Neoadjuvant Chemotherapy in Breast Cancer: An Advanced Personalized Multidisciplinary Prehabilitation Model (APMP-M) to Optimize Outcomes
Abstract
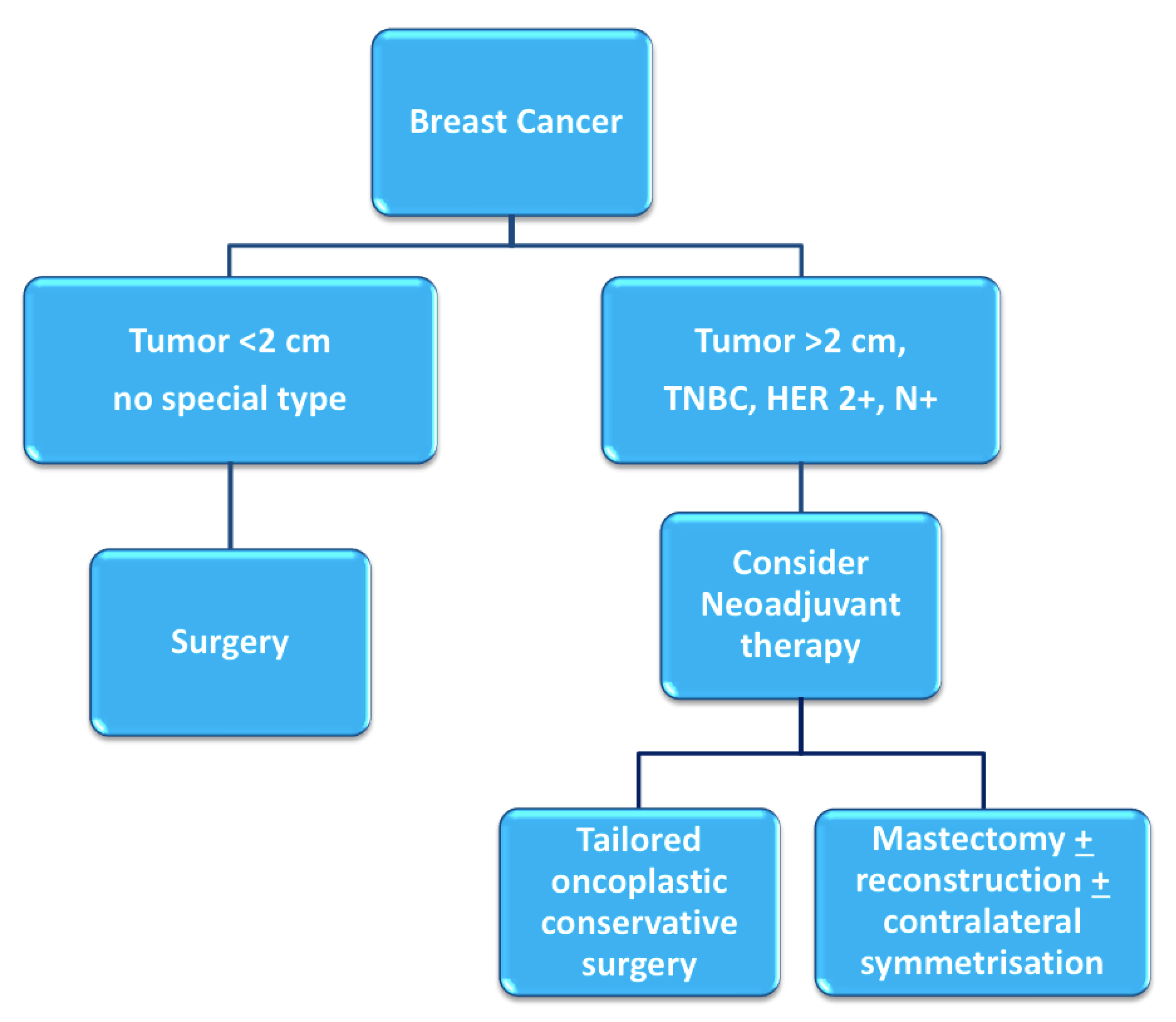
1. Introduction
2. Materials and Methods
3. Breast Unit and Outpatient Neoadjuvant Care Prehabilitation Clinic
4. The Neoadjuvant Oncologic Treatment Team
- Clinical breast examination, mammography, breast ultrasound, and breast MRI;
- Ultrasound- or stereotactic-guided tissue sampling of breast lesions and suspicious lymph nodes. Markers are positioned in the breast tissue and pathologic lymph nodes in order to ensure a correct pre-surgical localization in case of pathologic complete response or regression to a non-palpable lesion;
- Complete histopathological and prognostic characterization (ER, PgR, AR, Ki67, HER2 status);
- Photographical documentation of pre-NAC patient breasts. After clinical and ultrasound evaluation, the surgeon draws the tumor’s projection and measurements on the skin surface and takes two photographs in frontal and lateral projection (Figure 4). Pictures are re-evaluated after NAC and assist in surgical planning [13];
- Systemic staging is completed by performing either a whole-body CT scan and bone scintigraphy, or a PET/CT scan.
4.1. Cardiovascular Assessment
4.2. Genetic Counselling
4.3. Multiparametric Geriatric Assessment in Elderly Patients
4.4. Gynecologic and Fertility Counselling in Younger Patients
5. The Neoadjuvant Supportive Care Team
5.1. Lifestyle and Nutrition Counseling
5.2. Psychological Counselling
Emotional Eating Prevention during NAC
5.3. Integrative Oncology during Neoadjuvant Therapy
5.3.1. Acupuncture
5.3.2. Mindfulness
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-Term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: Meta-Analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018, 19, 27–39. [Google Scholar] [CrossRef]
- O’Halloran, N.; Lowery, A.; Curran, C.; McLaughlin, R.; Malone, C.; Sweeney, K.; Keane, M.; Kerin, M. A Review of the impact of neoadjuvant chemotherapy on breast surgery practice and outcomes. Clin. Breast Cancer 2019, 19, 377–382. [Google Scholar] [CrossRef]
- Franceschini, G.; Di Leone, A.; Natale, M.; Sanchez, A.M.; Masetti, R. Conservative surgery after neoadjuvant chemotherapy in patients with operable breast cancer. Ann. Ital. Chir. 2018, 89, 290. [Google Scholar]
- Franceschini, G.; Visconti, G.; Masetti, R. Oncoplastic breast surgery with oxidized regenerated cellulose: Appraisals based on five-year experience. Breast J. 2014, 20, 447–448. [Google Scholar] [CrossRef]
- Lo-Fo-Wong, D.N.; de Haes, H.C.; Aaronson, N.K.; van Abbema, D.L.; den Boer, M.D.; van Hezewijk, M.; Immink, M.; Kaptein, A.A.; Menke-Pluijmers, M.B.; Reyners, A.K.; et al. Risk factors of unmet needs among women with breast cancer in the post-treatment phase. Psychooncology 2020, 29, 539–549. [Google Scholar] [CrossRef]
- Brahmbhatt, P.; Sabiston, M.C.; Lopez, C.; Chang, E.; Goodman, J.; Jones, J.; McCready, D.; Randall, I.; Rotstein, S.; Santa Mina, D. Feasibility of prehabilitation prior to breast cancer surgery: A mixed-methods study. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Lyman, G.H.; Greenlee, H.; Bohlke, K.; Bao, T.; DeMichele, A.M.; Deng, G.E.; Fouladbakhsh, J.M.; Gil, B.; Hershman, D.L.; Mansfield, S.; et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J. Clin. Oncol. 2018, 36, 2647–2655. [Google Scholar] [CrossRef]
- Franceschini, G.; di Leone, A.; Masetti, R. The breast unit update on advantages and the open issues. Ann. Ital. Chir. 2014, 85, 407–412. [Google Scholar] [PubMed]
- Nardone, L.; Valentini, V.; Marino, L.; De Santis, M.C.; Terribile, D.; Franceschini, G.; Balducci, M.; Mantini, G.; Mattiucci, G.; Mulè, A.; et al. A feasibility study of neo-adjuvant low-dose fractionated radiotherapy with two different concurrent anthracycline-docetaxel schedules in stage IIA/B-IIIA breast cancer. Tumori 2012, 98, 79–85. [Google Scholar] [CrossRef]
- Grimmett, C.; Bradbury, K.; Dalton, S.O.; Fecher-Jones, I.; Hoedjes, M.; Varkonyi-Sepp, J.; Short, C.E. The role of behavioral science in personalized multimodal prehabilitation in cancer. Front. Psychol. 2021, 12. [Google Scholar] [CrossRef]
- Shao, J.; Rodrigues, M.; Corter, A.L.; Baxter, N.N. Multidisciplinary care of breast cancer patients: A scoping review of multidisciplinary styles, processes, and outcomes. Curr. Oncol. 2019, 26, e385–e397. [Google Scholar] [CrossRef]
- Franceschini, G.; Terribile, D.; Fabbri, C.; Magno, S.; D’Alba, P.; Chiesa, F.; Di Leone, A.; Masetti, R. Management of locally advanced breast cancer: Mini-Review. Minerva Chir. 2007, 62, 249–255. [Google Scholar]
- Salgarello, M.; Visconti, G.; Barone-Adesi, L.; Franceschini, G.; Masetti, R. Contralateral breast symmetrisation in immediate prosthetic breast reconstruction after unilateral nipple-sparing mastectomy: The tailored reduction/augmentation mammaplasty. Arch. Plast. Surg. 2015, 42, 302–308. [Google Scholar] [CrossRef]
- Franceschini, G.; Sanchez, A.M.; Di Leone, A.; Magno, S.; Moschella, F.; Accetta, C.; Natale, M.; Di Giorgio, D.; Scaldaferri, A.; D’Archi, S.; et al. Update on the surgical management of breast cancer. Ann. Ital. Chir. 2015, 86, 89–99. [Google Scholar]
- Shan, K.; Lincoff, A.M.; Young, J.B. Anthracycline-Induced cardiotoxicity. Ann. Intern. Med. 1996, 125, 47–58. [Google Scholar] [CrossRef]
- Felker, G.M.; Thompson, R.E.; Hare, J.M.; Hruban, R.H.; Clemetson, D.E.; Howard, D.L.; Baughman, K.L.; Kasper, E.K. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N. Engl. J. Med. 2000, 342, 1077–1084. [Google Scholar] [CrossRef]
- Patnaik, J.L.; Byers, T.; DiGuiseppi, C.; Dabelea, D.; Denberg, T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011, 13, R64. [Google Scholar] [CrossRef] [PubMed]
- Tischler, J.; Crew, K.D.; Chung, W.K. Cases in precision medicine: The role of tumor and germline genetic testing in breast cancer management. Ann. Intern. Med. 2019, 171, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.J.; Ward, K.C.; Hamilton, A.S.; McLeod, M.C.; Wallner, L.P.; Morrow, M.; Jagsi, R.; Hawley, S.T.; Kurian, A.W. Gaps in receipt of clinically indicated genetic counseling after diagnosis of breast cancer. J. Clin. Oncol. 2018, 36, 1218–1224. [Google Scholar] [CrossRef]
- Associazione Italiana di Oncologia Medica (AIOM). Neoplasia della Mammella; Linee Guida; Associazione Italiana di Oncologia Medica (AIOM): Milano, Italy, 2020. [Google Scholar]
- Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.; et al. NCCN Clinical Practice Guidelines in Oncology—Breast Cancer; National Comprehensive Cancer NetworkNCCN: Plymouth Meeting, PA, USA, 2020. [Google Scholar]
- Christian, N.; Zabor, E.C.; Cassidy, M.; Flynn, J.; Morrow, M.; Gemignani, M.L. Contralateral prophylactic mastectomy use after neoadjuvant chemotherapy. Ann. Surg. Oncol. 2020, 27, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Terkelsen, T.; Rønning, H.; Skytte, A.B. Impact of genetic counseling on the uptake of contralateral prophylactic mastectomy among younger women with breast cancer. Acta Oncol. 2020, 59, 60–65. [Google Scholar] [CrossRef]
- Okonji, D.O.; Sinha, R.; Phillips, I.; Fatz, D.; Ring, A. Comprehensive geriatric assessment in 326 older women with early breast cancer. Br. J. Cancer 2017, 117, 925–931. [Google Scholar] [CrossRef]
- Halfter, K.; Ditsch, N.; Kolberg, H.C.; Fischer, H.; Hauzenberger, T.; von Koch, F.E.; Bauerfeind, I.; von Minckwitz, G.; Funke, I.; Crispin, A.; et al. Prospective cohort study using the breast cancer spheroid model as a predictor for response to neoadjuvant therapy—The SpheroNEO study. BMC Cancer 2015, 15, 519. [Google Scholar] [CrossRef]
- von Waldenfels, G.; Loibl, S.; Furlanetto, J.; Machlei, A.; Lederer, B.; Denkert, C.; Hanusch, C.; Kümmel, S.; von Minckwitz, G.; Schneeweiss, A.; et al. Outcome after neoadjuvant chemotherapy in elderly breast cancer patients—A pooled analysis of individual patient data from eight prospectively randomized controlled trials. Oncotarget 2018, 9, 15168–15179. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Bhatia, S.; Katheria, V.; et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J. Clin. Oncol. 2011, 29, 3457–3465. [Google Scholar] [CrossRef] [PubMed]
- Arecco, L.; Perachino, M.; Damassi, A.; Latocca, M.M.; Soldato, D.; Vallome, G.; Parisi, F.; Razeti, M.G.; Solinas, C.; Tagliamento, M.; et al. Burning questions in the oncofertility counseling of young breast cancer patients. Breast Cancer Basic Clin. Res. 2020, 14. [Google Scholar] [CrossRef]
- Peccatori, F.A.; Azim, H.A.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G. Pentheroudakis, Cancer, pregnancy and fertility: ESMO Clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi160–vi170. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- Magno, S.; Alessio, F.; Scaldaferri, A.; Sacchini, V.; Chiesa, F. Integrative approaches in breast cancer patients: A mini-review. Integr. Cancer Sci. Ther. 2016, 3, 460–464. [Google Scholar] [CrossRef]
- Bozzetti, F.; Arends, J.; Lundholm, K.; Micklewright, A.; Zurcher, G.; Muscaritoli, M. ESPEN guidelines on parenteral nutrition: Non-Surgical oncology. Clin. Nutr. 2009, 28, 445–454. [Google Scholar] [CrossRef]
- Kondrup, J.E.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Ní Bhuachalla, E.; Prado, C.M. Cancer-Associated malnutrition, cachexia and sarcopenia: The skeleton in the hospital closet 40 years later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. World Cancer Research Fund/American Institute for Cancer Research Continous Update Project Report: Diet, Nutrition, Physical Activity and Breast Cancer; World Cancer Research Fund International: London, UK, 2018; Available online: https://www.wcrf.org/dietandcancer (accessed on 1 April 2021).
- Prado, C.M.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- Carbognin, L.; Trestini, I.; Sperduti, I.; Bonaiuto, C.; Zambonin, V.; Fiorio, E.; Tregnago, D.; Parolin, V.; Pilotto, S.; Scambia, G.; et al. Prospective trial in early-stage breast cancer (EBC) patients (pts) submitted to nutrition evidence-based educational intervention: Early results of adherence to dietary guidelines (ADG) and body weight change (BWC). J. Clin. Oncol. 2019, 37 (Suppl. 15), 11575. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Zhou, X.K.; Gucalp, A.; Morris, P.G.; Howe, L.R.; Giri, D.D.; Morrow, M.; Wang, H.; Pollak, M.; Jones, L.W.; et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin. Cancer Res. 2016, 22, 2283–2289. [Google Scholar] [CrossRef]
- Pierce, J.P.; Stefanick, M.L.; Flatt, S.W.; Natarajan, L.; Sternfeld, B.; Madlensky, L.; Al-Delaimy, W.K.; Thomson, C.A.; Kealey, S.; Hajek, R.; et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J. Clin. Oncol. 2007, 25, 2345–2351. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Lohse, T.; Faeh, D.; Bopp, M.; Rohrmann, S. Adherence to the cancer prevention recommendations of the World Cancer Research Fund/American Institute for Cancer Research and mortality: A census-linked cohort. Am. J. Clin. Nutr. 2016, 104, 678–685. [Google Scholar] [CrossRef]
- Courneya, K.S.; McKenzie, D.C.; Mackey, J.R.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Cook, D.; Jespersen, D.; Proulx, C.; et al. Effects of exercise dose and type during breast cancer chemotherapy: Multicenter randomized trial. J. Natl. Cancer Inst. 2013, 105, 1821–1832. [Google Scholar] [CrossRef]
- Irwin, M.L.; Cartmel, B.; Gross, C.P.; Ercolano, E.; Li, F.; Yao, X.; Fiellin, M.; Capozza, S.; Rothbard, M.; Zhou, Y.; et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J. Clin. Oncol. 2015, 33, 1104–1111. [Google Scholar] [CrossRef]
- Lee, A.; Chiu, C.H.; Cho, M.W.A.; Gomersall, C.D.; Lee, K.F.; Cheung, Y.S.; Lai, P.B.S. Factors associated with failure of enhanced recovery protocol in patients undergoing major hepatobiliary and pancreatic surgery: A retrospective cohort study. BMJ Open 2014, 4, e005330. [Google Scholar] [CrossRef]
- Velthuis, M.J.; Agasi-Idenburg, S.C.; Aufdemkampe, G.; Wittink, H.M. The effect of physical exercise on cancer-related fatigue during cancer treatment: A meta-analysis of randomised controlled trials. Clin. Oncol. 2010, 22, 208–221. [Google Scholar] [CrossRef]
- Knols, R.; Aaronson, N.K.; Uebelhart, D.; Fransen, J.; Aufdemkampe, G. Physical exercise in cancer patients during and after medical treatment: A systematic review of randomized and controlled clinical trials. J. Clin. Oncol. 2005, 23, 3830–3842. [Google Scholar] [CrossRef]
- Beaver, K.; Williamson, S.; Briggs, J. Exploring patient experiences of neo-adjuvant chemotherapy for breast cancer. Eur. J. Oncol. Nurs. 2015, 20, 77–86. [Google Scholar] [CrossRef]
- Ganz, P.A.; Desmond, K.A.; Leedham, B.; Rowland, J.H.; Meyerowitz, B.E.; Belin, T.R. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. J. Natl. Cancer Inst. 2002, 94, 39–49. [Google Scholar] [CrossRef]
- Magno, S.; Carnevale, S.; Dentale, F.; Belella, D.; Linardos, M.; Masetti, R. Neo-Adjuvant chemotherapy and distress in breast cancer patients: The moderating role of generalized self-efficacy. J. Clin. Oncol. 2017, 35 (Suppl. 15), e21570. [Google Scholar] [CrossRef]
- Gil, F.; Grassi, L.; Travado, L.; Tomamichel, M.; Gonzalez, J.R.; Zanotti, P.; Lluch, P.; Hollenstein, M.F.; Maté, J.; Magnani, K.; et al. Use of distress and depression thermometers to measure psychosocial morbidity among southern European cancer patients. Support Care Cancer 2005, 13, 600–606. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Luszczynska, A.; Scholz, U.; Schwarzer, R. The general self-efficacy scale: Multicultural validation studie. J. Psychol. Interdiscip. Appl. 2005, 139, 439–457. [Google Scholar] [CrossRef]
- Magno, S.; Filippone, A.; Scaldaferri, A. Evidence-Based usefulness of integrative therapies in breast cancer. Transl. Cancer Res. 2018, 7, S379–S389. [Google Scholar] [CrossRef]
- Maunsell, E.; Drolet, M.; Brisson, J.; Robert, J.; Deschênes, L. Dietary change after breast cancer: Extent, predictors, and relation with psychological distress. J. Clin. Oncol. 2002, 20, 1017–1025. [Google Scholar] [CrossRef]
- Ozier, A.D.; Kendrick, O.W.; Leeper, J.D.; Knol, L.L.; Perko, M.; Burnham, J. Overweight and obesity are associated with emotion and stress—Related eating as measured by the eating and appraisal due to emotions and stress questionnaire. J. Am. Diet. Assoc. 2008, 108, 49–56. [Google Scholar] [CrossRef]
- Greenlee, H.; DuPont-Reyes, M.J.; Balneaves, L.G.; Carlson, L.E.; Cohen, M.R.; Deng, G.; Johnson, J.A.; Mumber, M.; Seely, D.; Zick, S.M.; et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J. Clin. 2017, 67, 194–232. [Google Scholar] [CrossRef]
- Zia, F.Z.; Olaku, O.; Bao, T.; Berger, A.; Deng, G.; Fan, A.Y.; Garcia, M.K.; Herman, P.M.; Kaptchuk, T.J.; Ladas, E.J.; et al. The national cancer institute’s conference on acupuncture for symptom management in oncology: State of the science, evidence, and research gaps. J. Natl. Cancer Inst. Monogr. 2017, 2017, 68–73. [Google Scholar] [CrossRef]
- Mao, J.J.; Bowman, M.A.; Xie, S.X.; Bruner, D.; De Michele, A.; Farrar, J.T. Electroacupuncture versus gabapentin for hot flashes among breast cancer survivors: A randomized placebo-controlled trial. J. Clin. Oncol. 2015, 33, 3615–3620. [Google Scholar] [CrossRef]
- Lesi, G.; Razzini, G.; Musti, M.A.; Stivanello, E.; Petrucci, C.; Benedetti, B.; Rondini, E.; Ligabue, M.B.; Scaltriti, L.; Botti, A.; et al. Acupuncture as an integrative approach for the treatment of hot flashes in women with breast cancer: A prospective multicenter randomized controlled trial (AcCliMaT). J. Clin. Oncol. 2016, 34, 1795–1802. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Wang, K.; Cui, H.S.; Yao, Y.; Liu, S.M.; Zhou, J.; Chen, T.Y.; Xia, J. Acupuncture for neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 2017, CD012057. [Google Scholar] [CrossRef]
- Wardley, A.M.; Ryder, D.; Misra, V.; Hall, P.S.; Mackereth, P.; Stringer, J. ACUFOCIN: Randomized clinical trial of ACUpuncture plus standard care versus standard care alone FOr chemotherapy induced peripheral neuropathy (CIPN). J. Clin. Oncol. 2020, 38 (Suppl. 15), 12003. [Google Scholar] [CrossRef]
- Molassiotis, A.; Suen, L.K.P.; Cheng, H.L.; Mok, T.S.K.; Lee, S.C.Y.; Wang, C.H.; Lee, P.; Leung, H.; Chan, V.; Lau, T.K.H.; et al. A randomized assessor-blinded wait-list-controlled trial to assess the effectiveness of acupuncture in the management of chemotherapy-induced peripheral neuropathy. Integr. Cancer Ther. 2019, 18. [Google Scholar] [CrossRef]
- Bao, T.; Patil, S.; Chen, C.; Zhi, I.W.; Li, Q.S.; Piulson, L.; Mao, J.J. Effect of acupuncture vs sham procedure on chemotherapy—Induced peripheral neuropathy symptoms: A randomized clinical trial. JAMA Netw. Open 2020, 3, e200681. [Google Scholar] [CrossRef]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.J.B.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO clinical practice guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef]
- Hoffman, C.J.; Ersser, S.J.; Hopkinson, J.B.; Nicholls, P.G.; Harrington, J.E.; Thomas, P.W. Effectiveness of mindfulness-based stress reduction in mood, breast-and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: A randomized, controlled trial. J. Clin. Oncol. 2012, 30, 1335–1342. [Google Scholar] [CrossRef]
- Henderson, V.P.; Clemow, L.; Massion, A.O.; Hurley, T.G.; Druker, S.; Hébert, J.R. The effects of mindfulness-based stress reduction on psychosocial outcomes and quality of life in early-stage breast cancer patients: A randomized trial. Breast Cancer Res. Treat. 2012, 131, 99–109. [Google Scholar] [CrossRef]
- Lerman, R.; Jarski, R.; Rea, H.; Gellish, R.; Vicini, F. Improving symptoms and quality of life of female cancer survivors: A randomized controlled study. Ann. Surg. Oncol. 2012, 19, 373–378. [Google Scholar] [CrossRef]
- Gross, C.R.; Kreitzer, M.J.; Reilly-Spong, M.; Wall, M.; Winbush, N.Y.; Patterson, R.; Mahowald, M.; Cramer-Bornemann, M. Mindfulness-Based stress reduction versus pharmacotherapy for chronic primary insomnia: A randomized controlled clinical trial. Explor. J. Sci. Heal. 2011, 7, 76–87. [Google Scholar] [CrossRef][Green Version]
- Shapiro, S.L.; Bootzin, R.R.; Figueredo, A.J.; Lopez, A.M.; Schwartz, G.E. The efficacy of mindfulness-based stress reduction in the treatment of sleep disturbance in women with breast cancer. An exploratory study. J. Psychosom. Res. 2003, 54, 85–91. [Google Scholar] [CrossRef]
- Carlson, L.E.; Garland, S.N. Impact of mindfulness-based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. Int. J. Behav. Med. 2005, 12, 278–285. [Google Scholar] [CrossRef]
- Carlson, L.E.; Speca, M.; Patel, K.D.; Goodey, E. Mindfulness-Based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom. Med. 2003, 65, 571–581. [Google Scholar] [CrossRef]
- Würtzen, H.; Dalton, S.O.; Elsass, P.; Sumbundu, A.D.; Steding-Jensen, M.; Karlsen, R.V.; Andersen, K.K.; Flyger, H.L.; Pedersen, A.E.; Johansen, C. Mindfulness significantly reduces self-reported levels of anxiety and depression: Results of a randomised controlled trial among 336 Danish women treated for stage I–III breast cancer. Eur. J. Cancer 2013, 49, 1365–1373. [Google Scholar] [CrossRef]
- Lengacher, C.A.; Reich, R.R.; Paterson, C.L.; Ramesar, S.; Park, J.Y.; Alinat, C.; Johnson-Mallard, V.; Moscoso, M.; Budhrani-Shani, P.; Miladinovic, B.; et al. Examination of broad symptom improvement resulting from mindfulness-based stress reduction in breast cancer survivors: A randomized controlled trial. J. Clin. Oncol. 2016, 34, 2827–2834. [Google Scholar] [CrossRef]
- Andersen, S.R.; Würtzen, H.; Steding-Jessen, M.; Christensen, J.; Andersen, K.K.; Flyger, H.; Mitchelmore, C.; Johansen, C.; Dalton, S.O. Effect of mindfulness-based stress reduction on sleep quality: Results of a randomized trial among Danish breast cancer patients. Acta Oncol. 2013, 52, 336–344. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Leone, A.; Terribile, D.; Magno, S.; Sanchez, A.M.; Scardina, L.; Mason, E.J.; D’Archi, S.; Maggiore, C.; Rossi, C.; Di Micco, A.; et al. Neoadjuvant Chemotherapy in Breast Cancer: An Advanced Personalized Multidisciplinary Prehabilitation Model (APMP-M) to Optimize Outcomes. J. Pers. Med. 2021, 11, 324. https://doi.org/10.3390/jpm11050324
Di Leone A, Terribile D, Magno S, Sanchez AM, Scardina L, Mason EJ, D’Archi S, Maggiore C, Rossi C, Di Micco A, et al. Neoadjuvant Chemotherapy in Breast Cancer: An Advanced Personalized Multidisciplinary Prehabilitation Model (APMP-M) to Optimize Outcomes. Journal of Personalized Medicine. 2021; 11(5):324. https://doi.org/10.3390/jpm11050324
Chicago/Turabian StyleDi Leone, Alba, Daniela Terribile, Stefano Magno, Alejandro Martin Sanchez, Lorenzo Scardina, Elena Jane Mason, Sabatino D’Archi, Claudia Maggiore, Cristina Rossi, Annalisa Di Micco, and et al. 2021. "Neoadjuvant Chemotherapy in Breast Cancer: An Advanced Personalized Multidisciplinary Prehabilitation Model (APMP-M) to Optimize Outcomes" Journal of Personalized Medicine 11, no. 5: 324. https://doi.org/10.3390/jpm11050324
APA StyleDi Leone, A., Terribile, D., Magno, S., Sanchez, A. M., Scardina, L., Mason, E. J., D’Archi, S., Maggiore, C., Rossi, C., Di Micco, A., Carnevale, S., Paris, I., Marazzi, F., Masiello, V., Orlandi, A., Palazzo, A., Fabi, A., Masetti, R., & Franceschini, G. (2021). Neoadjuvant Chemotherapy in Breast Cancer: An Advanced Personalized Multidisciplinary Prehabilitation Model (APMP-M) to Optimize Outcomes. Journal of Personalized Medicine, 11(5), 324. https://doi.org/10.3390/jpm11050324
















