Radiology 2010, 256, 32–61. [Google Scholar] [CrossRef]
- Sica, G.T.; Ji, H.; Ros, P.R. CT and MR Imaging of Hepatic Metastases. AJR Am. J. Roentgenol. 2000, 174, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, T.G.; Bulagahapitiya, S. Contrast-induced Nephropathy. Review 2004, 183, 1673–1689. [Google Scholar] [CrossRef]
- Cohan, R.H.; Dunnick, N.R. Intravascular contrast media: Adverse reactions. AJR Am. J. Roentgenol. 1987, 149, 665–670. [Google Scholar] [CrossRef]
- Kondo, H.; Kanematsu, M.; Goshima, S.; Tomita, Y.; Miyoshi, T.; Hatcho, A.; Moriyama, N.; Onozuka, M.; Shiratori, Y.; Bae, K.T. Abdominal Multidetector CT in Patients with Varying Body Fat Percentages: Estimation of Optimal Contrast Material Dose. Radiology 2008, 249, 872–877. [Google Scholar] [CrossRef]
- Martens, B.; Hendriks, B.M.F.; Eijsvoogel, N.G.; Wildberger, J.E.; Mihl, C. Individually Body Weight–Adapted Contrast Media Application in Computed Tomography Imaging of the Liver at 90 kVp. Investig. Radiol. 2019, 54, 177–182. [Google Scholar] [CrossRef]
- Mihl, C.; Kok, M.; Altintas, S.; Kietselaer, B.L.J.H.; Turek, J.; Wildberger, J.E.; Das, M. Evaluation of individually body weight adapted contrast media injection in coronary CT-angiography. Eur. J. Radiol. 2016, 85, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, B.M.F.; Kok, M.; Mihl, C.; Bekkers, S.C.A.M.; Wildberger, J.E.; Das, M. Individually tailored contrast enhancement in CT pulmonary angiography. Br. J. Radiol. 2016, 89, 20150850. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.T.; Heiken, J.P.; Brink, J.A. Aortic and hepatic contrast medium enhancement at CT. Part I. Prediction with a computer model. Radiology 1998, 207, 647–655. [Google Scholar] [CrossRef]
- Ho, L.M.; Nelson, R.C.; Delong, D.M. Determining Contrast Medium Dose and Rate on Basis of Lean Body Weight: Does This Strategy Improve Patient-to-Patient Uniformity of Hepatic Enhancement during Multi–Detector Row CT? Radiology 2007, 243, 431–437. [Google Scholar] [CrossRef]
- Kondo, H.; Kanematsu, M.; Goshima, S.; Tomita, Y.; Kim, M.-J.; Moriyama, N.; Onozuka, M.; Shiratori, Y.; Bae, K.T. Body Size Indexes for Optimizing Iodine Dose for Aortic and Hepatic Enhancement at Multidetector CT: Comparison of Total Body Weight, Lean Body Weight, and Blood Volume. Radiology 2010, 254, 163–169. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Masuda, T.; Sato, T.; Arataki, K.; Nakamura, Y.; Tatsugami, F.; Awai, K. Contrast Material Injection Protocol with the Dose Determined According to Lean Body Weight at Hepatic Dynamic Computed Tomography: Comparison Among Patients with Different Body Mass Indices. J. Comput. Assist. Tomogr. 2019, 43, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Kok, M.; Mihl, C.; Mingels, A.A.; Kietselaer, B.L.; Mühlenbruch, G.; Seehofnerova, A.; Wildberger, J.E.; Das, M. Influence of contrast media viscosity and temperature on injection pressure in computed tomographic angiography: A phantom study. Investig. Radiol. 2014, 49, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Moeskops, P.; de Vos, B.; Veldhuis, W.B.; de Jong, P.A.; Išgum, I.; Leiner, T. Automatic quantification of body composition at L3 vertebra level with convolutional neural networks. Eur. Congr. Radiol. 2020. [Google Scholar] [CrossRef]
- Morsbach, F.; Zhang, Y.-H.Y.H.; Martin, L.; Lindqvist, C.; Brismar, T. Body composition evaluation with computed tomography: Contrast media and slice thickness cause methodological errors. Nutrition 2019, 59, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Awai, K.; Takada, K.; Onishi, H.; Hori, S. Aortic and Hepatic Enhancement and Tumor-to-Liver Contrast: Analysis of the Effect of Different Concentrations of Contrast Material at Multi-Detector Row Helical CT. Radiology 2002, 242, 757–763. [Google Scholar] [CrossRef]
- RIVM Trends in het aantal CT-onderzoeken. Available online: https://www.rivm.nl/medische-stralingstoepassingen/trends-en-stand-van-zaken/diagnostiek/computer-tomografie/trends-in-aantal-ct-onderzoeken (accessed on 5 August 2020).
- James, W.P.T.; Waterlow, J.C. Research on Obesity: A Report of the DHSS/MRC Group; Her Majesty’s Stationery Office: London, UK, 1976. [Google Scholar]
- Boer, P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am. J. Physiol. Ren. Fluid Electrolyte Physiol. 1984, 247, F632–F636. [Google Scholar] [CrossRef]
- Janmahasatian, S.; Duffull, S.B.; Ash, S.; Ward, L.C.; Byrne, N.M.; Green, B. Quantification of Lean Bodyweight. Clin. Pharmacokinet. 2005, 44, 1051–1065. [Google Scholar] [CrossRef]
- Kok, M.; Turek, J.; Mihl, C.; Reinartz, S.D.; Gohmann, R.F.; Nijssen, E.C.; Kats, S.; van Ommen, V.G.; Kietselaer, B.L.J.H.; Wildberger, J.E. Low contrast media volume in pre-TAVI CT examinations. Eur. Radiol. 2016, 26, 2426–2435. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Nakaura, T.; Funama, Y.; Sato, T.; Higaki, T.; Matsumoto, Y.; Yamashita, Y.; Imada, N.; Kiguchi, M.; Baba, Y. Contrast enhancement on 100-and 120 kVp hepatic CT scans at thin adults in a retrospective cohort study: Bayesian inference of the optimal enhancement probability. Medicine (Baltimore). 2019, 98. [Google Scholar] [CrossRef]
- Nakamoto, A.; Yamamoto, K.; Sakane, M.; Nakai, G.; Higashiyama, A.; Juri, H.; Yoshikawa, S.; Narumi, Y. Reduction of the radiation dose and the amount of contrast material in hepatic dynamic CT using low tube voltage and adaptive iterative dose reduction 3-dimensional. Medicine (Baltimore) 2018, 97. [Google Scholar] [CrossRef]
- Araki, K.; Yoshizako, T.; Yoshida, R.; Tada, K.; Kitagaki, H. Low-voltage (80-kVp) abdominopelvic computed tomography allows 60% contrast dose reduction in patients at risk of contrast-induced nephropathy. Clin. Imaging 2018, 51, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Yu, L.; Fletcher, J.G.; McCollough, C.H. Maximizing iodine contrast-to-noise ratios in abdominal CT imaging through use of energy domain noise reduction and virtual monoenergetic dual-energy CT. Radiology 2015, 276, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Lu, Z.; Yin, J.; Gao, Y.; Chen, X.; Guo, Q. Effects of radiation dose levels and spectral iterative reconstruction levels on the accuracy of iodine quantification and virtual monochromatic CT numbers in dual-layer spectral detector CT: An iodine phantom study. Quant. Imaging Med. Surg. 2019, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, T.; Cicero, G.; Mazziotti, S.; Ascenti, G.; Albrecht, M.H.; Martin, S.S.; Othman, A.E.; Vogl, T.J.; Wichmann, J.L. Dual energy computed tomography virtual monoenergetic imaging: Technique and clinical applications. Br. J. Radiol. 2019, 92, 20180546. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Lee, J.M.; Lee, S.M.; Yang, H.K.; Kim, R.H.; Nam, J.G.; Karnawat, A.; Han, J.K. Value of virtual monochromatic spectral image of dual-layer spectral detector CT with noise reduction algorithm for image quality improvement in obese simulated body phantom. BMC Med. Imaging 2019, 19, 76. [Google Scholar] [CrossRef]
- Kawahara, D.; Ozawa, S.; Yokomachi, K.; Tanaka, S.; Higaki, T.; Fujioka, C.; Suzuki, T.; Tsuneda, M.; Nakashima, T.; Ohno, Y. Accuracy of the raw-data-based effective atomic numbers and monochromatic CT numbers for contrast medium with a dual-energy CT technique. Br. J. Radiol. 2018, 91, 20170524. [Google Scholar] [CrossRef]
- Raman, S.P.; Johnson, P.T.; Deshmukh, S.; Mahesh, M.; Grant, K.L.; Fishman, E.K. CT dose reduction applications: Available tools on the latest generation of CT scanners. J. Am. Coll. Radiol. 2013, 10, 37–41. [Google Scholar] [CrossRef]
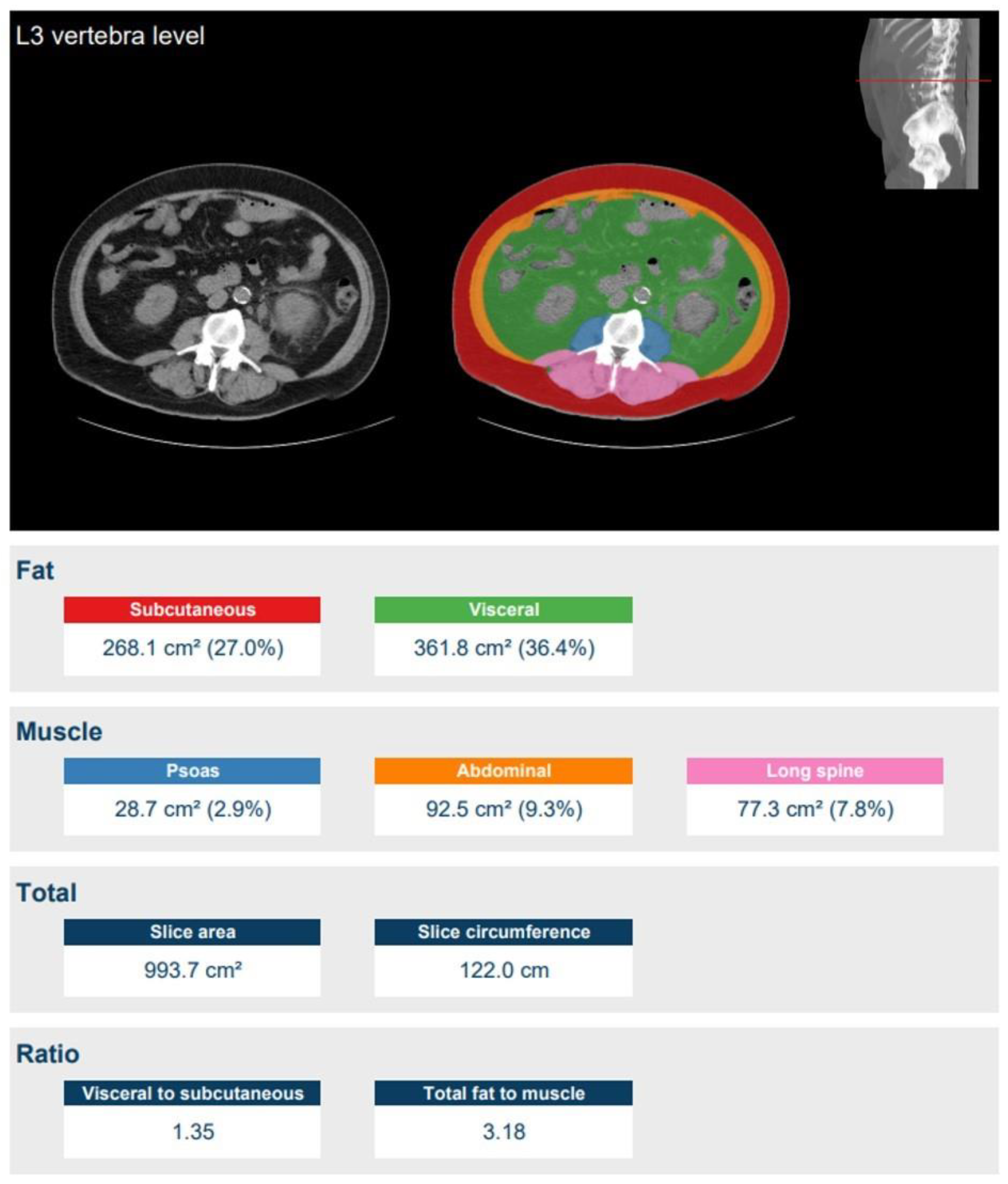
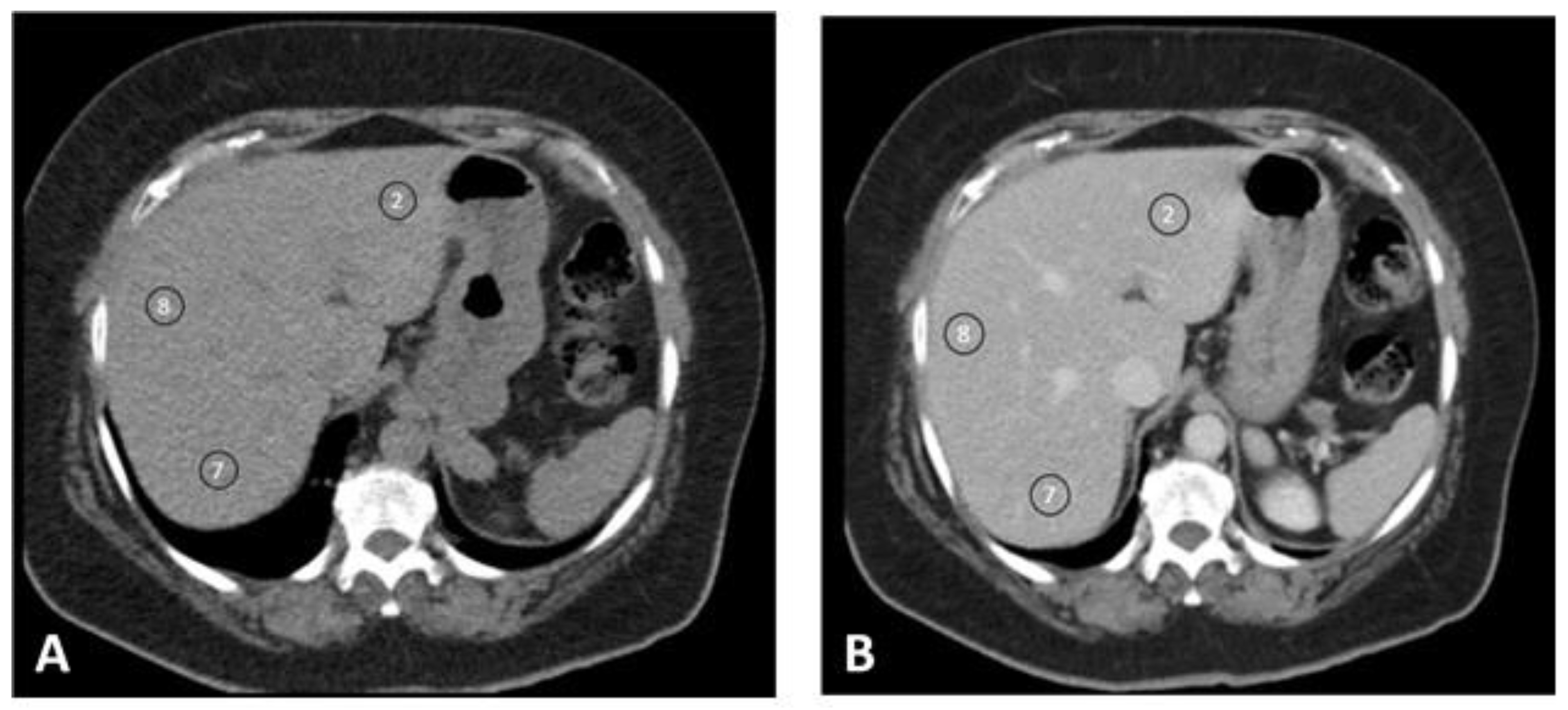

| Characteristic | Group ≤ 70 kg | Group 70–90 kg | Group ≥ 90 kg | Total |
|---|---|---|---|---|
| No participants | 20 | 62 | 20 | 102 |
| Sex male | 45.0% | 75.8% | 80.0% | 70.6% |
| Age (year) | 70 (59–76) | 69 (56–74) | 64 (59–73) | 68 (57–74) |
| TBW (kg) | 62.5 (56.3–64.8) | 81.0 (75.8–85.0) | 101 (94.7–110) | 81.0 (72.8–90.0) |
| LBW (kg) | 40.8 (32.2–46.2) | 40.8 (34.9–44.0) | 41.4 (38.9–45.9) | 41.1 (35.8–44.1) |
| %LBW | 69.6 (55.3–73.8) | 51.0 (43.8–53.7) | 40.5 (37.5–44.5) | 49.8 (42.1–55.3) |
| Height (cm) | 168 (±13.1) | 176 (±8.02) | 180 (±10.9) | 176 (±9.11) |
| BMI | 21.3 (±2.01) | 26.3 (±2.47) | 31.5 (±4.10) | 26.3 (±4.18) |
| Grams of iodine (mean) | 38.7 (±3.88) | 42.6 (±3.62) | 46.3 (±3.96) | 42.6 (±4.42) |
| Grams of iodine (median) | 36.0 (36.0–43.5) | 45.0 (39.0–45.0) | 45.0 (45.0–45.7) | 45.0 (39.0–45.0) |
| Grams of iodine/TBW | 0.632 (±0.693) | 0.530 (±0.534) | 0.453 (±0.060) | 0.532 (±0.081) |
| Grams of iodine/LBW | 1.00 (±0.281) | 1.07 (±0.176) | 1.12 (±0.139) | 1.07 (±0.196) |
| Mean (± SD) or Median (IQR) |
| Enhancement | Group ≤ 70 kg | Group 70–90 kg | Group ≥ 90 kg | Total | p-Value |
|---|---|---|---|---|---|
| S2 blanco | 60.5 (±5.77) | 56.7 (±5.02) | 53.6 (±6.30) | 56.8 (±5.83) | 0.000 |
| S2 PV | 120.6 (±11.6) | 109.8 (±11.7) | 105.7 (±9.86) | 111.1 (±12.3) | 0.000 |
| S2 SD | 9.57 (±1.43) | 11.1 (±1.89) | 12.1 (±1.91) | 11.0 (±1.92) | 0.000 |
| Δ S2 | 60.0 (±10.6) | 53.1 (±10.7) | 52.1 (±6.73) | 54.3 (±10.3) | 0.014 |
| S8 blanco | 60.7 (±5.24) | 55.7 (±5.84) | 51.0 (±6.61) | 55.7 (±6.61) | 0.000 |
| S8 PV | 120.9 (±14.2) | 109.2 (±10.9) | 104.4 (±9.52) | 110.5 (±12.5) | 0.000 |
| S8 SD | 9.19 (±0.981) | 10.2 (±1.47) | 11.2 (±2.18) | 10.3 (±1.75) | 0.000 |
| Δ S8 | 60.1 (±12.6) | 53.5 (±10.8) | 53.4 (±8.18) | 54.8 (±10.9) | 0.043 |
| S7 blanco | 59.5 (±5.56) | 54.5 (±5.35) | 50.8 (±6.82) | 54.7 (±6.32) | 0.000 |
| S7 PV | 118.7 (±10.8) | 107.8 (±10.1) | 103.1 (±8.34) | 109.0 (±11.1) | 0.000 |
| S7 SD | 9.29 (±1.10) | 10.6 (±1.70) | 12.2 (±2.35) | 10.7 (±2.09) | 0.000 |
| Δ S7 | 60.1 (±12.6) | 53.3 (±9.25) | 52.4 (±7.45) | 54.3 (±9.30) | 0.022 |
| Δ S2 Δ S8 Δ S7 | 0.667 | 0.939 | 0.520 | 0.114 | |
| Mean Δ | 60.7 (±12.4) | 53.3 (±9.75) | 52.6 (±6.63) | 54.6 (±10.2) | 0.007 * |
| Mean (± SD) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jong, D.J.; Veldhuis, W.B.; Wessels, F.J.; de Vos, B.; Moeskops, P.; Kok, M. Towards Personalised Contrast Injection: Artificial-Intelligence-Derived Body Composition and Liver Enhancement in Computed Tomography. J. Pers. Med. 2021, 11, 159. https://doi.org/10.3390/jpm11030159
de Jong DJ, Veldhuis WB, Wessels FJ, de Vos B, Moeskops P, Kok M. Towards Personalised Contrast Injection: Artificial-Intelligence-Derived Body Composition and Liver Enhancement in Computed Tomography. Journal of Personalized Medicine. 2021; 11(3):159. https://doi.org/10.3390/jpm11030159
Chicago/Turabian Stylede Jong, Daan J., Wouter B. Veldhuis, Frank J. Wessels, Bob de Vos, Pim Moeskops, and Madeleine Kok. 2021. "Towards Personalised Contrast Injection: Artificial-Intelligence-Derived Body Composition and Liver Enhancement in Computed Tomography" Journal of Personalized Medicine 11, no. 3: 159. https://doi.org/10.3390/jpm11030159
APA Stylede Jong, D. J., Veldhuis, W. B., Wessels, F. J., de Vos, B., Moeskops, P., & Kok, M. (2021). Towards Personalised Contrast Injection: Artificial-Intelligence-Derived Body Composition and Liver Enhancement in Computed Tomography. Journal of Personalized Medicine, 11(3), 159. https://doi.org/10.3390/jpm11030159






