Is Metformin a Possible Beneficial Treatment for Psoriasis? A Scoping Review
Abstract
:1. Introduction
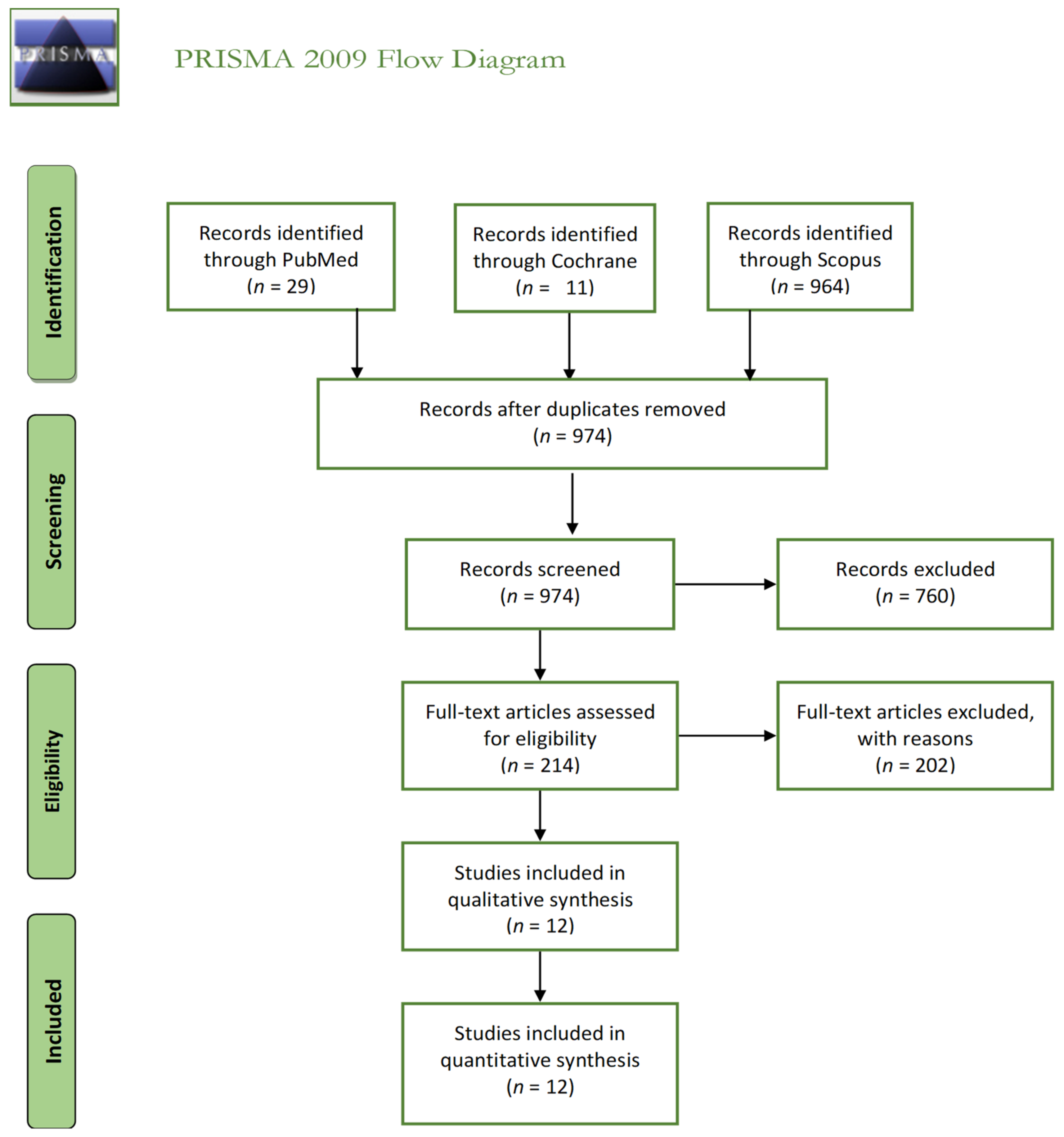
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
3. Results
3.1. Evidence from Experimental and Skin Cell Culture Studies Supporting the Use of Metformin for Psoriasis
3.2. Evidence from Clinical Studies Supporting Add-On Metformin for Psoriasis
3.3. Safety of Metformin Treatment in Psoriasis
3.4. Evidence against Metformin Treatment in Patients with Psoriasis
4. Discussion
- Metformin plays an important role in the treatment of autoimmune diseases.
- Better results are achieved with metformin and methotrexate combined, compared with methotrexate therapy alone.
- Good results have also resulted from topical therapy combined with metformin.
- Metformin decreases the risk of developing psoriasis in diabetic patients.
- Good results have been achieved with metformin treatment in patients with psoriasis and metabolic syndrome, for both psoriasis and metabolic syndrome.
- Metformin is generally safe for administration in patients with psoriasis.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.-F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to2025. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Schwartz, S.S.; Epstein, S.; Corkey, B.E.; Grant, S.F.; Iii, J.R.G.; Aguilar, R.B.; Herman, M.E. A Unified Pathophysiological Construct of Diabetes and its Complications. Trends Endocrinol. Metab. 2017, 28, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.S.; Chi, Y.; Metzendorf, M.-I.; Richter, B.; Hemmingsen, B. Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2019, 2019, CD008558. [Google Scholar] [CrossRef] [PubMed]
- Shpakov, A.O. Improvement Effect of Metformin on Female and Male Reproduction in Endocrine Pathologies and Its Mechanisms. Pharmaceuticals 2021, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Meyerhardt, A.J.; Irwin, M.L.; Jones, L.W.; Zhang, S.; Campbell, N.; Brown, J.C.; Pollak, M.; Sorrentino, A.; Cartmel, B.; Harrigan, M.; et al. Randomized Phase II Trial of Exercise, Metformin, or Both on Metabolic Biomarkers in Colorectal and Breast Cancer Survivors. JNCI Cancer Spectr. 2020, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- Mu, N.; Xu, T.; Gao, M.; Dong, M.; Tang, Q.; Hao, L.; Wang, G.; Li, Z.; Wang, W.; Yang, Y.; et al. Therapeutic effect of metformin in the treatment of endometrial cancer (Review). Oncol. Lett. 2020, 20, 1. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Zheng, Z.-J.; Kan, H.; Song, Y.; Cui, W.; Zhao, G.; Kip, K.E. Reduced Risk of Colorectal Cancer with Metformin Therapy in Patients with Type 2 Diabetes: A Meta-Analysis. Diabetes Care 2011, 34, 2323–2328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pircher, A.; Zieher, M.; Eigentler, A.; Pichler, R.; Schäfer, G.; Fritz, J.; Puhr, M.; Steiner, E.; Horninger, W.; Klocker, H.; et al. Antidiabetic drugs influence molecular mechanisms in prostate cancer. Cancer Biol. Ther. 2018, 19, 1153–1161. [Google Scholar] [CrossRef]
- Podhorecka, M.; Ibanez, B.; Dmoszyńska, A. Metformin—Its potential anti-cancer and anti-aging effects. Postępy Higieny i Medycyny Doświadczalnej 2017, 71, 170–175. [Google Scholar] [CrossRef]
- LaVine, J.E. Effect of Vitamin E or Metformin for Treatment of Nonalcoholic Fatty Liver Disease in Children and Adolescents. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, S.; Farran, B.; McGurnaghan, S.; McCrimmon, R.J.; Leese, G.P.; Petrie, J.R.; McKeigue, P.M.; Sattar, N.; Wild, S.; McKnight, J.; et al. Risk of acute kidney injury and survival in patients treated with Metformin: An observational cohort study. BMC Nephrol. 2017, 18, 163. [Google Scholar] [CrossRef] [Green Version]
- Dziubak, A.; Wójcicka, G.; Wojtak, A.; Bełtowski, J. Metabolic Effects of Metformin in the Failing Heart. Int. J. Mol. Sci. 2018, 19, 2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, M.; Al-Talabany, S.; McKinnie, A.; Mordi, I.R.; Singh, J.S.S.; Gandy, S.J.; Baig, F.; Hussain, M.S.; Bhalraam, U.; Khan, F.; et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: The MET-REMODEL trial. Eur. Heart J. 2019, 40, 3409–3417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Mathur, D.K.; Paliwal, V.; Bhargava, P. Efficacy of Metformin in the Treatment of Acne in Women with Polycystic Ovarian Syndrome: A Newer Approach to Acne Therapy. J. Clin. Aesthet. Derm. 2019, 12, 34–38. [Google Scholar]
- Sharpe, A.; Morley, L.C.; Tang, T.; Norman, R.J.; Balen, A.H. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, 2019, CD013505. [Google Scholar] [CrossRef]
- Vashist, S.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Yadav, R.S.; Sharma, S.B.; Sharma, V.; Sharma, A.; Chowdhary, B.; Kumar, P. Association of Psoriasis with Autoimmune Disorders: Results of a Pilot Study. Indian Dermatol. Online J. 2020, 11, 753–759. [Google Scholar]
- National Psoriasis Foundation. Statistics. Available online: https://www.psoriasis.org/content/statistics (accessed on 20 April 2020).
- World Health Organization. Global Report on Psoriasis. Available online: https://apps.who.int/iris/handle/10665/ (accessed on 1 February 2021).
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [Green Version]
- Abramczyk, R.; Queller, J.N.; Rachfal, A.W.; Schwartz, S.S. Diabetes and Psoriasis: Different Sides of the Same Prism. Diabetes Metab. Syndr. Obesity Targets Ther. 2020, 13, 3571–3577. [Google Scholar] [CrossRef] [PubMed]
- Glossmann, H.; Reider, N. A marriage of two “Methusalem” drugs for the treatment of psoriasis? Derm. Endocrinol. 2013, 5, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Arnone, M.; Takahashi, M.D.F.; De Carvalho, A.V.E.; Bernardo, W.M.; Bressan, A.L.; Ramos, A.M.C.; Terena, A.C.; Souza, C.D.S.; Nunes, D.H.; Bortoletto, M.C.D.C.; et al. Diagnostic and therapeutic guidelines for plaque psoriasis—Brazilian Society of Dermatology. An. Bras. Dermatol. 2019, 94, 76–107. [Google Scholar] [CrossRef] [Green Version]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Rizzuto, F.; Dastoli, S.; Patruno, C.; Bianchi, L.; Nisticò, S.P. A novel vehicle for the treatment of psoriasis. Dermatol. Ther. 2020, 33, e13185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannone, L.F.; Bennardo, L.; Palleria, C.; Roberti, R.; De Sarro, C.; Naturale, M.D.; Dastoli, S.; Donato, L.; Manti, A.; Valenti, G.; et al. Safety profile of biologic drugs for psoriasis in clinical practice: An Italian prospective pharmacovigilance study. PLoS ONE 2020, 15, e0241575. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Tamburi, F.; Amoruso, G.F.; Bennardo, L.; Nisticò, S.P. Emerging role of anti-IL23 in the treatment of psoriasis: When humanized is very promising. Dermatol. Ther. 2020, 33, e14504. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, A.M.A.; Simionescu, A.A.; Diaconu, C.C. Oral Vitamin D Therapy in Patients with Psoriasis. Nutrients 2021, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tricco, A.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, F.; Ma, W.; Sun, Q. Metformin inhibits proliferation and proinflammatory cytokines of human keratinocytesin vitrovia mTOR-signaling pathway. Pharm. Biol. 2015, 54, 1173–1178. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, R.; Zhao, X.; Yu, X.; Sun, Q. Metformin Promotes HaCaT Cell Apoptosis through Generation of Reactive Oxygen Species via Raf-1-ERK1/2-Nrf2 Inactivation. Inflammation 2018, 41, 948–958. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, S.; Ren, J.; Zhang, D. A unified mitochondria mechanistic target of rapamycin acyl-coenzyme A dehydrogenase 10 signal relay modulation for metformin growth inhibition in human immortalized keratinocytes cells. J. Cell. Biochem. 2018, 120, 1773–1782. [Google Scholar] [CrossRef]
- Kamiński, M.M.; Sauer, S.W.; Klemke, C.-D.; Süss, D.; Okun, J.G.; Krammer, P.H.; Gülow, K. Mitochondrial Reactive Oxygen Species Control T Cell Activation by Regulating IL-2 and IL-4 Expression: Mechanism of Ciprofloxacin-Mediated Immunosuppression. J. Immunol. 2010, 184, 4827–4841. [Google Scholar] [CrossRef] [Green Version]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Ip, W.; Kirchhof, M.G. Glycemic Control in the Treatment of Psoriasis. Dermatology 2017, 233, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Ba, W.; Xu, Y.; Yin, G.; Yang, J.; Wang, R.; Chi, S.; Wang, Y.; Li, C. Metformin inhibits pro-inflammatory responses via targeting nuclear factor-κB in HaCaT cells. Cell Biochem. Funct. 2019, 37, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, G.; Hashimoto-Hachiya, A.; Yen, V.H.; Takemura, M.; Yumine, A.; Furue, K.; Furue, M.; Nakahara, T. Metformin inhibits IL-1β secretion via impairment of NLRP3 inflammasome in keratinocytes: Implications for preventing the development of psoriasis. Cell Death Discov. 2020, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, G.; Nakahara, T.; Furue, M. 458 The antidiabetic agent metformin prevents the development of psoriasis via inhibition of caspase-1 inflammasome. J. Investig. Dermatol. 2019, 139, S79. [Google Scholar] [CrossRef] [Green Version]
- Al-Amran, F.G.; Hadi, N.R.; Swadi, A. Metformin ameliorates methotrexate-induced hepatotoxicity. J. Pharmacol. Pharmacother. 2012, 3, 248–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, T.H.T.; Thi, V.B.; Ngoc, A.T.; Van, E.D.; Dang, Q.T.; Van, T.N.; Minh, P.P.T.; Thi, L.P.; Huu, N.D.; Gandolfi, M.; et al. Quality of Life in Psoriasis Vietnamese Patients Treated with Metformin in Combination with Methotrexate. Open Access Maced. J. Med. Sci. 2019, 7, 302–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou-Raya, A.; Helmii, M. SAT0384 Metformin: A Valid ADD-ON Drug in the Treatment of Psoriatic Arthritis—Randomized Controlled Trial. Ann. Rheum. Dis. 2014, 73, 733. [Google Scholar] [CrossRef]
- Robert, F.; Fendri, S.; Hary, L.; Lacroix, C.; Andréjak, M.; Lalau, J.D. Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects. Diabetes Metab. 2003, 29, 279–283. [Google Scholar] [CrossRef]
- Brauchli, Y.B.; Jick, S.S.; Curtin, F.; Meier, C.R. Association between use of thiazolidinediones or other oral antidiabetics and psoriasis: A population based case-control study. J. Am. Acad. Dermatol. 2008, 58, 421–429. [Google Scholar] [CrossRef]
- Kim, S.C.; Schneeweiss, S.; Glynn, R.J.; Doherty, M.; Goldfine, A.B.; Solomon, D.H. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: A population-based cohort study. Ann. Rheum. Dis. 2015, 74, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Shieh, J.-J.; Shen, J.-L.; Liu, Y.-Y.; Chang, Y.-T.; Chen, Y.-J. Association between antidiabetic drugs and psoriasis risk in diabetic patients: Results from a nationwide nested case-control study in Taiwan. J. Am. Acad. Dermatol. 2015, 72, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhansali, A. Randomized placebo control study of insulin sensitizers (Metformin and Pioglitazone) in psoriasis patients with metabolic syndrome (Topical Treatment Cohort). BMC Dermatol. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Bhansali, A. Randomized Placebo Control Study of Metformin in Psoriasis Patients with Metabolic Syndrome (Systemic Treatment Cohort). Indian J. Endocrinol. Metab. 2017, 21, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Parasramani, S.G. Itolizumab in the Management of Psoriasis with Metabolic Syndrome. J. Clin. Diagn. Res. 2017, 11, WD01–WD02. [Google Scholar] [CrossRef]
- Łebkowska, A.; Krentowska, A.; Adamska, A.; Lipińska, D.; Piasecka, B.; Kowal-Bielecka, O.; Górska, M.; Semple, R.K.; Kowalska, I. Type B insulin resistance syndrome associated with connective tissue disease and psoriasis. Endocrinol. Diabetes Metab. Case Rep. 2020, 2020, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.F.; Soper, T.; Jones, N.; Grevelink, J.; Abourizk, N. Psoriatic Exacerbation Associated with Insulin Therapy. Diabetes Care 2008, 31, e31. [Google Scholar] [CrossRef] [Green Version]
- El-Gharabawy, R.M.; Ahmed, A.S.; Al-Najjar, A.H. Mechanism of action and effect of immune-modulating agents in the treatment of psoriasis. Biomed. Pharmacother. 2017, 85, 141–147. [Google Scholar] [CrossRef]
- Su, Y.-J.; Chen, T.-H.; Hsu, C.-Y.; Chiu, W.-T.; Lin, Y.-S.; Chi, C.-C. Safety of Metformin in Psoriasis Patients with Diabetes Mellitus: A 17-Year Population-Based Real-World Cohort Study. J. Clin. Endocrinol. Metab. 2019, 104, 3279–3286. [Google Scholar] [CrossRef]
- Zibar, L.; Zibar, K. Hemodialysis-refractory metformin-associated lactate acidosis with hypoglycemia, hypothermia, and bradycardia in a diabetic patient with belated diagnosis and chronic kidneydisease. Int. J. Clin Pharmacol. Ther. 2017, 55, 348–351. [Google Scholar] [CrossRef]
- Rhee, C.M.; Kovesdy, C.P.; Kalantar-Zadeh, K. Risks of metformin intype 2 diabetes and chronic kidney disease: Lessons learned from Taiwanese data. Nephron 2017, 135, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubna, A.K. Metformin—For the dermatologist. Indian J. Pharmacol. 2016, 48, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Sung, C.; Chao, T.; Lee, A.; Foulad, D.; Choi, F.; Juhasz, M.; Dobry, A.; Mesinkovska, N. Oral Metformin for Treating Dermatological Diseases: A Systematic Review. J. Drugs Dermatol. JDD 2020, 19, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-E.; Choi, M.S. A Molecular Perspective on the Potential Benefits of Metformin for the Treatment of Inflammatory Skin Disorders. Int. J. Mol. Sci. 2020, 21, 8960. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Lee, C.; Liu, C.; Wang, S.; O’Donnell, F.T.; Tung, T. Effects of antidiabetic drugs on psoriasis: A meta-analysis. Eur. J. Clin. Investig. 2021, 51. [Google Scholar] [CrossRef]
- Mittal, R.; Malhotra, S.; Pandhi, P.; Kaur, I.; Dogra, S. Efficacy and safety of combination acitretin and pioglitazone therapy in patients with moderate to severe chronic plaque-type psoriasis: A randomized, double-blind, placebo-controlled clinical trial. Arch. Dermatol. 2009, 145, 387–393. [Google Scholar] [CrossRef]
- Shafiq, N.; Malhotra, S.; Pandhi, P.; Gupta, M.; Kumar, B.; Sandhu, K. Pilot trial: Pioglitazone versus placebo in patients with plaque psoriasis (the P6). Int. J. Dermatol. 2005, 44, 328–333. [Google Scholar] [CrossRef]
- Ghiasi, M.; Ebrahimi, S.; Lajevardi, V.; Taraz, M.; Azizpour, A. Efficacy and safety of pioglitazone plus phototherapy versus phototherapy in patients with plaque type psoriasis: A Double Blinded Randomized Controlled Trial. J. Dermatol. Treat. 2018, 30, 664–667. [Google Scholar] [CrossRef]
- Hafez, V.G.; Bosseila, M.; Halim, M.R.E.A.; Shaker, O.G.; Kamal, M.; Kareem, H.S. Clinical effects of “pioglitazone”, an insulin sensitizing drug, on psoriasis vulgaris and its co-morbidities, a double blinded randomized controlled trialx1. J. Dermatol. Treat. 2014, 26, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lajevardi, V.; Hallaji, Z.; Daklan, S.; Abedini, R.; Goodarzi, A.; Abdolreza, M. The efficacy of methotrexate plus pioglitazone vs. methotrexate alone in the management of patients with plaque-type psoriasis: A single-blinded randomized controlled trial. Int. J. Dermatol. 2014, 54, 95–101. [Google Scholar] [CrossRef]
- Ellis, C.N.; Barker, J.N.; Haig, A.E.; Parker, C.A.; Daly, S.; Jayawardene, D.A. Placebo response in two long-term randomized psoriasis studies that were negative for rosiglitazone. Am. J. Clin. Dermatol. 2007, 8, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, A.; Gyldenløve, M.; Rohde, U.; Thyssen, J.; Zachariae, C.; Skov, L.; Knop, F.; Vilsbøll, T. Lack of effect of the glucagon-like peptide-1 receptor agonist liraglutide on psoriasis in glucose-tolerant patients—A randomized placebo-controlled trial. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Koca, R.; Altinyazar, H.C.; Yenidünya, S.; Tekin, N.S. Psoriasiform drug eruption associated with metformin hydrochloride: A case report. Dermatol. Online J. 2003, 9, 11. [Google Scholar]
- Voore, P.; Odigwe, C.; Mirrakhimov, A.E.; Rifai, D.; Iroegbu, N.A. DRESS Syndrome Following Metformin Administration: A Case Report and Review of the Literature. Am. J. Ther. 2016, 23, e1970–e1973. [Google Scholar] [CrossRef]
- Cochran, R.E.I.; Thomson, J.; Fleming, K.; McQueen, A. The Psoriasiform Eruption Induced by Practolol. J. Cutan. Pathol. 1975, 2, 314–319. [Google Scholar] [CrossRef]
- Leonard, J.C. Letter: Oxprenolol and a psoriasis-like eruption. Lancet 1975, 1, 630. [Google Scholar] [CrossRef]
- Halevy, S.; Feuerman, E.J. Psoriasiform eruption induced by propranolol. Cutis 1979, 24, 95–98. [Google Scholar]
- A Neumann, H.; Van Joost, T.; Westerhof, W. Dermatitis as side-effect of long-term metoprolol. Lancet 1979, 2, 745. [Google Scholar] [CrossRef]
- Gawkrodger, D.; Beveridge, G. Psoriasiform reaction to atenolol. Clin. Exp. Dermatol. 1984, 9, 92–94. [Google Scholar] [CrossRef]
- Tsankov, N.; Angelova, I.; Kazandjieva, J. Drug-Induced Psoriasis. Am. J. Clin. Dermatol. 2000, 1, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Dorfman, B.; Krakowski, A. Psoriasiform eruption induced by captopril and chlorthalidone. Cutis 1987, 40, 162–164. [Google Scholar] [PubMed]
- Brenner, S.; Wolf, R.; Landau, M.; Politi, Y. Psoriasiform eruption induced by anticonvulsants. Isr. J. Med. Sci. 1994, 30, 283–286. [Google Scholar]
- Bharath, L.P.; Nikolajczyk, B.S. The Intersection of Metformin and Inflammation. Am. J. Physiol. Physiol. 2021. [Google Scholar] [CrossRef]
- Bartosińska, J.; Przepiórka-Kosińska, J.; Sarecka-Hujar, B.; Raczkiewicz, D.; Kowal, M.; Chyl-Surdacka, K.; Bartosiński, J.; Kosiński, J.; Krasowska, D.; Chodorowska, G. Osteopontin Serum Concentration and Metabolic Syndrome in Male Psoriatic Patients. J. Clin. Med. 2021, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Lønnberg, A.S.; Skov, L. Co-morbidity in psoriasis: Mechanisms and implications for treatment. Expert Rev. Clin. Immunol. 2016, 13, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sodek, J.; Da Silva, A.P.B.; Zohar, R. Osteopontin and Mucosal Protection. J. Dent. Res. 2006, 85, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, L.; Kähäri, V.-M. Matrix metalloproteinases in inflammation. Biochim. et Biophys. Acta BBA Gen. Subj. 2014, 1840, 2571–2580. [Google Scholar] [CrossRef]
- Kahles, F.; Findeisen, H.M.; Bruemmer, D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol. Metab. 2014, 3, 384–393. [Google Scholar] [CrossRef]
- Yang, X.; Yang, T.; Li, J.; Yang, R.; Qi, S.; Zhao, Y.; Li, L.; Li, J.; Zhang, X.; Yang, K.; et al. Metformin prevents nephrolithiasis formation by inhibiting the expression of OPN and MCP-1 in vitro and in vivo. Int. J. Mol. Med. 2019, 43, 1611–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Author | Year of Publication | Country | Type of Study | Mechanism and Effectiveness of Metformin Therapy in Psoriasis |
|---|---|---|---|---|
| Liu et al. [29] | 2015 | China | human keratinocytes HaCaT | - Metformin treatment significantly inhibited proliferation and proinflammatory responses (dose-dependently) in HaCaT cells, by a mechanism associated with inhibition of the mTOR signaling pathway. - Metformin inhibited the expression of IL-6, TNF-a, and VEGF proteins in HaCaT cells. - Metformin induced HaCaT cell apoptosis. |
| Wang et al. [30] | 2018 | China | human keratinocytes HaCaT | - Metformin could attenuate Raf-1-ERK1/2 signaling in HaCaT cells. - Metformin suppressed the expression and phosphorylation levels of Nrf2, which contributed to intracellular ROS generation and pro-apoptotic effects. |
| Wu et al. [31] | 2017 | China | Human HaCaT | - Metformin has antiproliferative and proapoptotic effects through the upregulation of ACAD10 expression, which is mediated by the negative regulation of mitochondria-mTORC1 signaling via the induction of cell-cycle arrest and apoptosis in human keratinocytes. |
| Ba et al. [35] | 2018 | China | Human cell culture | - Metformin significantly decreased the production of inflammatory cytokines and inhibited the nuclear localization of p65. - Metformin inhibits TNFα-induced inflammatory responses in HaCaT cells via nuclear factor kappa B (NF-κB) signaling. |
| - Metformin suppresses the transcriptional activity of NF-κB by suppressing the degradation of IκBα. Furthermore, metformin’s inhibitory effect on NF-κB is comparable to that of the specific IKKβ inhibitor, BI605906. | ||||
| Tsuji et al. [36] | 2020 | Japan | Animal tissue culture | - Metformin has immunomodulatory effects in an induced-psoriasis mouse model associated with type 2 diabetes mellitus. - TNF-α and IL-17A induce inflammatory responses by keratinocytes by blocking NLRP3 inflammasome activation in vitro. - Oral metformin treatment significantly attenuates IMQ-induced psoriasis-like inflammation in vivo. The therapeutic benefits can be partially attributed to its interference with mature IL-1β secretion by keratinocytes. |
| Author | Year of Publication | Country | Type of Study | Diseases |
|---|---|---|---|---|
| Kim et al. [43] | 2014 | US | Cohort | Autoimmune diseases (rheumatoid arthritis, systemic lupus erythematosus, psoriasis, multiple sclerosis, and inflammatory bowel disease). |
| Wu et al. [44] | 2015 | Taiwan | Case-control | Diabetic patients, especially among those who have never used insulin. |
| Author | Year of Publication | Country | Type of Study | Metformin Therapy in Psoriasis | Dose |
|---|---|---|---|---|---|
| Brauchli et al. [42] | 2008 | Switzerland | Case-control | Effect of long-term use of metformin in obese patients associated with thiazolidinediones | |
| Singh and Bhansali [45] | 2016 | India | Randomized | Metformin improved features of metabolic syndrome (MS). Metformin and pioglitazone improving MS parameters might account for the improved efficacy in psoriasis itself. The anti-proliferative and anti-inflammatory action of metformin might have resulted in improvement of the psoriasis. There is significant reduction in weight with the use of metformin, and due to controversy about increased risk of bladder cancer associated with pioglitazone, metformin can be preferred over pioglitazone in psoriasis patients with MS. | Metformin 1000 mg once daily (O.D) or pioglitazone 30 mg |
| Singh et al. [46] | 2017 | India | Randomized | Metformin has shown improvement in psoriasis and parameters of MS; hence, it can be used for the benefit of psoriasis patients having MS. The metformin group had greater percentage reduction in mean PASI, ESI, and PGA scores as compared with placebo. In total, 45% of the patients had complete improvement in MS in the metformin group as compared with 33.3% of patients in the placebo group. Patients taking metformin had statistically significant decreased weight, BMI, waist circumference, fasting plasma glucose, serum triglycerides, total cholesterol, and LDL-C as compared with patients taking placebo. | |
| El-Gharabawy et al. [50] | 2016 | Saudi Arabia | Randomized Study | Metformin modulates the immune system (causing a significant decline in CD4+ T cells) in psoriasis and MS or impaired glucose tolerance and has a remarkable effect in the early stages of psoriasis. Therefore, either pioglitazone or metformin in combination with traditional anti-psoriatic drugs provides better results in the treatment of psoriasis than does either alone. | 850 mg twice daily |
| Su et al. [51] | 2019 | Taiwan | Retrospective cohort study 1995–2014 | Metformin can be prescribed for diabetic psoriasis patients without chronic kidney disease. |
| Antidiabetic Drug | Dose | Period | Country | Year | Authors |
|---|---|---|---|---|---|
| Metformin | 1000 mg | 12 weeks | India India | 2016 2017 | Singh and Bhansali [45] Singh and Bhansali [46] |
| Pioglitazone | 15–30 mg | 10–16 weeks | India India India Iran Egypt Iran | 2016 2009 2005 2019 2015 2015 | Singh and Bhansali [45] Mittal at al. [58] Shafiq et al. [59] Ghiasi et al. [60] Hafez et al. [61] Lajevardi et al. [62] |
| Rosiglitazone | 2–8 mg | 26 weeks | SUA | 2007 | Ellis et al. [63] |
| Liraglutide subcutaneous injection | Initial dose: 0.6 mg—1 week 1.2 mg—1 week 1.8 mg—6 weeks | 8 weeks | Denmark | 2015 | Faurschou et al. [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanescu, A.M.A.; Simionescu, A.A.; Florea, M.; Diaconu, C.C. Is Metformin a Possible Beneficial Treatment for Psoriasis? A Scoping Review. J. Pers. Med. 2021, 11, 251. https://doi.org/10.3390/jpm11040251
Stanescu AMA, Simionescu AA, Florea M, Diaconu CC. Is Metformin a Possible Beneficial Treatment for Psoriasis? A Scoping Review. Journal of Personalized Medicine. 2021; 11(4):251. https://doi.org/10.3390/jpm11040251
Chicago/Turabian StyleStanescu, Ana Maria Alexandra, Anca Angela Simionescu, Mira Florea, and Camelia Cristina Diaconu. 2021. "Is Metformin a Possible Beneficial Treatment for Psoriasis? A Scoping Review" Journal of Personalized Medicine 11, no. 4: 251. https://doi.org/10.3390/jpm11040251
APA StyleStanescu, A. M. A., Simionescu, A. A., Florea, M., & Diaconu, C. C. (2021). Is Metformin a Possible Beneficial Treatment for Psoriasis? A Scoping Review. Journal of Personalized Medicine, 11(4), 251. https://doi.org/10.3390/jpm11040251








