A Systematic Literature Review on the Application of Machine-Learning Models in Behavioral Assessment of Autism Spectrum Disorder
Abstract
:1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Selection Criteria
2.3. Quality Assessment
2.4. Data Extraction
- Author(s) (year),
- Number of citations,
- Source(s) of the research data,
- Data collection/assessment instrument,
- ML model(s)developed,
- Best performing model(s),
- The key finding(s).
3. Results
3.1. Descriptive Analysis on Trends and Status of the Study on ML in ASD Assessment
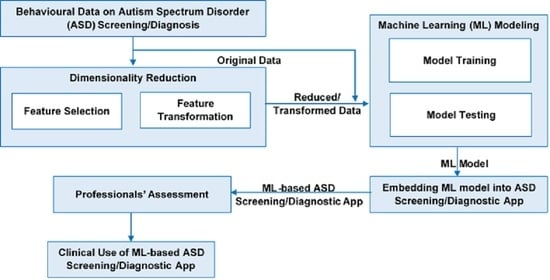
3.2. Dimensionality Reduction Techniques
3.3. Models Implementation
3.4. Data Collection/Assessment Instruments
3.5. Sources of Data
3.6. Research Procedures
| Article/ Citations | Aim | Tool | Data Source | FS/FT | FS/FT Method | Modeling Algorithms | Key Findings |
|---|---|---|---|---|---|---|---|
| Goel et al. [51] C = 10 | Proposed Optimization Algorithm for improved performance over common ML | AQ-10 (child, adolescent, adult) | ASDTest | - | - | GOA, BACO, LR, NB, KNN, RF-CART + ID3, * MGOA | The proposed MGOA (GOA with Random Forest classifier) predicted ASD cases with approximate accuracy, specificity, and sensitivity of 100%. |
| Shahamiri and Thabtah [11] C = 0 | Implementation and evaluation of CNN-based ASD scoring system | Q-CHAT-10, AQ-10 | ASDTest | - | - | C4.5, Bayes Net, RIDOR, * CNN | The performance evaluation showed the superior performance of CNN over other algorithms; indicating the robustness of the implemented system. |
| Thabtah and Peebles [52] C = 28 | Demonstrate the superiority of Rules-based ML over other models | Q-CHAT-10, AQ-10 (child, Adolescent, adult) | ASDTest | - | - | RIPPER, RIDOR, Nnge, Bagging, CART, C4.5, and PRISM, * RML | Empirically evaluated rule induction, Bagging, Boosting, and decision trees algorithms on different ASD datasets. The superiority of the RML model was reported in not only classifying ASD but also offer rules that can be utilized in understanding the reasons behind the classification. |
| Wall et al. [35] C = 106 | Streamlining ADR-I and evaluate ML performance | ADI-R | AGRE, SSC, AC | FS | Trial-error | * ADTree, BFTree, ConjunctiveRule, DecisionStump, FilteredClassifier, J48, J48graft, JRip, LADTree, Nnge, OneR, OrdinalClassClassifier, PART, Ridor, and SimpleCart | The best model utilized 7 of the 93 items contained in the ADI-R in classifying ASD with 99.9% accuracy. |
| Duda et al. [39] C = 50 | Streamlining ADOS and demonstrate the superior performance of ADTree over common hand-crafted methods | ADOS | AC, AGRE, SSC, NDAR, SVIP | FS | Trial-error | ADTree | 72% reduction in the items from ADOS-G with >97% accuracy. |
| Küpper et al. [40] C = 2 | Streamlining ADOS and demonstrate the performance of SVM | ADOS | ASD outpatient clinics in Germany | FS | Recursive Feature Selection | SVM | SVM achieved good sensitivity and specificity with fewer ADOS items pointing to 5 behavioral features. |
| Wall et al. [34] C = 160 | Streamlining ADOS and evaluate ML performance | ADOS | AC, AGRE, SSC | FS | Trial-error | * ADTree, BFTree, Decision Stump, Functional Tree, J48, J48graft, Jrip, LADTree, LMT, Nnge, OneR, PART, Random Tree, REPTree, Ridor, Simple Cart | The ADTree model utilized 8 of the 29 items in Module 1 of the ADOS and classified ASD with 100% accuracy. |
| Levy et al. [50] C = 21 | Streamlining ADOS and evaluate ML performance | ADOS | AC, AGRE, SSC, SVIP | FS | Sparsity/parsimony enforcing regularization techniques | LR, Lasso, Ridge, Elastic net, Relaxed Lasso, Nearest shrunken centroids, LDA, * LR, * SVM, ADTree, RF, Gradient boosting, AdaBoost | With at most 10 features from ADOS′s Module 3 and Module 2, AUC of 0.95 and 0.93 was achieved, respectively. |
| Kosmicki et al. [37] C = 84 | Streamlining ADOS and evaluate ML performance | ADOS | AC, AGRE, SSC, NDAR, SVIP | FS | Stepwise Backward Feature Selection | ADTree, * SVM, Logistic Model Tree, * LR, NB, NBTree, RF | The best performing models have utilized 9 of the 28 items from module 2, and 12 of the 28 items from module 3 in classifying ASD with 98.27% and 97.66% accuracy, respectively. |
| Thabtah [13] C = 31 | Propose ASDTest; AQ-based mobile screening app, streamline AQ-10 items, and evaluate the performance of 2 ML models | AQ-10 (child, adolescent, adult) | ASDTest | FS | Trial-error | NB, * LR | Feature and predictive analyses demonstrate small groups of autistic traits improving the efficiency and accuracy of screening processes. |
| Thabtah et al. [46] C = 47 | Demonstrate the superiority of Va over other FS methods based on the performance of ML models on the streamlined datasets | Q-CHAT-10, and AQ-10 (child, adolescent, adult) | ASDTest | FS | Va, IG, Correlation, CFS, and CHI | Repeated Incremental Pruning to Produce Error Reduction (RIPPER), C4.5 (Decision Tree) | Va derived fewer features from adults, adolescents, and child datasets with optimal model performance. Demonstrate the efficacy of Va over IG, Correlation, CFS, and CHI in reducing AQ-10 items |
| Thabtah et al. [48] C = 13 | Streamlining AQ-10 and demonstrate the superior performance of LR over common hand-crafted methods | AQ-10 (adolescent, adult) | ASDTest | FS | IG, CHI | LR | LR showed acceptable performance in terms of sensitivity, specificity, and accuracy among others. |
| Suresh Kumar and Renugadevi [49] C = 0 | Algorithm Optimization (improvement in accuracy compared to common ML) | AQ-10 (child, adolescent, adult) | ASDTest | FS | SFS | SVM, ANN, * DE SVM, DE ANN | DE optimized SVM outperformed ANN and DE optimized ANN in classifying ASD. DE is effective. |
| Pratama et al. [47] C = 0 | Input Optimization using Va | AQ-10 (child, adolescent, adult) | ASDTest | FS | Va | SVM, * RF, ANN | RF succeeded in producing higher adult AQ sensitivity (87.89%), and a rise in the specificity level of AQ-Adolescents was better produced using SVM (86.33%). |
| Usta et al. [45] C = 9 | ML Performance Evaluation | Autism Behavior Checklist, Aberrant Behavior Checklist, Clinical Global Impression | Ondokuz Mayis University Samsun | FS | Trial-error | NB, LR, * ADTree | The ML modeling revealed the significant influence of other demographic parameters in ASD classification. |
| Wingfield et al. [12] C = 3 | Propose PASS; a culturally sensitive app embedded with ML model | PASS | VPASS app | FS | CFS, mRMR | * RF, NB, Adaboost, Multilayer Perceptron, J48, PART, SMO | PASS app overcomes the cultural variation in interpreting ASD symptoms, and the study demonstrated the possibility of removing feature redundancy. |
| Duda et al. [36] C = 89 | ML Performance Evaluation in classifying ASD from ADHD | SRS | AC, AGRE, SSC | FS | Forward Feature Selection | ADTree, RF, SVM, LR, Categorical lasso, LDA | All the models could classify ASD from ADHD by utilizing 5 of the 65 items of SRS with high average accuracy (AUC = 0.965). |
| Duda et al. [53] C = 25 | Improve models’ reliability using expanded datasets for classifying ASD from ADHD | SRS | AC, AGRE, SSC, and crowdsourced data | FS | - | SVM, LR, * LDA | LDA model achieved an AUC of 0.89 with 15 items. |
| Bone et al. [38] C = 77 | Demonstrate the improved accuracy of SVM over common hand-crafted rules | ADI-R, SRS | Balanced Independent Dataset | FT | Tuned parameters across multiple levels of cross-validation | SVM | The SVM model utilized five of the fused ADI-R and SRS items and classified ASD sufficiently with below (above) 89.2% (86.7%) sensitivity and 59.0% (53.4%) specificity. |
| Puerto et al. [42] C = 17 | Propose MFCM-ASD and evaluate its performance against other ML models | ADOS, ADI-R | APADA | FT | Inputs fuzzification | * MFCM-ASD, SVM, Random forest, NB | The superior performance of MFCM characterized by its robustness makes it an effective ASD diagnostic technique. |
| Akter et al. [44] C = 6 | Compare FT methods and evaluate the performance of ML models on the transformed datasets | Q-CHAT-10, and AQ-10 (child, adolescent, adult) | ASDTest | FT | Log, Z-score, and Sine FT | Adaboost, FDA, C5.0, LDA, MDA, PDA, SVM, and CART | Varying superior performances of the ML models and FT approaches were achieved across the datasets. |
| Baadel et al. [43] C = 2 | Input Optimization using a clustering approach | AQ-10 (child, adolescent, adult) | ASDTest | FT | CATC | OMCOKE, RIPPER, PART, * RF, RT, ANN | CATC showed significant improvement in screening ASD based on traits′ similarity as opposed to scoring functions. The improvement was more pronounced with RF classifier. |
4. Discussion
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Publishing: Philadelphia, PA, USA, 2013. [Google Scholar]
- Chauhan, A.; Sahu, J.; Jaiswal, N.; Kumar, K.; Agarwal, A.; Kaur, J.; Singh, S.; Singh, M. Prevalence of autism spectrum disorder in Indian children: A systematic review and meta-analysis. Neurol. India 2019, 67, 100. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Rosenberg, C.R.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.S.; Elsabbagh, M.; Barbaro, J.; Gladstone, M.; Happe, F.; Hoekstra, R.A.; Lee, L.C.; Rattazzi, A.; Stapel-Wax, J.; Stone, W.L.; et al. Autism screening and diagnosis in low resource settings: Challenges and opportunities to enhance research and services worldwide. Autism Res. 2015, 8, 473–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matson, J.L.; Konst, M.J. Early intervention for autism: Who provides treatment and in what settings. Res. Autism Spectr. Disord. 2014, 8, 1585–1590. [Google Scholar] [CrossRef]
- Case-Smith, J.; Weaver, L.L.; Fristad, M.A. A systematic review of sensory processing interventions for children with autism spectrum disorders. Autism 2015, 19, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guthrie, W.; Wallis, K.; Bennett, A.; Brooks, E.; Dudley, J.; Gerdes, M.; Pandey, J.; Levy, S.E.; Schultz, R.T.; Miller, J.S. Accuracy of autism screening in a large pediatric network. Pediatrics 2019, 144, e20183963. [Google Scholar] [CrossRef]
- Øien, R.A.; Candpsych, S.S.; Volkmar, F.R.; Shic, F.; Cicchetti, D.V.; Nordahl-Hansen, A.; Stenberg, N.; Hornig, M.; Havdahl, A.; Øyen, A.S.; et al. Clinical features of children with autism who passed 18-month screening. Pediatrics 2018, 141, e20173596. [Google Scholar] [CrossRef] [Green Version]
- Surén, P.; Saasen-Havdahl, A.; Bresnahan, M.; Hirtz, D.; Hornig, M.; Lord, C.; Reichborn-Kjennerud, T.; Schjølberg, S.; Øyen, A.-S.; Magnus, P.; et al. Sensitivity and specificity of early screening for autism. BJPsych Open 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Yuen, T.; Carter, M.T.; Szatmari, P.; Ungar, W.J. Cost-Effectiveness of Universal or High-Risk Screening Compared to Surveillance Monitoring in Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 2968–2979. [Google Scholar] [CrossRef]
- Shahamiri, S.R.; Thabtah, F. Autism AI: A New Autism Screening System Based on Artificial Intelligence. Cognit. Comput. 2020, 12, 766–777. [Google Scholar] [CrossRef]
- Wingfield, B.; Miller, S.; Yogarajah, P.; Kerr, D.; Gardiner, B.; Seneviratne, S.; Samarasinghe, P.; Coleman, S. A predictive model for paediatric autism screening. Health Inform. J. 2020, 26, 2538–2553. [Google Scholar] [CrossRef] [Green Version]
- Thabtah, F. An accessible and efficient autism screening method for behavioural data and predictive analyses. Health Inform. J. 2019, 25, 1739–1755. [Google Scholar] [CrossRef]
- Campbell, K.; Carpenter, K.L.H.; Espinosa, S.; Hashemi, J.; Qiu, Q.; Tepper, M.; Calderbank, R.; Sapiro, G.; Egger, H.L.; Baker, J.P.; et al. Use of a Digital Modified Checklist for Autism in Toddlers–Revised with Follow-up to Improve Quality of Screening for Autism. J. Pediatr. 2017, 183, 133–139.e1. [Google Scholar] [CrossRef] [Green Version]
- Ghafouri-Fard, S.; Taheri, M.; Omrani, M.D.; Daaee, A.; Mohammad-Rahimi, H.; Kazazi, H. Application of Single-Nucleotide Polymorphisms in the Diagnosis of Autism Spectrum Disorders: A Preliminary Study with Artificial Neural Networks. J. Mol. Neurosci. 2019, 68, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Sekaran, K.; Sudha, M. Predicting autism spectrum disorder from associative genetic markers of phenotypic groups using machine learning. J. Ambient Intell. Humaniz. Comput. 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Jack, A. Neuroimaging in neurodevelopmental disorders: Focus on resting-state fMRI analysis of intrinsic functional brain connectivity. Curr. Opin. Neurol. 2018, 31, 140–148. [Google Scholar] [CrossRef]
- Fu, C.H.Y.; Costafreda, S.G. Neuroimaging-based biomarkers in psychiatry: Clinical opportunities of a paradigm shift. Can. J. Psychiatry 2013, 58, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.J.; Hwang, J.; Kana, R.; Torous, J.; Kim, J.W. Accuracy of machine learning algorithms for the diagnosis of autism spectrum disorder: Systematic review and meta-analysis of brain magnetic resonance imaging studies. J. Med. Internet Res. 2019, 6, e14108. [Google Scholar] [CrossRef] [Green Version]
- Sarabadani, S.; Schudlo, L.C.; Samadani, A.A.; Kushski, A. Physiological Detection of Affective States in Children with Autism Spectrum Disorder. IEEE Trans. Affect. Comput. 2020, 11, 588–600. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Yi, L. Identifying children with autism spectrum disorder based on their face processing abnormality: A machine learning framework. Autism Res. 2016, 9, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Alcañiz Raya, M.; Chicchi Giglioli, I.A.; Marín-Morales, J.; Higuera-Trujillo, J.L.; Olmos, E.; Minissi, M.E.; Teruel Garcia, G.; Sirera, M.; Abad, L. Application of Supervised Machine Learning for Behavioral Biomarkers of Autism Spectrum Disorder Based on Electrodermal Activity and Virtual Reality. Front. Hum. Neurosci. 2020, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, J.; Dawson, G.; Carpenter, K.L.H.; Campbell, K.; Qiu, Q.; Espinosa, S.; Marsan, S.; Baker, J.P.; Egger, H.L.; Sapiro, G. Computer Vision Analysis for Quantification of Autism Risk Behaviors. IEEE Trans. Affect. Comput. 2018, 3045, 1–12. [Google Scholar] [CrossRef]
- Dahiya, A.V.; McDonnell, C.; DeLucia, E.; Scarpa, A. A systematic review of remote telehealth assessments for early signs of autism spectrum disorder: Video and mobile applications. Pract. Innov. 2020, 5, 150–164. [Google Scholar] [CrossRef]
- Thabtah, F. Machine learning in autistic spectrum disorder behavioral research: A review and ways forward. Inform. Health Soc. Care 2019, 44, 278–297. [Google Scholar] [CrossRef]
- Alahmari, F. A Comparison of Resampling Techniques for Medical Data Using Machine Learning. J. Inf. Knowl. Manag. 2020, 19, 1–13. [Google Scholar] [CrossRef]
- Abdelhamid, N.; Padmavathy, A.; Peebles, D.; Thabtah, F.; Goulder-Horobin, D. Data Imbalance in Autism Pre-Diagnosis Classification Systems: An Experimental Study. J. Inf. Knowl. Manag. 2020, 19, 1–16. [Google Scholar] [CrossRef]
- Song, D.-Y.; Kim, S.Y.; Bong, G.; Kim, J.M.; Yoo, H.J. The Use of Artificial Intelligence in Screening and Diagnosis of Autism Spectrum Disorder: A Literature Review. J. Korean Acad. Child Adolesc. Psychiatry 2019, 30, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Low, D.M.; Bentley, K.H.; Ghosh, S.S. Automated assessment of psychiatric disorders using speech: A systematic review. Laryngoscope Investig. Otolaryngol. 2020, 5, 96–116. [Google Scholar] [CrossRef] [Green Version]
- Lebersfeld, J.B.; Swanson, M.; Clesi, C.D.; Kelley, S.E.O. Systematic Review and Meta-Analysis of the Clinical Utility of the ADOS-2 and the ADI-R in Diagnosing Autism Spectrum Disorders in Children. J. Autism Dev. Disord. 2021, 51, 1–14. [Google Scholar] [CrossRef]
- Kulage, K.M.; Goldberg, J.; Usseglio, J.; Romero, D.; Bain, J.M.; Smaldone, A.M. How has DSM-5 Affected Autism Diagnosis? A 5-Year Follow-Up Systematic Literature Review and Meta-analysis. J. Autism Dev. Disord. 2020, 50, 2102–2127. [Google Scholar] [CrossRef]
- Smith, I.C.; Reichow, B.; Volkmar, F.R. The Effects of DSM-5 Criteria on Number of Individuals Diagnosed with Autism Spectrum Disorder: A Systematic Review. J. Autism Dev. Disord. 2015, 45, 2541–2552. [Google Scholar] [CrossRef]
- Wall, D.; Kosmicki, J.; Deluca, T.; Harstad, E.; Fusaro, V. Use of machine learning to shorten observation-based screening and diagnosis of autism. Transl. Psychiatry 2012, 2, e100-8. [Google Scholar] [CrossRef]
- Wall, D.; Dally, R.; Luyster, R.; Jung, J.Y.; DeLuca, T. Use of artificial intelligence to shorten the behavioral diagnosis of autism. PLoS ONE 2012, 7, e43855. [Google Scholar] [CrossRef] [Green Version]
- Duda, M.; Ma, R.; Haber, N.; Wall, D. Use of machine learning for behavioral distinction of autism and ADHD. Transl. Psychiatry 2016, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kosmicki, J.; Sochat, V.; Duda, M.; Wall, D. Searching for a minimal set of behaviors for autism detection through feature selection-based machine learning. Transl. Psychiatry 2015, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bone, D.; Bishop, S.L.; Black, M.P.; Goodwin, M.S.; Lord, C.; Narayanan, S.S. Use of machine learning to improve autism screening and diagnostic instruments: Effectiveness, efficiency, and multi-instrument fusion. J. Child Psychol. Psychiatry Allied Discip. 2016, 57, 927–937. [Google Scholar] [CrossRef] [Green Version]
- Duda, M.; Kosmicki, J.; Wall, D. Testing the accuracy of an observation-based classifier for rapid detection of autism risk. Transl. Psychiatry 2015, 5, e556. [Google Scholar] [CrossRef]
- Küpper, C.; Stroth, S.; Wolff, N.; Hauck, F.; Kliewer, N.; Schad-Hansjosten, T.; Kamp-Becker, I.; Poustka, L.; Roessner, V.; Schultebraucks, K.; et al. Identifying predictive features of autism spectrum disorders in a clinical sample of adolescents and adults using machine learning. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Bellesheim, K.R.; Cole, L.; Coury, D.L.; Yin, L.; Levy, S.E.; Guinnee, M.A.; Klatka, K.; Malow, B.A.; Katz, T.; Taylor, J.; et al. Family-driven goals to improve care for children with autism spectrum disorder. Pediatrics 2018, 142, e20173225. [Google Scholar] [CrossRef] [Green Version]
- Puerto, E.; Aguilar, J.; López, C.; Chávez, D. Using Multilayer Fuzzy Cognitive Maps to diagnose Autism Spectrum Disorder. Appl. Soft Comput. J. 2019, 75, 58–71. [Google Scholar] [CrossRef]
- Baadel, S.; Thabtah, F.; Lu, J. A clustering approach for autistic trait classification. Inform. Health Soc. Care 2020, 45, 309–326. [Google Scholar] [CrossRef]
- Akter, T.; Shahriare Satu, M.; Khan, M.I.; Ali, M.H.; Uddin, S.; Lio, P.; Quinn, J.M.W.; Moni, M.A. Machine Learning-Based Models for Early Stage Detection of Autism Spectrum Disorders. IEEE Access 2019, 7, 166509–166527. [Google Scholar] [CrossRef]
- Usta, M.B.; Karabekiroglu, K.; Sahin, B.; Aydin, M.; Bozkurt, A.; Karaosman, T.; Aral, A.; Cobanoglu, C.; Kurt, A.D.; Kesim, N.; et al. Use of machine learning methods in prediction of short-term outcome in autism spectrum disorders. Psychiatry Clin. Psychopharmacol. 2019, 29, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Thabtah, F.; Kamalov, F.; Rajab, K. A new computational intelligence approach to detect autistic features for autism screening. Int. J. Med. Inform. 2018, 117, 112–124. [Google Scholar] [CrossRef]
- Pratama, T.G.; Hartanto, R.; Setiawan, N.A. Machine learning algorithm for improving performance on 3 AQ-screening classification. Commun. Sci. Technol. 2019, 4, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Thabtah, F.; Abdelhamid, N.; Peebles, D. A machine learning autism classification based on logistic regression analysis. Health Inf. Sci. Syst. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Suresh Kumar, R.; Renugadevi, M. Differential evolution tuned support vector machine for autistic spectrum disorder diagnosis. Int. J. Recent Technol. Eng. 2019, 8, 3861–3870. [Google Scholar] [CrossRef]
- Levy, S.; Duda, M.; Haber, N.; Wall, D. Sparsifying machine learning models identify stable subsets of predictive features for behavioral detection of autism. Mol. Autism 2017, 8, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Goel, N.; Grover, B.; Gupta, D.; Khanna, A.; Sharma, M. Modified Grasshopper Optimization Algorithm for detection of Autism Spectrum Disorder. Phys. Commun. 2020, 41, 101115. [Google Scholar] [CrossRef]
- Thabtah, F.; Peebles, D. A new machine learning model based on induction of rules for autism detection. Health Inform. J. 2020, 26, 264–286. [Google Scholar] [CrossRef] [Green Version]
- Duda, M.; Haber, N.; Daniels, J.; Wall, D. Crowdsourced validation of a machine-learning classification system for autism and ADHD. Transl. Psychiatry 2017, 7, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Alhaj, T.A.; Siraj, M.M.; Zainal, A.; Elshoush, H.T.; Elhaj, F. Feature Selection Using Information Gain for Improved Structural-Based Alert Correlation. PLoS ONE 2016, 11, e0166017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roobaert, D.; Karakoulas, G.; Chawla, N.V. Information Gain, Correlation and Support Vector Machines. In Feature Extraction; Springer: Berlin/Heidelberg, Germany, 2006; Volume 207, pp. 463–470. [Google Scholar]
- Wiesen, J. Benefits, Drawbacks, and Pitfalls of z-Score Weighting. In Proceedings of the 30th Annual IPMAAC Conference, Las Vegas, NV, USA, 27 June 2006; pp. 1–41. [Google Scholar]
- Lapteacru, I. On the Consistency of the Z-Score to Measure the Bank Risk. SSRN Electron. J. 2016, 4, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Curtis, A.; Smith, T.; Ziganshin, B.; Elefteriades, J. The Mystery of the Z-Score. AORTA 2016, 4, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, H.; Lu, N.; Chen, T.; He, H.; Lu, Y.; Tu, X.M. Log-transformation and its implications for data analysis. Shanghai Arch. Psychiatry 2014, 26, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Plitt, M.; Barnes, K.A.; Martin, A. Functional connectivity classification of autism identifies highly predictive brain features but falls short of biomarker standards. NeuroImage Clin. 2015, 7, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.; Smith, L.E.; Hong, J.; Greenberg, J.S.; Mailick, M.R. Validating the social responsiveness scale for adults with autism. Autism Res. 2017, 10, 1663–1671. [Google Scholar] [CrossRef]
- Becker, M.M.; Wagner, M.B.; Bosa, C.A.; Schmidt, C.; Longo, D.; Papaleo, C.; Riesgo, R.S. Translation and validation of Autism Diagnostic Interview-Revised (ADI-R) for autism diagnosis in Brazil. Arq. Neuropsiquiatr. 2012, 70, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Falkmer, T.; Anderson, K.; Falkmer, M.; Horlin, C. Diagnostic procedures in autism spectrum disorders: A systematic literature review. Eur. Child Adolesc. Psychiatry 2013, 22, 329–340. [Google Scholar] [CrossRef]
- Medda, J.E.; Cholemkery, H.; Freitag, C.M. Sensitivity and Specificity of the ADOS-2 Algorithm in a Large German Sample. J. Autism Dev. Disord. 2019, 49, 750–761. [Google Scholar] [CrossRef] [Green Version]
- Chojnicka, I.; Pisula, E. Adaptation and Validation of the ADOS-2, Polish Version. Front. Psychol. 2017, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Achenie, L.E.K.; Scarpa, A.; Factor, R.S.; Wang, T.; Robins, D.L.; McCrickard, D.S. A Machine Learning Strategy for Autism Screening in Toddlers. J. Dev. Behav. Pediatr. 2019, 40, 369–376. [Google Scholar] [CrossRef]
- Torres, E.B.; Rai, R.; Mistry, S.; Gupta, B. Hidden aspects of the research ADOS are bound to affect autism science. Neural Comput. 2020, 32, 515–561. [Google Scholar] [CrossRef]





| Inclusion Criteria |
|---|
| Journal articles published in the English language |
| Documents published within the last ten years from 2011 to date |
| Full-text papers that are accessible and downloadable |
| Studies that utilized behavioral data |
| Studies that employed machine learning as the main technique |
| Studies that considered autism as the main disorder assessed |
| Exclusion criteria |
| Papers that are written in other languages |
| Duplicated papers |
| Full-text of the document is not accessible on the internet |
| The study aim is not clearly defined |
| Studies that are not relevant to the stated research question |
| Relevant studies, but machine learning is not the main method |
| Relevant studies, but autism is not the main disorder assessed |
| Conferences papers, editorial materials, and literature reviews |
| Studies that utilized data from either brain imaging, genetic, or physical/metabolic biomarkers. |
| Intervention studies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavus, N.; Lawan, A.A.; Ibrahim, Z.; Dahiru, A.; Tahir, S.; Abdulrazak, U.I.; Hussaini, A. A Systematic Literature Review on the Application of Machine-Learning Models in Behavioral Assessment of Autism Spectrum Disorder. J. Pers. Med. 2021, 11, 299. https://doi.org/10.3390/jpm11040299
Cavus N, Lawan AA, Ibrahim Z, Dahiru A, Tahir S, Abdulrazak UI, Hussaini A. A Systematic Literature Review on the Application of Machine-Learning Models in Behavioral Assessment of Autism Spectrum Disorder. Journal of Personalized Medicine. 2021; 11(4):299. https://doi.org/10.3390/jpm11040299
Chicago/Turabian StyleCavus, Nadire, Abdulmalik A. Lawan, Zurki Ibrahim, Abdullahi Dahiru, Sadiya Tahir, Usama Ishaq Abdulrazak, and Adamu Hussaini. 2021. "A Systematic Literature Review on the Application of Machine-Learning Models in Behavioral Assessment of Autism Spectrum Disorder" Journal of Personalized Medicine 11, no. 4: 299. https://doi.org/10.3390/jpm11040299
APA StyleCavus, N., Lawan, A. A., Ibrahim, Z., Dahiru, A., Tahir, S., Abdulrazak, U. I., & Hussaini, A. (2021). A Systematic Literature Review on the Application of Machine-Learning Models in Behavioral Assessment of Autism Spectrum Disorder. Journal of Personalized Medicine, 11(4), 299. https://doi.org/10.3390/jpm11040299