Existence of a Neutral-Impact Maxillo-Mandibular Displacement on Upper Airways Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patient Population
2.3. Data Acquisition
3. Study Design
3.1. Image Segmentation
3.2. Pre- to Post-Operative Model Registration
- Alignment of 3D cranial base models through the Align (point pairs picking) tool (Tool > Registration > Align). Specifically, two pairs of landmarks unaltered by surgery were manually defined: most medial point of the frontozygomatic sutures bilaterally; most concave point of the inner posterior border of each zygomatic arch. The pre-operative mesh was defined as the Reference entity (fixed), the post-operative as Aligned (moving). The alignment of the pre- to post-operative mesh was then refined via iterative closest point (ICP) alignment; reference and aligned entities were set as in the previous step. Finally, a colorimetric surface map was generated to visually inspect the obtained superimposition for misalignments due to segmentation artifacts.
- Digital model sectioning to obtain a detached upper maxilla and mandible.
- Point-by-point alignment of the bone sections via four point pairs picking for each bony segment considered: most mesio-vestibular cusp of the first molar bilaterally and canine cusp bilaterally. The alignment was refined via ICP following the aforementioned workflow. The transformation matrices referring to each bony fragment were analysed to express the corresponding rigid body transformation (i.e., resulting bone displacement) in terms of Euler angles convention, which is composed of three angles (pitch, roll, yaw) and three translations (x, y, z, or lateral, anteroposterior and craniocaudal). The signed discrepancies were tabulated applying conventional signs.
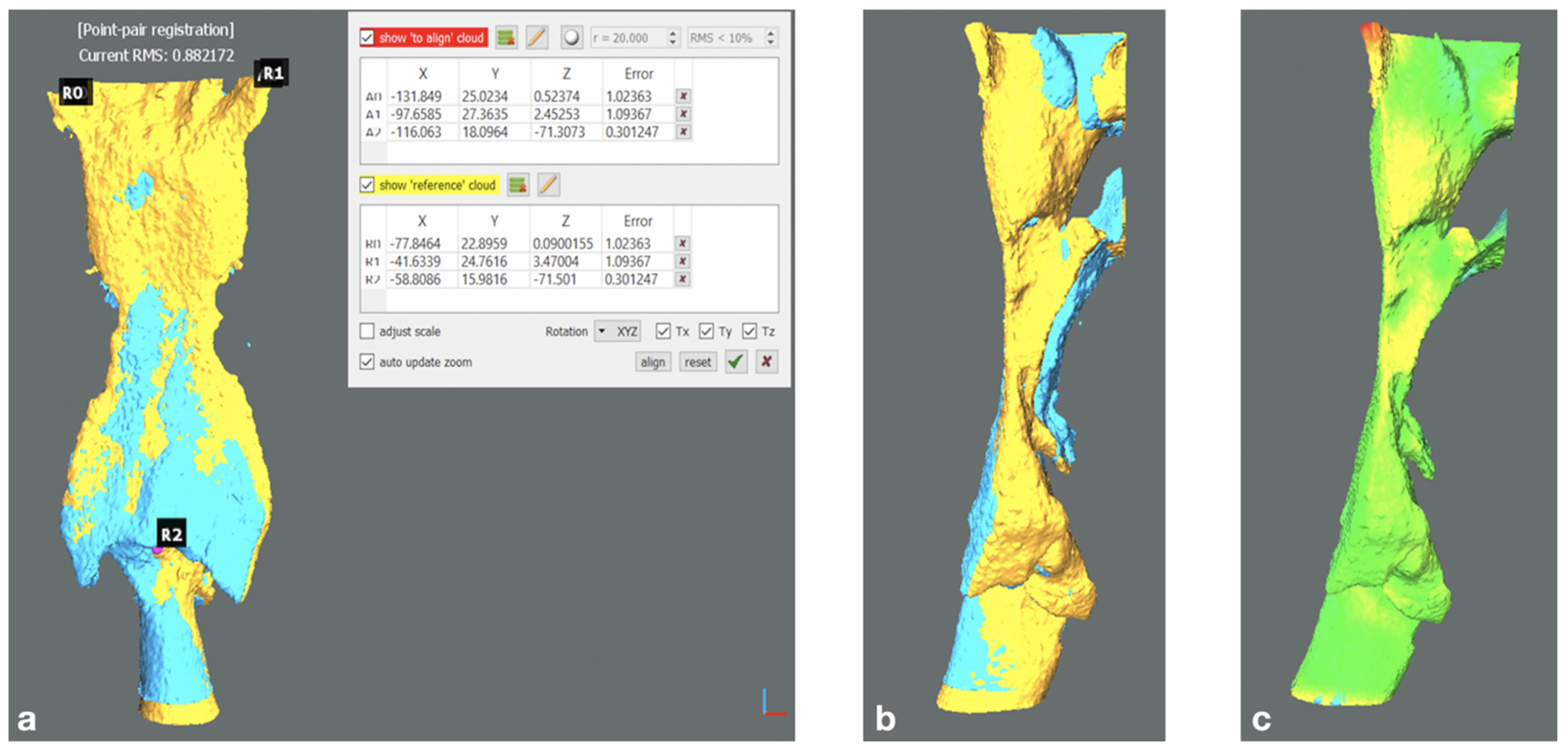
- Point-by-point manual alignment of the upper airways models via three point pairs (pharyngeal recess bilaterally, interarithenoid notch) followed by ICP refinement and colorimetric surface map inspection. (Figure 2a–c)
3.3. Three-Dimensional (3D) Analysis
- Partitioning of the UA models into 25 bounding boxes, defined as the smallest volume able to contain a set of points, with sides parallel to the three axes of the reference system. (Figure 3)
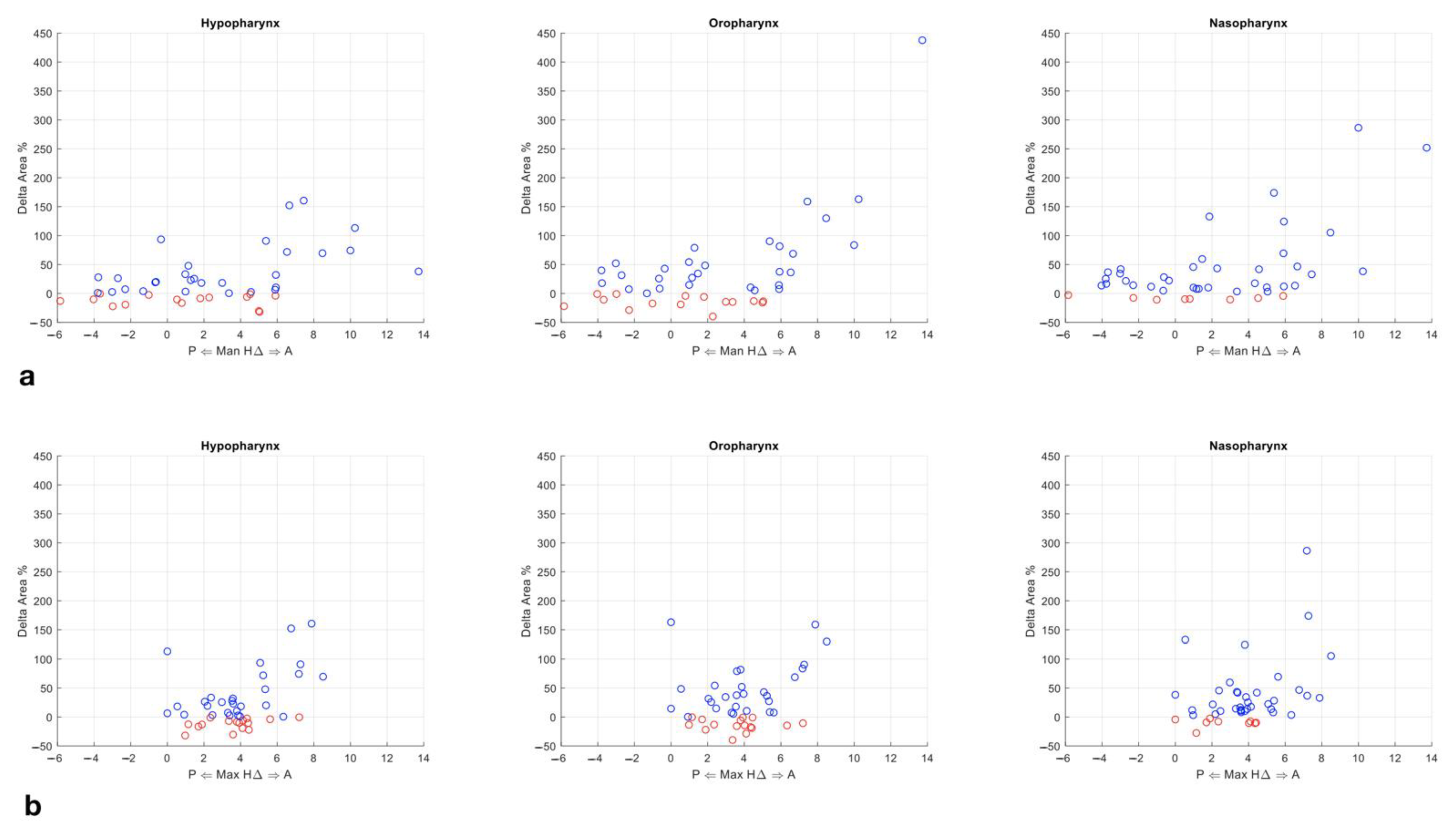
- Subdivision of the pharynx into three sectors according to its anatomical regions: naso-, oro-, hypopharynx. Each region was assigned a corresponding number of Bounding Boxes (5, 9, 11; respectively).
- Computation of the projected surface area (mm2) of the UA section delimited by each bounding box.
- Computation of the total volume (mm3) of the pharyngeal airway space.
3.4. Statistical Analysis
- MAMS vs. MMA with Upper Maxilla AP < 5 mm
- MAMS vs. MMA with Mandible AP < 5 mm
- MAMS vs. MMA with Upper Maxilla AP ≥ 5 mm
- MAMS vs. MMA with Mandible AP ≥ 5 mm
4. Results
4.1. Analysis of Variance (ANOVA) Test
4.2. Games–Howell Test
4.3. Mann–Whitney Test
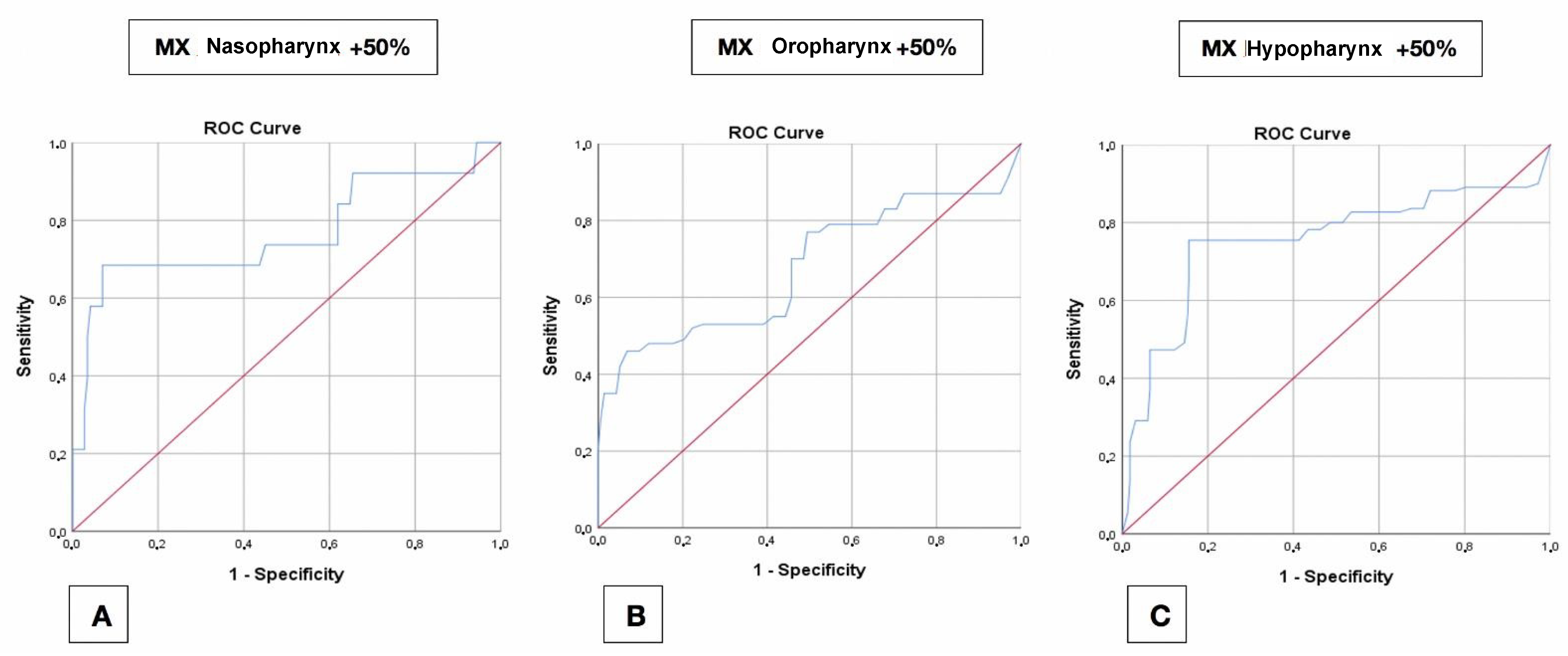
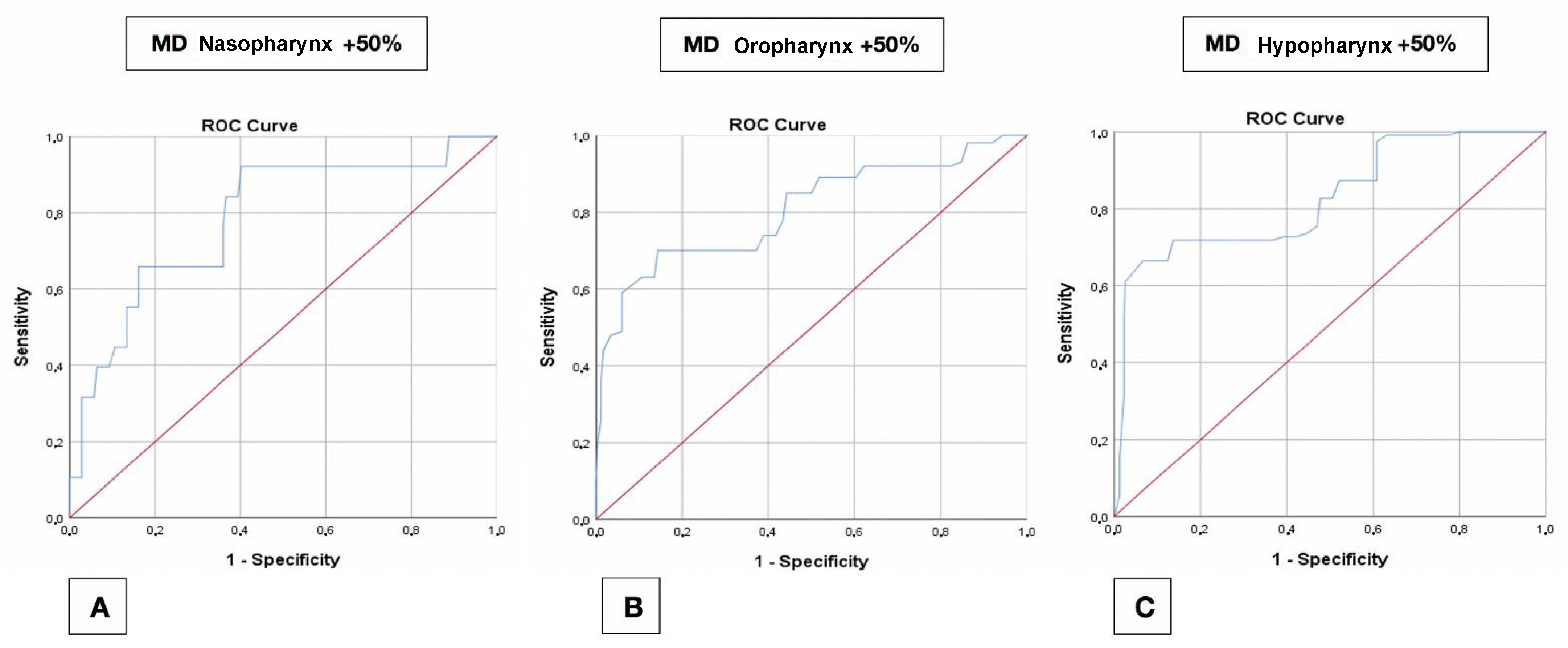
4.4. Receiver Operating Characteristic (ROC) Curves
4.4.1. Maxilla
4.4.2. Mandible
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hernández-Alfaro, F.; Guijarro-Martínez, R.; Mareque-Bueno, J. Effect of Mono- and Bimaxillary Advancement on Pharyngeal Airway Volume: Cone-Beam Computed Tomography Evaluation. J. Oral Maxillofac. Surg. 2011, 69, e395–e400. [Google Scholar] [CrossRef]
- Bianchi, A.; Betti, E.; Tarsitano, A.; Morselli-Labate, A.M.; Lancellotti, L.; Marchetti, C. Volumetric three-dimensional computed tomographic evaluation of the upper airway in patients with obstructive sleep apnoea syndrome treated by maxillomandib-ular advancement. Br. J. Oral Maxillofac. Surg. 2014, 52, 831–837. [Google Scholar] [CrossRef]
- Yamashita, A.L.; Filho, L.I.; Leite, P.C.C.; Navarro, R.D.L.; Ramos, A.L.; Previdelli, I.T.S.; Ribeiro, M.H.D.M.; Iwaki, L.C.V. Three-dimensional analysis of the pharyngeal airway space and hyoid bone position after orthognathic surgery. J. Cranio-Maxillofacial Surg. 2017, 45, 1408–1414. [Google Scholar] [CrossRef]
- Guijarro-Martínez, R.; Swennen, G. Cone-beam computerized tomography imaging and analysis of the upper airway: A systematic review of the literature. Int. J. Oral Maxillofac. Surg. 2011, 40, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Swennen, R.J. 3D Virtual Treatment Planning of Orthognathic Surgery; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Gurani, S.F.; Di Carlo, G.; Cattaneo, P.M.; Thorn, J.J.; Pinholt, E.M. Effect of Head and Tongue Posture on the Pharyngeal Airway Dimensions and Morphology in Three-Dimensional Imaging: A Systematic Review. J. Oral Maxillofac. Res. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, G.; Gurani, S.F.; Pinholt, E.M.; Cattaneo, P.M. A new simple three-dimensional method to characterize upper airway in orthognathic surgery patient. Dentomaxillofacial Radiol. 2017, 46. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Phillips, C.; Paniagua, B. The use of SPHARM-PDM and mean latitude axis to evaluate airway changes. Angle Orthod. 2016, 86, 943–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guijarro-Martínez, R.; Swennen, G.R. Three-dimensional cone beam computed tomography definition of the anatomical subre-gions of the upper airway: A validation study. Int. J. Oral Maxillofac. Surg. 2013, 42, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.N.; Vora, S.R.; Pliska, B.T. Reliability of upper airway assessment using CBCT. Eur. J. Orthod. 2018, 41, 101–108. [Google Scholar] [CrossRef]
- Ryan, D.P.O.; Bianchi, J.; Ignácio, J.; Wolford, L.M.; Gonçalves, J.R. Cone-beam computed tomography airway measurements: Can we trust them? Am. J. Orthod. Dentofac. Orthop. 2019, 156, 53–60. [Google Scholar] [CrossRef]
- Wiedemeyer, V.; Berger, M.; Martini, M.; Kramer, F.-J.; Heim, N. Predictability of pharyngeal airway space dimension changes after orthognathic surgery in class II patients: A mathematical approach. J. Cranio-Maxillofac. Surg. 2019, 47, 1504–1509. [Google Scholar] [CrossRef]
- Tan, S.K.; Tang, A.T.H.; Leung, W.K.; Zwahlen, R.A. Three-dimensional pharyngeal airway changes in dento-skeletal class II pa-tients after two-jaw orthognathic surgery with segmentation—A pilot study. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 461–468. [Google Scholar] [CrossRef]
- Rosário, H.D.; de Oliveira, B.G.; Pompeo, D.D.; de Freitas, P.H.L.; Paranhos, L.R. Surgical Maxillary Advancement Increases Upper Airway Volume in Skeletal Class III Patients: A Cone Beam Computed Tomography-Based Study. J. Clin. Sleep Med. 2016, 12, 1527–1533. [Google Scholar] [CrossRef] [Green Version]
- Torre, D.D.; Burtscher, D.; Widmann, G.; Rasse, M.; Puelacher, T.; Puelacher, W. Long-term influence of mandibular advancement on the volume of the posterior airway in skeletal Class II-patients:A retrospective analysis. Br. J. Oral Maxillofac. Surg. 2017, 55, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, H.S.; Choi, S.H.; Jung, Y.S.; Jung, H.D. Changes in position of the hyoid bone and volume of the phar-yngeal airway after mandibular setback: Three-dimensional analysis. Br. J. Oral Maxillofac. Surg. 2019, 57, 29–35. [Google Scholar] [CrossRef]
- Lee, S.-T.; Park, J.-H.; Kwon, T.-G. Influence of mandibular setback surgery on three-dimensional pharyngeal airway changes. Int. J. Oral Maxillofac. Surg. 2019, 48, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Muto, T.; Takeda, S.; Kanazawa, M.; Yamazaki, A.; Fujiwara, Y.; Mizoguchi, I. The effect of head posture on the pharyngeal airway space (PAS). Int. J. Oral Maxillofac. Surg. 2002, 31, 579–583. [Google Scholar] [CrossRef]
- Park, J.-E.; Bae, S.-H.; Choi, Y.-J.; Choi, W.-C.; Kim, H.-W.; Lee, U.-L. The structural changes of pharyngeal airway contributing to snoring after orthognathic surgery in skeletal class III patients. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterfield, K.J.; Marks, P.L.; McLean, L.; Newton, J. Linear and Volumetric Airway Changes After Maxillomandibular Advancement for Obstructive Sleep Apnea. J. Oral Maxillofac. Surg. 2015, 73, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Hu, H.; Liao, Q.; Zhang, W.; Xiang, X.; Fan, X. Impact on the upper airway space of different types of orthognathic surgery for the correction of skeletal class III malocclusion: A systematic review and meta-analysis. Int. J. Surg. 2017, 38, 31–40. [Google Scholar] [CrossRef]
- Tan, S.K.; Leung, W.K.; Tang, A.T.H.; Zwahlen, R.A. Effects of mandibular setback with or without maxillary advancement osteotomies on pharyngeal airways: An overview of systematic reviews. PLoS ONE 2017, 12, e0185951. [Google Scholar] [CrossRef] [PubMed]
- Christovam, I.; Lisboa, C.; Ferreira, D.M.T.P.; Cury-Saramago, A.; Mattos, C.T. Upper airway dimensions in patients undergoing orthognathic surgery: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Havron, A.G.; Aronovich, S.; Shelgikar, A.V.; Kim, H.L.; Conley, R.S. 3D Airway changes using CBCT in patients fol-lowing mandibular setback surgery ± maxillary advancement. Orthod. Craniofacial Res. 2019, 22, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Borojeni, A.A.T.; Garcia, G.J.M.; Moghaddam, M.G.; Frank-Ito, D.O.; Kimbell, J.S.; Laud, P.W.; Koenig, L.J.; Rhee, J.S. Normative ranges of nasal airflow variables in healthy adults. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 87–98. [Google Scholar] [CrossRef] [PubMed]


| N | Max | Min | Mean | S.D. | Median | IQR | ||
|---|---|---|---|---|---|---|---|---|
| MAMS | MX AP | 16 | 7.20 | 0.90 | 3.58 | 1.66 | 3.90 | 2.40 |
| MD AP | 16 | 0.00 | −5.80 | −2.39 | 1.63 | −2.50 | 3.00 | |
| MMA | MX AP | 29 | 15.10 | 0.00 | 4.36 | 3.07 | 3.80 | 3.50 |
| MD AP | 29 | 13.70 | 0.50 | 4.66 | 3.25 | 4.60 | 4.55 | |
| MMA—MX < 5 | MX AP | 19 | 4.40 | 0.00 | 2.69 | 1.42 | 3.40 | 2.10 |
| MD AP | 19 | 10.20 | 0.50 | 3.49 | 2.52 | 3.00 | 3.70 | |
| MMA—MX ≥ 5 | MX AP | 10 | 15.10 | 5.20 | 7.52 | 2.88 | 7.00 | 2.52 |
| MD AP | 10 | 13.70 | 1.10 | 6.86 | 3.46 | 6.60 | 3.98 | |
| MMA—MD < 5 | MX AP | 16 | 6.30 | 0.50 | 3.39 | 1.38 | 3.50 | 1.65 |
| MD AP | 16 | 5.00 | 0.50 | 2.38 | 1.54 | 1.85 | 3.05 | |
| MMA—MD ≥ 5 | MX AP | 13 | 15.10 | 0.00 | 5.54 | 4.1 | 5.60 | 5.30 |
| MD AP | 13 | 13.70 | 5.00 | 7.46 | 2.51 | 6.50 | 3.35 | |
| Delta Area % | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypopharynx | Oropharynx | Nasopharynx | n Patients | ||||||||||
| Mean | SD | min | Max | Mean | SD | min | Max | Mean | SD | min | Max | ||
| MAMS | 10 | 35 | −37 | 176 | 12 | 27 | −36 | 91 | 18 | 22 | −10 | 118 | 16 |
| MMA | 37 | 66 | −49 | 317 | 54 | 91 | −57 | 769 | 44 | 61 | −70 | 336 | 29 |
| MMA—Mn < 5 mm | 6 | 30 | −44 | 176 | 12 | 37 | −57 | 221 | 23 | 48 | −70 | 336 | 16 |
| MMA—Mn ≥ 5 mm | 74 | 79 | −49 | 317 | 106 | 142 | −22 | 769 | 70 | 71 | −13 | 272 | 13 |
| MMA—Mx < 5 mm | 12 | 42 | −49 | 176 | 24 | 50 | −57 | 221 | 24 | 45 | −66 | 336 | 19 |
| MMA—Mx ≥ 5 mm | 84 | 77 | −26 | 317 | 110 | 159 | −17 | 769 | 81 | 77 | −70 | 272 | 10 |
| Nasopharynx | Oropharynx | Hypopharynx | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Δ Area | Δ AP (mm) | Youden’s Index | ROC AUC | Δ AP (mm) | Youden’s Index | ROC AUC | Δ AP (mm) | Youden’s Index | ROC AUC | |
| Maxilla | 30% | 5.5 | 0.478 | 0.702 | 6.6 | 0.287 | 0.629 | 4.8 | 0.477 | 0.700 |
| 50% | 5.5 | 0.614 | 0.766 | 6.6 | 0.391 | 0.677 | 4.8 | 0.599 | 0.753 | |
| 100% | 6.6 | 0.815 | 0.890 | 7.0 | 0.597 | 0.735 | 4.8 | 0.566 | 0.728 | |
| Mandible | 30% | (1.4) 5.2 | (0.407) 0.380 | 0.723 | 5.2 | 0.384 | 0.687 | 5.9 | 0.494 | 0.746 |
| 50% | (1.4) 5.2 | (0.520) 0.496 | 0.785 | 5.2 | 0.557 | 0.805 | 5.9 | 0.596 | 0.826 | |
| 100% | 5.2 | 0.630 | 0.810 | 5.2 | 0.751 | 0.935 | 6.2 | 0.776 | 0.902 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badiali, G.; Lunari, O.; Bevini, M.; Bortolani, B.; Cercenelli, L.; Lorenzetti, M.; Marcelli, E.; Bianchi, A.; Marchetti, C. Existence of a Neutral-Impact Maxillo-Mandibular Displacement on Upper Airways Morphology. J. Pers. Med. 2021, 11, 177. https://doi.org/10.3390/jpm11030177
Badiali G, Lunari O, Bevini M, Bortolani B, Cercenelli L, Lorenzetti M, Marcelli E, Bianchi A, Marchetti C. Existence of a Neutral-Impact Maxillo-Mandibular Displacement on Upper Airways Morphology. Journal of Personalized Medicine. 2021; 11(3):177. https://doi.org/10.3390/jpm11030177
Chicago/Turabian StyleBadiali, Giovanni, Ottavia Lunari, Mirko Bevini, Barbara Bortolani, Laura Cercenelli, Manuela Lorenzetti, Emanuela Marcelli, Alberto Bianchi, and Claudio Marchetti. 2021. "Existence of a Neutral-Impact Maxillo-Mandibular Displacement on Upper Airways Morphology" Journal of Personalized Medicine 11, no. 3: 177. https://doi.org/10.3390/jpm11030177








