The Challenging Diagnosis of Pediatric Multisystem Inflammatory Syndrome Associated with Sars-Cov-2 Infection-Two Case Reports and Literature Review
Abstract
1. Introduction
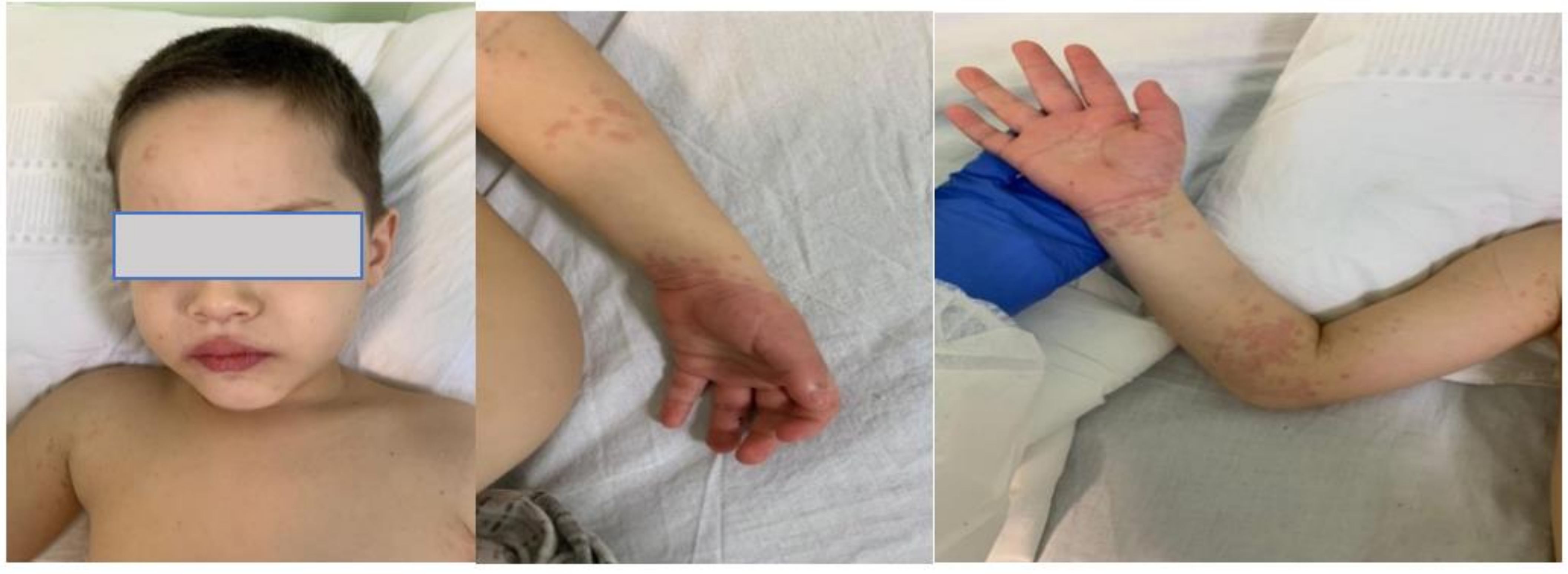
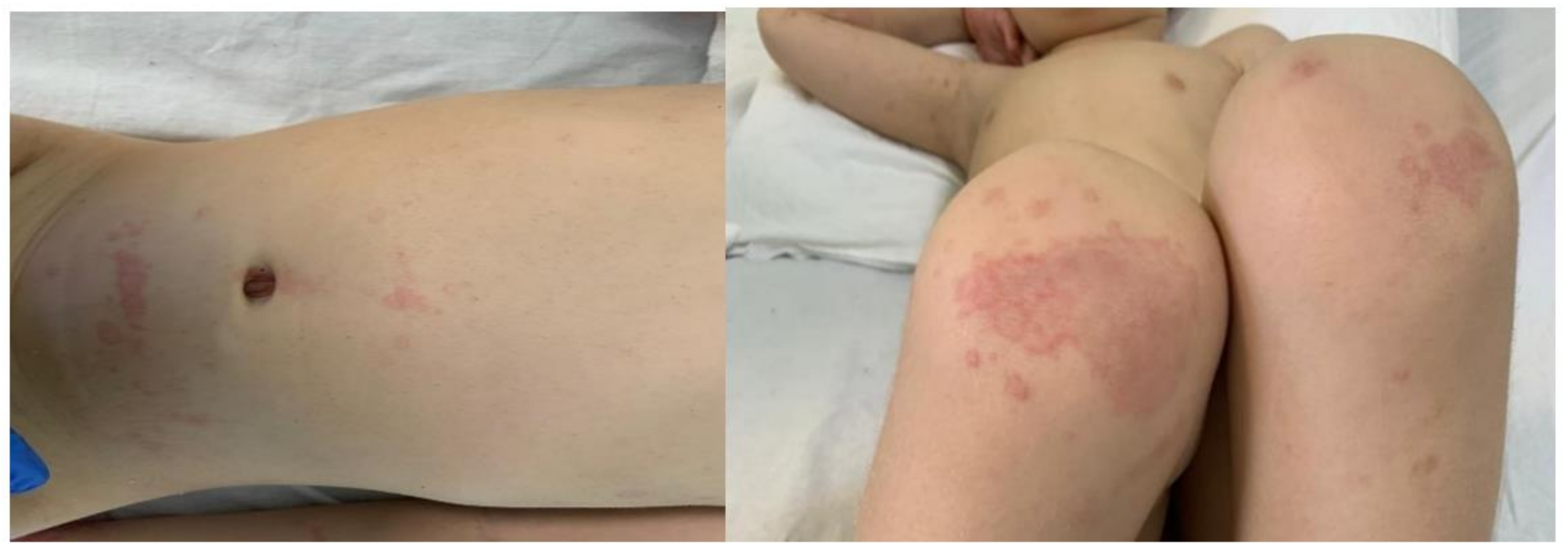
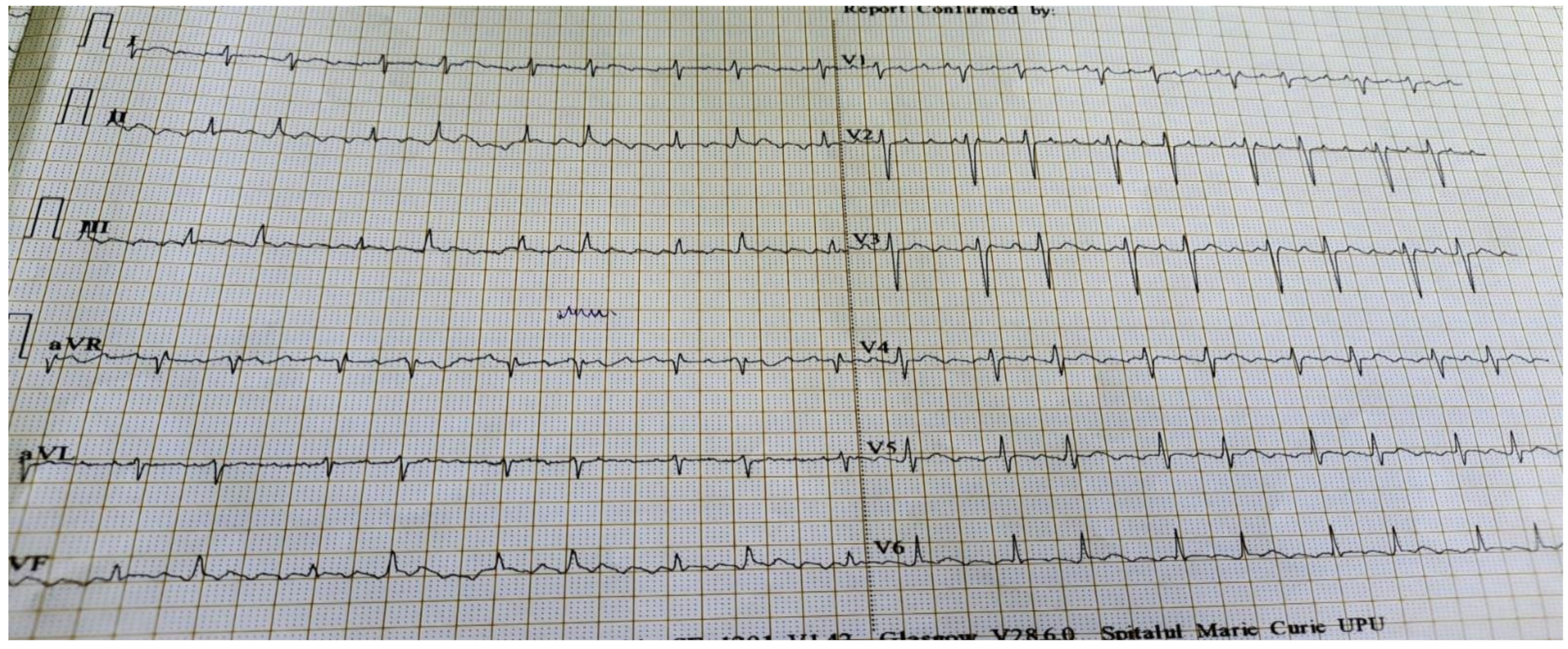
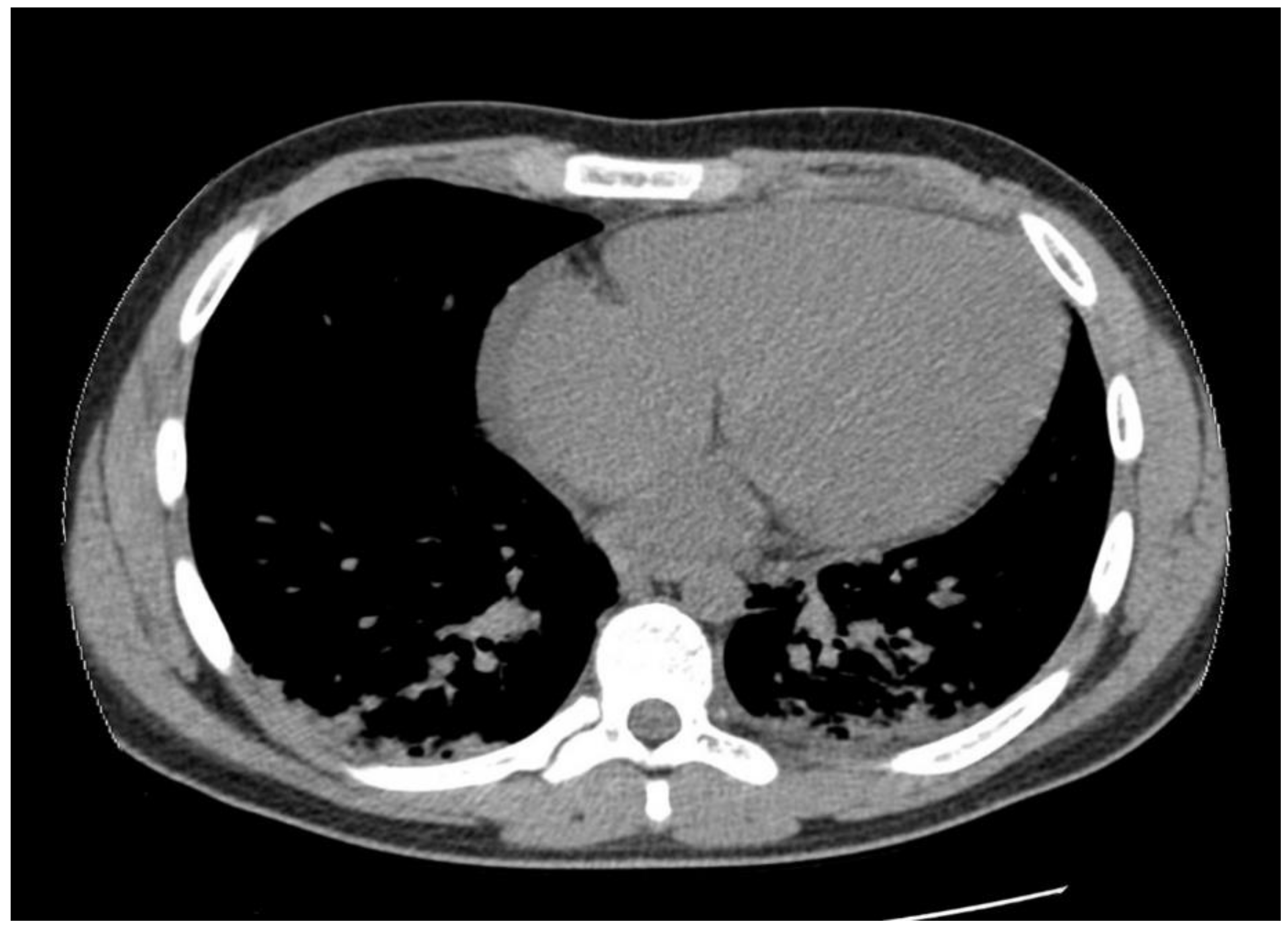
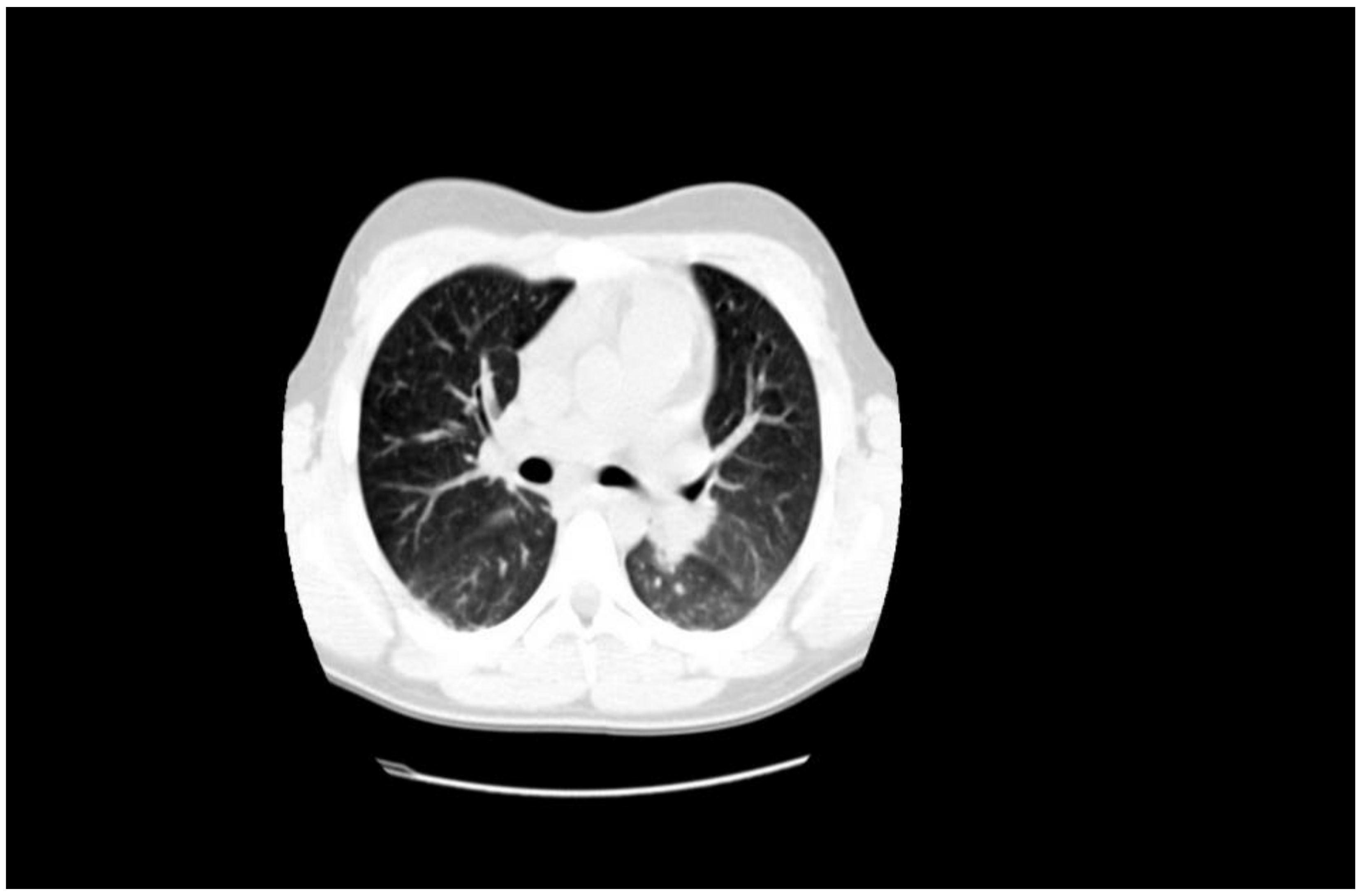
1.1. Case Presentation #1
1.2. Case Presentation #2
2. Literature Review
2.1. Coronavirus Disease 2019
2.2. Kawasaki Disease
2.3. MIS-C
2.4. KD versus MIS-C
2.5. COVID19 and Atrial Flutter
3. Discussion
Case Particularities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe acute respiratory coronavirus 2; |
| COVID19 | coronavirus disease 2019; |
| KD | Kawasaki disease; |
| MIS-C | multisystemic inflammatory syndrome in children; |
| PIMS-TS | pediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 infection; |
| UK | United Kingdom; |
| USA | United States of America; |
| CRP | C-reactive protein; |
| IL-6 | interleukin-6; |
| IgG | immunoglobulin G; |
| IgM | immunoglobulin M; |
| PT | prothrombin time; |
| APTT | activated partial thromboplastin time; |
| INR | international normalized ratio; |
| CK | creatine kinase; |
| PCR | polymerase chain reaction; |
| ECG | electrocardiogram; |
| CDC | Center for Disease Control; |
| BNP | brain natriuretic peptide; |
| ESR | erythrocyte sedimentation rate; |
| NT-proBNP | N-terminal pro brain natriuretic peptide; |
| TGO | glutamic-oxaloacetic transaminase; |
| TGP | glutamic pyruvic transaminase; |
| GGT | gamma glutamyl transferase; |
| CT | computer tomography; |
| IL1RA | IL1 receptor antagonist; |
| LDH | lactate dehydrogenase; |
| IgA | immunoglobulin A; |
| IFN-ϒ | gamma interferon; |
| TNF-α | alpha tumor necrosis factor; |
| CXCL10 | C-X-C motif chemokine ligand 10. |
References
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Outbreak Live Update. Available online: https://www.worldometers.info/coronavirus/ (accessed on 14 March 2020).
- Toubiana, J.; Poirault, C.; Corsia, A.; Bajolle, F.; Fourgeaud, J.; Angoulvant, F.; Debray, A.; Basmaci, R.; Salvador, E.; Biscardi, S.; et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: Prospective observational study. BMJ 2020, 369, m2094. [Google Scholar] [CrossRef] [PubMed]
- Götzinger, F.; Santiago-García, B.; Noguera-Julián, A.; Lanaspa, M.; Lancella, L.; Carducci, F.I.C.; Gabrovska, N.; Velizarova, S.; Prunk, P.; Osterman, V.; et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc. Health 2020, 4, 653–661. [Google Scholar] [CrossRef]
- Rapid risk assessment: Paediatric inflammatory multisystem syndrome and SARS -CoV-2 infection in children. European Centre for Disease Prevention and Control. 15 May 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment (accessed on 17 December 2020).
- PIMS-TS in children. 2020, p. 18. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-risk-assessment-paediatric-inflammatory-multisystem-syndrome-15-May-2020.pdf (accessed on 17 December 2020).
- Nelson, C.; Ishimine, P.; Hayden, S.R.; Correia, M.; Wardi, G. Multisystem Inflammatory Syndrome in Children (MIS-C) in an Adolescent that Developed Coronary Aneurysms: A Case Report and Review of the Literature. J. Emerg. Med. 2020, 59, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Pouletty, M.; Borocco, C.; Ouldali, N.; Caseris, M.; Basmaci, R.; Lachaume, N.; Bensaid, P.; Pichard, S.; Kouider, H.; Morelle, G.; et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): A multicentre cohort. Ann. Rheum. Dis. 2020, 79, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef]
- Grimaud, M.; Starck, J.; Levy, M.; Marais, C.; Chareyre, J.; Khraiche, D.; Leruez-Ville, M.; Quartier, P.; Léger, P.L.; Geslain, G.; et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann. Intensiv. Care 2020, 10, 1–5. [Google Scholar] [CrossRef]
- Davies, P.; Evans, C.; Kanthimathinathan, H.K.; Lillie, J.; Brierley, J.; Waters, G.; Johnson, M.; Griffiths, B.; du Pré, P.; Mohammad, Z.; et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: A multicentre observational study. Lancet Child Adolesc. Health 2020, 4, 669–677. [Google Scholar] [CrossRef]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef]
- Cokugras, H.; Onal, P. SARS-CoV-2 infection in children. Turk. Arch. Pediatr. 2021, 55, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Capone, C.A.; Subramony, A.; Sweberg, T.; Schneider, J.; Shah, S.; Rubin, L.; Schleien, C.; Epstein, S.; Johnson, J.C.; Kessel, A.; et al. Characteristics, Cardiac Involvement, and Outcomes of Multisystem Inflammatory Syndrome of Childhood Associated with severe acute respiratory syndrome coronavirus 2 Infection. J. Pediatr. 2020, 224, 141–145. [Google Scholar] [CrossRef] [PubMed]
- CDC. Multisystem Inflammatory Syndrome in Children (MIS-C); Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020. Available online: https://www.cdc.gov/mis-c/hcp/ (accessed on 18 December 2020).
- Su, J.A.; Weisert, M.A.; Silka, M.J.; Bar-Cohen, Y.; Menteer, J. SARS-CoV-2 infection presenting as sustained atrial flutter and advanced ventricular dysfunction. Clin. Case Stud. Rep. 2020, 3. [Google Scholar] [CrossRef]
- Yonker, L.M.; Neilan, A.M.; Bartsch, Y.; Patel, A.B.; Regan, J.; Arya, P.; Gootkind, E.; Park, G.; Hardcastle, M.; John, A.S.; et al. Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical Presentation, Infectivity, and Immune Responses. J. Pediatr. 2020, 227, 45–52.e5. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, S.; Hedrich, C.M. SARS-CoV-2 infections in children and young people. Clin. Immunol. 2020, 220, 108588. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.G.; Mills, M.; Suarez, D.; Hogan, C.A.; Yeh, D.; Segal, J.B.; Nguyen, E.L.; Barsh, G.R.; Maskatia, S.; Mathew, R. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hosp. Pediatr. 2020, 10, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Akca, U.K.; Kesici, S.; Ozsurekci, Y.; Aykan, H.H.; Batu, E.D.; Atalay, E.; Demir, S.; Sag, E.; Vuralli, D.; Bayrakci, B.; et al. Kawasaki-like disease in children with COVID-19. Rheumatol. Int. 2020, 40, 2105–2115. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, K.; Levin, M.; Irfan, O.; Morris, S.K.; Wilson, K.; Klein, J.D.; A Bhutta, Z. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020, 20, e276–e288. [Google Scholar] [CrossRef]
- Schnabel, A.; Hedrich, C.M. Childhood Vasculitis. Front. Pediatr. 2019, 6. [Google Scholar] [CrossRef]
- Kliegman, R.M. Nelson Textbook of Pediatrics; Elsevier: Philadelphia, PA, USA, 2020; p. 15739. [Google Scholar]
- Kanegaye, J.T.; Wilder, M.S.; Molkara, D.; Frazer, J.R.; Pancheri, J.; Tremoulet, A.H.; Watson, V.E.; Best, B.M.; Burns, J.C. Recognition of a Kawasaki Disease Shock Syndrome. Pediatrics 2009, 123, e783–e789. [Google Scholar] [CrossRef]
- Levin, M. Childhood Multisystem Inflammatory Syndrome—A New Challenge in the Pandemic. N. Engl. J. Med. 2020, 383, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Ferris, A.; Kernan, K.F.; Schulert, G.S.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 1. Arthritis Rheumatol. 2020, 72, 1791–1805. [Google Scholar] [CrossRef] [PubMed]
- Dufort, E.M.; Koumans, E.H.; Chow, E.J.; Rosenthal, E.M.; Muse, A.; Rowlands, J.; Barranco, M.A.; Maxted, A.M.; Rosenberg, E.S.; Easton, D.; et al. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020, 383, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat. Rev. Immunol. 2020, 20, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Elsevier Enhanced Reader. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Available online: https://reader.elsevier.com/reader/sd/pii/S0092867420311570?token=C76035267893E8797C4E5344AE39E225D70803D7F8958176ADACFD03D35AD3889E6DBC9F2884FFBD683311514FD15B16 (accessed on 3 March 2021).
- Brodsky, N.N.; Ramaswamy, A.; Lucas, C.L. The Mystery of MIS-C Post-SARS-CoV-2 Infection. Trends Microbiol. 2020, 28, 956–958. [Google Scholar] [CrossRef]
- Rowley, A.H.; Shulman, S.T.; Arditi, M. Immune pathogenesis of COVID-19–related multisystem inflammatory syndrome in children. J. Clin. Investig. 2020, 130, 5619–5621. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.-H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J.; et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021, 22, 25–31. [Google Scholar] [CrossRef]
- Gruber, C.N.; Patel, R.S.; Trachtman, R.; Lepow, L.; Amanat, F.; Krammer, F.; Wilson, K.M.; Onel, K.; Geanon, D.; Tuballes, K.; et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020, 183, 982–995.e14. [Google Scholar] [CrossRef]
- Shulman, S.T.; Rowley, A.H. Kawasaki disease: Insights into pathogenesis and approaches to treatment. Nat. Rev. Rheumatol. 2015, 11, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Rowley, A.H.; Baker, S.C.; Orenstein, J.M.; Shulman, S.T. Searching for the cause of Kawasaki disease—Cytoplasmic inclusion bodies provide new insight. Nat. Rev. Genet. 2008, 6, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Cantor, A.; Zachariah, P.; Ahn, D.; Martinez, M.; Margolis, K.G. Gastrointestinal Symptoms as a Major Presentation Component of a Novel Multisystem Inflammatory Syndrome in Children That Is Related to Coronavirus Disease 2019: A Single Center Experience of 44 Cases. Gastroenterology 2020, 159, 1571–1574.e2. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Advani, S.; Moreira, A.; Zoretic, S.; Martinez, J.; Chorath, K.; Acosta, S.; Naqvi, R.; Burmeister-Morton, F.; Burmeister, F.; et al. Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine 2020, 26, 100527. [Google Scholar] [CrossRef]
- Sperotto, F.; Friedman, K.G.; Son, M.B.F.; VanderPluym, C.J.; Newburger, J.W.; Dionne, A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: A comprehensive review and proposed clinical approach. Eur. J. Nucl. Med. Mol. Imaging 2021, 180, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Morris, S.B. Multisystem Inflammatory Syndrome in Adults. Chest 2021, 159, 471–472. [Google Scholar] [CrossRef]



| Before Treatment | After Treatment | Normal Range | |
|---|---|---|---|
| Complete blood count | |||
| White blood cells count | 12.12 | 10.62 | 5.50–15.50 × 103/µL |
| Lymphocytes | 1.2 | 2.14 | 2–8 × 103/µL |
| Neutrophils | 10.38 | 7.42 | 1.5–8.5 × 103/µL |
| Thrombocytes | 94000 | 554000 | 150.000–450.000/µL mm3 |
| Hb | 13.10 | 12.3 | 11–14 g/dL |
| Rheumatology | |||
| C-reactive protein | 186.11 | 0.31 | 0–5 mg/L |
| Procalcitonin | 413.9 | 0.16 | <0.05 ng/mL |
| Ferritin | 218 | 4–67 ng/mL | |
| IL-6 | 31.51 | <7 pg/mL | |
| LDH | 251 | 223 | 120–300 U/L |
| Myocardium function | |||
| PT | 22.1 | 15.5 | 11.3–15.6 s |
| APPT | 38.2 | 29.5 | 24–37 s |
| INR | 1.7 | 1.17 | 0.84–1.2 |
| D-dimers | 1.96 | 0.49 | 0–0.5 µg/mL |
| Troponin T | <40 | <40 | <40 ng/mL |
| NT proBNP | 200 | 187 | <125 pg/mL |
| CK | 32 | 15 | 7–149 U/L |
| CK-MB | 23.5 | 22.5 | 7–25 U/L |
| Kidney function | |||
| Creatinine | 0.39 | 0.29 | <0.47 mg/dL |
| BUN | 30.7 | 29.2 | <39 mg/dL |
| Ionogram | |||
| Na+ | 129 | 136.3 | 138–145 mmol/L |
| K+ | 3.97 | 4.45 | 3.5–5.1 mmol/L |
| Liver function | |||
| TGO | 23.5 | 27.4 | 2–48 U/L |
| TGP | 27.1 | 30.5 | 2–29 U/L |
| Albuminemia | 3.35 | 3.75 | 3.8–5.4 g/dL |
| Proteinemia | 5.27 | 6.2 | 6–8 g/dL |
| Infectious disease | |||
| Blood cultures | Negative | Negative | |
| Urine culture | Negative | Negative | |
| PCR SARS-CoV-2 | Negative | Negative | |
| IgM SARS-CoV-2 | Negative | Negative | |
| IgG SARS-CoV-2 | Positive | Negative | |
|
| No alternative plausible diagnoses AND |
| Positive for current or recent SARS-CoV-2 infection (RT-PCR, antigen test, serology or Exposure to a suspected or confirmed COVID19 person in the last 4 weeks) |
| Initial Results | After Treatment | Reference Values | |
|---|---|---|---|
| Complete Blood Count | |||
| White blood cells count | 4.77 | 12.92 | 4.50–13 × 103/µL |
| Lymphocytes | 0.79 | 1.44 | 1.5–6.5 × 103/µL |
| Neutrophils | 3.64 | 10.85 | 1.8–8 × 103/µL |
| Platelets | 114,000 | 419,000 | 150.000–450.000/µL mm3 |
| Hemoglobin | 11.9 | 13.8 | 11.7–16.6 g/dL |
| Rheumathologic | |||
| C-reactive protein | 274.53 | 0.59 | 0–5 mg/L |
| ESR | 47 | 6 | 2–15 mm/h |
| Procalcitonin | 2.04 | 0.05 | <0.05 ng/mL |
| Ferritin | 3331 | 168 | 14–152 ng/mL |
| IL-6 | 113.9 | 1.5 | <7 pg/mL |
| LDH | 324 | 239 | 135–225 U/L |
| Cardiovascular | |||
| PT | 15.6 | 12.2 | 11.3–15.6 s |
| APPT | 43.2 | 25 | 24–37 s |
| INR | 1.18 | 0.91 | 0.84–1.2 |
| Fibrinogen | 555 | 182 | 160–390 mg/dL |
| D-dimers | 2.38 | 0.27 | 0–0.5 µg/mL |
| CK | 95 | 87 | 7–270 U/L |
| CK-MB | 13.9 | 26.9 | 7–25 U/L |
| Troponin T | <40 | <40 | <40 ng/mL |
| NT-proBNP | 6421 | 260 | <125 pg/mL |
| Renal | |||
| Creatinine | 1.28 | 0.57 | <1.2 mg/dL |
| BUN | 76.5 | 41.5 | <39 mg/dL |
| Ionogram | |||
| Na+ | 137.6 | 138–145 mmol/L | |
| K+ | 3.76 | 3.5–5.1 mmol/L | |
| Hepatic | |||
| TGO | 60.3 | 26.9 | 2–48 U/L |
| TGP | 60 | 145.3 | 2–29 U/L |
| GGT | 107 | 87 | 3–29 U/L |
| Albuminemia | 3.17 | 3.51 | 3.2–4.5 g/dL |
| Proteinemia | 6.27 | 6.26 | 6–8 g/dL |
| Infectious | |||
| Blood cultures | Negative | Negative | |
| Urine culture | Negative | Negative | |
| Influenza A + B antigen test | Negative | Negative | |
| Quantiferon TB Gold test | Negative | Negative | |
| PCR SARS-CoV-2 | Negative | Negative | |
| IgM SARS-CoV-2 | Negative | Negative | |
| IgG SARS-CoV-2 | Positive | Negative | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, M.D.; Taras, R.; Dombici, B.; Balgradean, M.; Berghea, E.C.; Nicolescu, A. The Challenging Diagnosis of Pediatric Multisystem Inflammatory Syndrome Associated with Sars-Cov-2 Infection-Two Case Reports and Literature Review. J. Pers. Med. 2021, 11, 318. https://doi.org/10.3390/jpm11040318
Ionescu MD, Taras R, Dombici B, Balgradean M, Berghea EC, Nicolescu A. The Challenging Diagnosis of Pediatric Multisystem Inflammatory Syndrome Associated with Sars-Cov-2 Infection-Two Case Reports and Literature Review. Journal of Personalized Medicine. 2021; 11(4):318. https://doi.org/10.3390/jpm11040318
Chicago/Turabian StyleIonescu, Marcela Daniela, Roxana Taras, Bianca Dombici, Mihaela Balgradean, Elena Camelia Berghea, and Alin Nicolescu. 2021. "The Challenging Diagnosis of Pediatric Multisystem Inflammatory Syndrome Associated with Sars-Cov-2 Infection-Two Case Reports and Literature Review" Journal of Personalized Medicine 11, no. 4: 318. https://doi.org/10.3390/jpm11040318
APA StyleIonescu, M. D., Taras, R., Dombici, B., Balgradean, M., Berghea, E. C., & Nicolescu, A. (2021). The Challenging Diagnosis of Pediatric Multisystem Inflammatory Syndrome Associated with Sars-Cov-2 Infection-Two Case Reports and Literature Review. Journal of Personalized Medicine, 11(4), 318. https://doi.org/10.3390/jpm11040318







