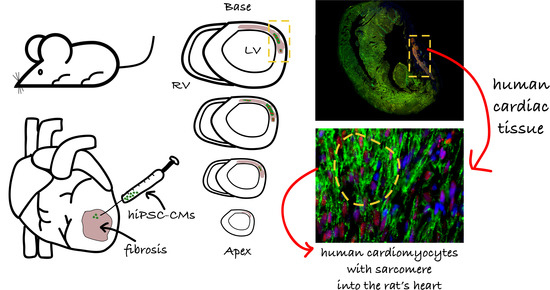
In Situ Maturated Early-Stage Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Improve Cardiac Function by Enhancing Segmental Contraction in Infarcted Rats
Abstract
:1. Introduction
2. Results
2.1. Descriptive Analysis of Mortality and Echocardiography-Based Randomization
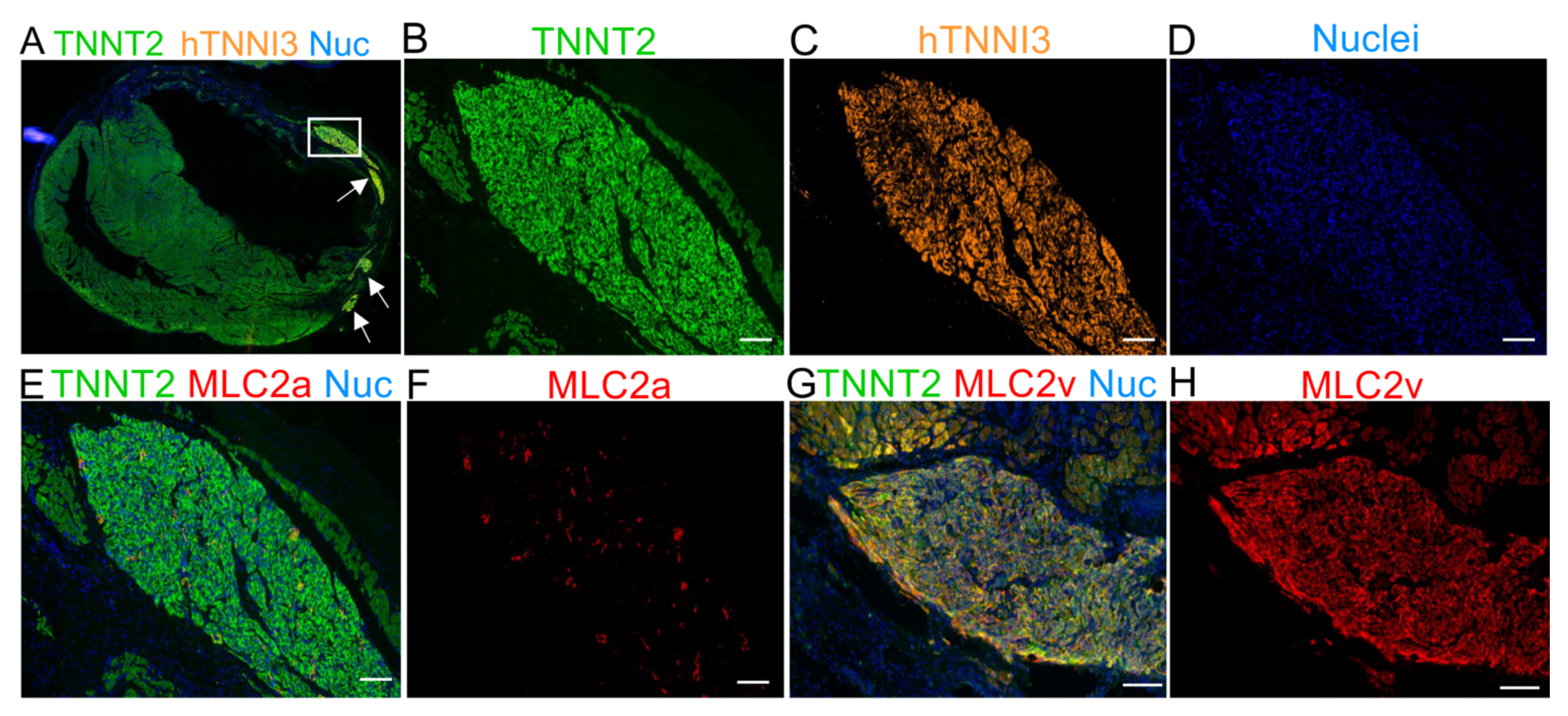
2.2. Early-Stage Human iPSC-CMs Are Predominantly MLC2a Positive (Atrial-Like Cardiomyocytes) at the Time of Injection
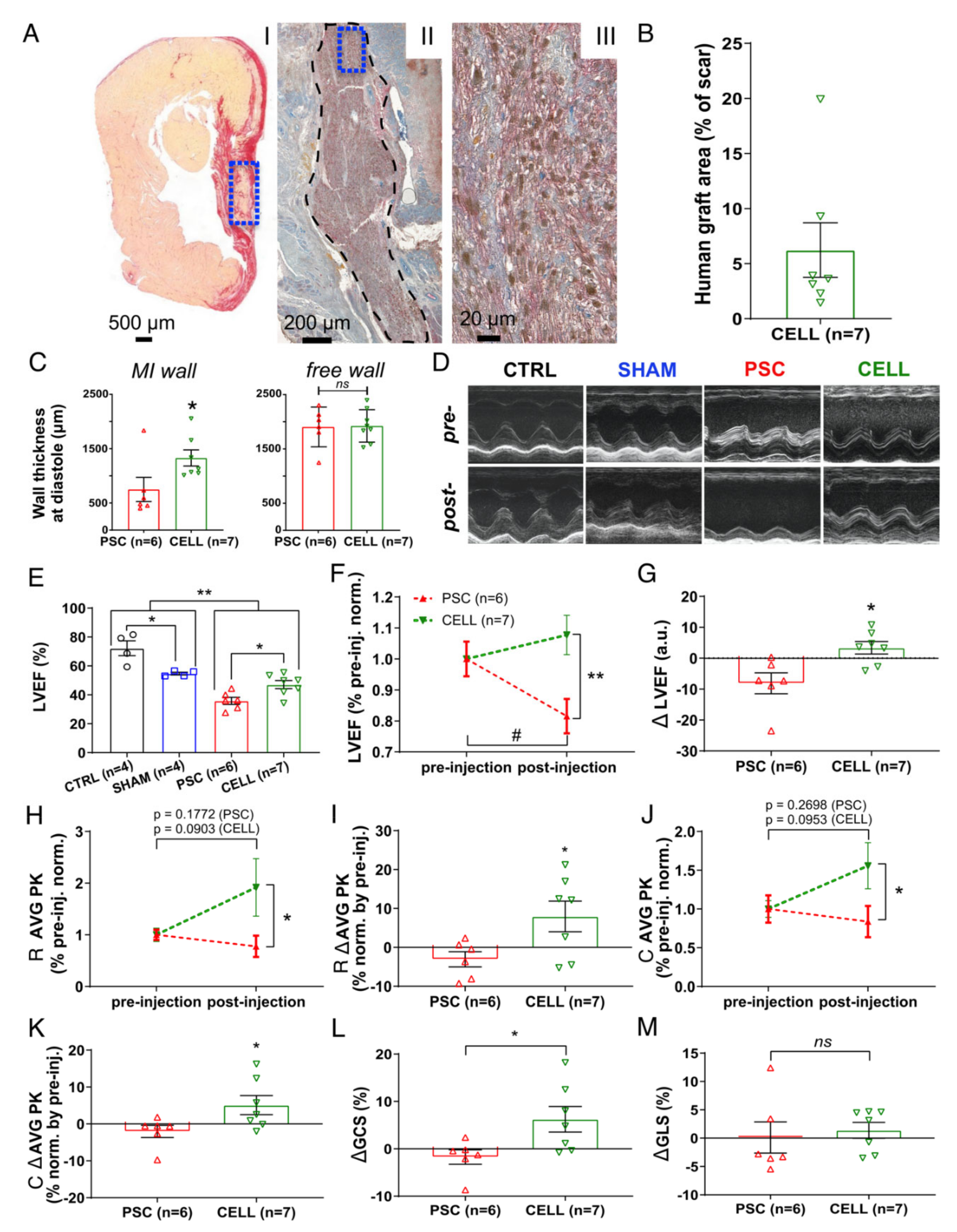
2.3. Early-Stage Human iPSC-CM Therapy Significantly Improves the Overall Cardiac Function of Immunosuppressed Infarcted Rats
2.4. The Human Grafts Are Composed of Cardiomyocytes That Preserve Certain Levels of Cell Cycling Activity
2.5. The Human Cardiac Grafts Maturate In Situ after Injection
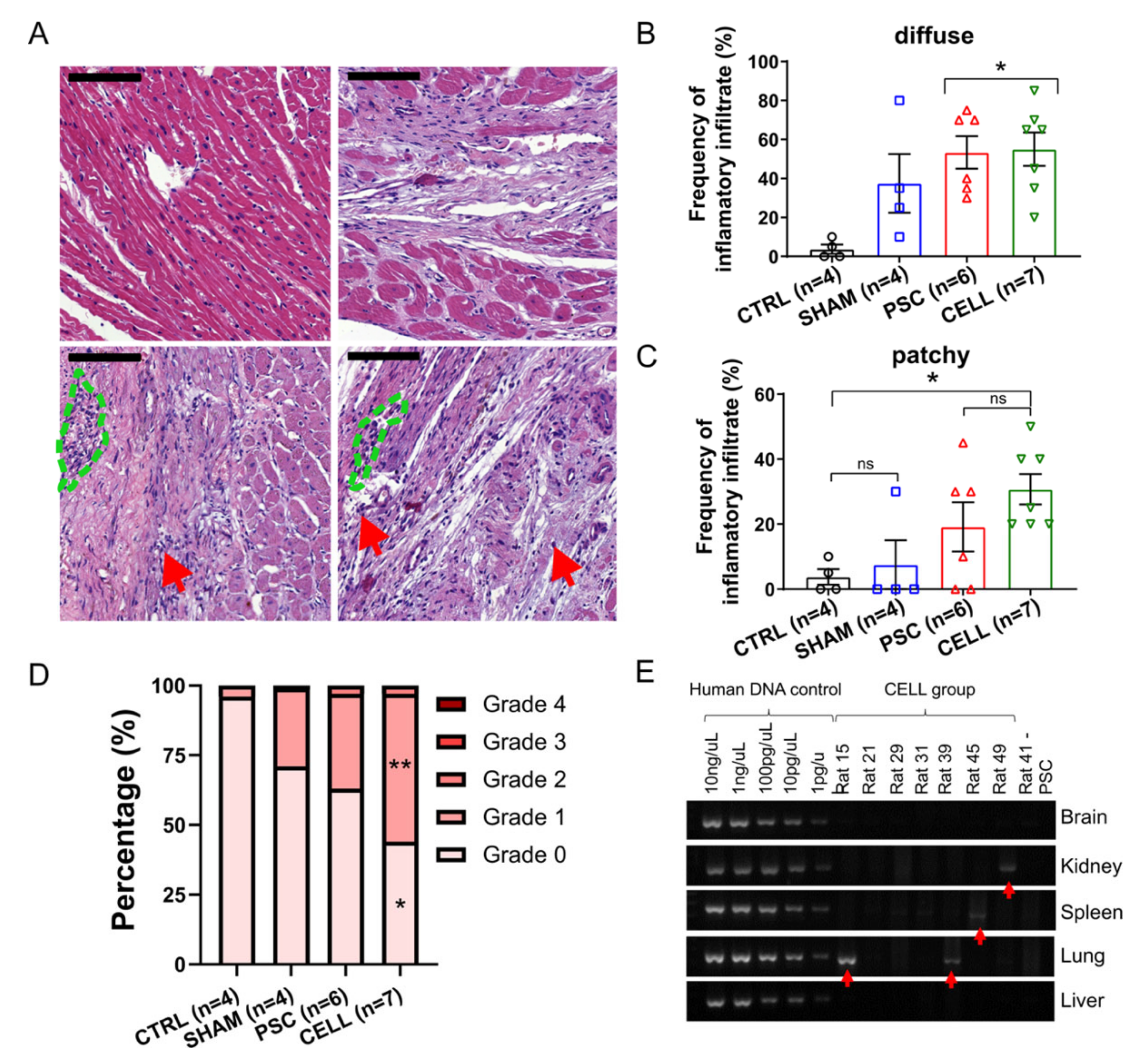
2.6. Immunosuppression Confidently Preserves Human Cardiac Grafts from Rejection or Ectopic Cellular Formations
3. Discussion
4. Material and Methods
4.1. Immunosuppression Confidently Preserves Human Cardiac Grafts from Rejection or Ectopic Cellular Formations
4.2. hiPSC-CM Differentiation
4.3. hiPSC-CM Characterization: Flow Cytometry and Immunofluorescence
4.4. Myocardial Infarction Induction
4.5. Immunosuppression
4.6. Early-Stage hiPSC-CM Priming and Intramyocardial Injection
4.7. Echocardiography, Randomization, and Exclusion Criteria
4.8. Euthanasia and Tissues Sampling
4.9. Histology, Immunohistochemistry (IHC), and Immunofluorescent (IF) Assays
4.10. Biodistribution
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teo, K.K.; Rafiq, T. Cardiovascular risk factors and prevention: A perspective from developing countries. Can. J. Cardiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update. Circulation 2021, 143. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [Green Version]
- Vivien, C.J.; Hudson, J.E.; Porrello, E.R. Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regen. Med. 2016, 1, 16012. [Google Scholar] [CrossRef] [Green Version]
- Mummery, C.L.; Davis, R.P.; Krieger, J.E. Challenges in using stem cells for cardiac repair. Sci. Transl. Med. 2010, 2, 27ps17. [Google Scholar] [CrossRef] [PubMed]
- Gowdak, L.H.W.; Schettert, I.T.; Baptista, E.; Lopes, N.L.G.; Rochitte, C.E.; Vieira, M.L.C.; Grupi, C.J.; César, L.A.M.; Krieger, J.E.; de Oliveira, S. a Intramyocardial injection of autologous bone marrow cells as an adjunctive therapy to incomplete myocardial revascularization--safety issues. Clinics 2008, 63, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Gowdak, L.H.W.; Schettert, I.T.; Rochitte, C.E.; Lisboa, L.A.F.; Dallan, L.A.O.; César, L.A.M.; de Oliveira, S.A.; Krieger, J.E. Early Increase in Myocardial Perfusion After Stem Cell Therapy in Patients Undergoing Incomplete Coronary Artery Bypass Surgery. J. Cardiovasc. Transl. Res. 2011, 4, 106–113. [Google Scholar] [CrossRef]
- Nicolau, J.C.; Furtado, R.H.M.; Silva, S.A.; Rochitte, C.E.; Rassi, A.; Moraes, J.B.M.C.; Quintella, E.; Costantini, C.R.; Korman, A.P.M.; Mattos, M.A.; et al. Stem-cell therapy in ST-segment elevation myocardial infarction with reduced ejection fraction: A multicenter, double-blind randomized trial. Clin. Cardiol. 2018, 41, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Nakamuta, J.S.; Danoviz, M.E.; Marques, F.L.N.; dos Santos, L.; Becker, C.; Gonçalves, G.A.; Vassallo, P.F.; Schettert, I.T.; Tucci, P.J.F.; Krieger, J.E. Cell therapy attenuates cardiac dysfunction post myocardial infarction: Effect of timing, routes of injection and a fibrin scaffold. PLoS ONE 2009, 4, e6005. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, L.; Santos, A.A.; Gonçalves, G.; Krieger, J.E.; Tucci, P.J.F. Bone marrow cell therapy prevents infarct expansion and improves border zone remodeling after coronary occlusion in rats. Int. J. Cardiol. 2010, 145, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Danoviz, M.E.; Nakamuta, J.S.; Marques, F.L.N.; dos Santos, L.; Alvarenga, E.C.; dos Santos, A.A.; Antonio, E.L.; Schettert, I.T.; Tucci, P.J.; Krieger, J.E. Rat adipose tissue-derived stem cells transplantation attenuates cardiac dysfunction post infarction and biopolymers enhance cell retention. PLoS ONE 2010, 5, e12077. [Google Scholar] [CrossRef] [Green Version]
- Dariolli, R.; Naghetini, M.V.; Marques, E.F.; Takimura, C.K.; Jensen, L.S.; Kiers, B.; Tsutsui, J.M.; Mathias, W.; Lemos Neto, P.A.; Krieger, J.E. Allogeneic pASC transplantation in humanized pigs attenuates cardiac remodeling post-myocardial infarction. PLoS ONE 2017, 12, e0176412. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Burridge, P.W.; Keller, G.; Gold, J.D.; Wu, J.C. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 2012, 10, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babiarz, J.E.; Ravon, M.; Sridhar, S.; Ravindran, P.; Swanson, B.; Bitter, H.; Weiser, T.; Chiao, E.; Certa, U.; Kolaja, K.L. Determination of the human cardiomyocyte mRNA and miRNA differentiation network by fine-scale profiling. Stem Cells Dev. 2012, 21, 1956–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundy, S.D.; Zhu, W.-Z.; Regnier, M.; Laflamme, M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013, 22, 1991–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, C.; Tran, D.D.; George, S.C. Concise review: Maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells 2013, 31, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funakoshi, S.; Miki, K.; Takaki, T.; Okubo, C.; Hatani, T.; Chonabayashi, K.; Nishikawa, M.; Takei, I.; Oishi, A.; Narita, M.; et al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 2016, 6, 19111. [Google Scholar] [CrossRef] [Green Version]
- Kadota, S.; Pabon, L.; Reinecke, H.; Murry, C.E. In Vivo Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Neonatal and Adult Rat Hearts. Stem Cell Rep. 2017, 8, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Gold, J.; Xu, C.; Hassanipour, M.; Rosler, E.; Police, S.; Muskheli, V.; Murry, C.E. Formation of human myocardium in the rat heart from human embryonic stem cells. Am. J. Pathol. 2005, 167, 663–671. [Google Scholar] [CrossRef] [Green Version]
- Ishida, M.; Miyagawa, S.; Saito, A.; Fukushima, S.; Harada, A.; Ito, E.; Ohashi, F.; Watabe, T.; Hatazawa, J.; Matsuura, K.; et al. Transplantation of Human-induced Pluripotent Stem Cell-derived Cardiomyocytes Is Superior to Somatic Stem Cell Therapy for Restoring Cardiac Function and Oxygen Consumption in a Porcine Model of Myocardial Infarction. Transplantation 2019, 103, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Romagnuolo, R.; Masoudpour, H.; Porta-Sánchez, A.; Qiang, B.; Barry, J.; Laskary, A.; Qi, X.; Massé, S.; Magtibay, K.; Kawajiri, H.; et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Rep. 2019, 12, 967–981. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef]
- Fernandes, S.; Naumova, A.V.; Zhu, W.Z.; Laflamme, M.A.; Gold, J.; Murry, C.E. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J. Mol. Cell. Cardiol. 2010, 49, 941–949. [Google Scholar] [CrossRef] [Green Version]
- Shiba, Y.; Fernandes, S.; Zhu, W.Z.; Filice, D.; Muskheli, V.; Kim, J.; Palpant, N.J.; Gantz, J.; Moyes, K.W.; Reinecke, H.; et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 2012, 489, 322–325. [Google Scholar] [CrossRef]
- Chong, J.J.H.; Yang, X.; Don, C.W.; Minami, E.; Liu, Y.-W.; Weyers, J.J.; Mahoney, W.M.; Van Biber, B.; Cook, S.M.; Palpant, N.J.; et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014, 510, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Citro, L.; Naidu, S.; Hassan, F.; Kuppusamy, M.L.; Kuppusamy, P.; Angelos, M.G.; Khan, M. Comparison of Human Induced Pluripotent Stem-Cell Derived Cardiomyocytes with Human Mesenchymal Stem Cells following Acute Myocardial Infarction. PLoS ONE 2014, 9, e116281. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.; Chong, J.J.H.; Paige, S.L.; Iwata, M.; Torok-Storb, B.; Keller, G.; Reinecke, H.; Murry, C.E. Comparison of Human Embryonic Stem Cell-Derived Cardiomyocytes, Cardiovascular Progenitors, and Bone Marrow Mononuclear Cells for Cardiac Repair. Stem Cell Rep. 2015, 5, 753–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef]
- Weinberger, F.; Breckwoldt, K.; Pecha, S.; Kelly, A.; Geertz, B.; Starbatty, J.; Yorgan, T.; Cheng, K.H.; Lessmann, K.; Stolen, T.; et al. Cardiac repair in Guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med. 2016, 8, 1–13. [Google Scholar] [CrossRef]
- Dariolli, R.; Bassaneze, V.; Nakamuta, J.S.; Omae, S.V.; Campos, L.C.G.; Krieger, J.E. Porcine Adipose Tissue-Derived Mesenchymal Stem Cells Retain Their Proliferative Characteristics, Senescence, Karyotype and Plasticity after Long-Term Cryopreservation. PLoS ONE 2013, 8, e67939. [Google Scholar] [CrossRef] [Green Version]
- Dariolli, R.; Takimura, C.K.; Campos, C.A.; Lemos, P.A.; Krieger, J.E. Development of a closed-artery catheter-based myocardial infarction in pigs using sponge and lidocaine hydrochloride infusion to prevent irreversible ventricular fibrillation. Physiol. Rep. 2014, 2, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruvinel, E.; Ogusuku, I.; Cerioni, R.; Rodrigues, S.; Gonçalves, J.; Góes, M.E.; Alvim, J.M.; Silva, A.C.; de Lino, V.S.; Boccardo, E.; et al. Long-term single-cell passaging of human iPSC fully supports pluripotency and high-efficient trilineage differentiation capacity. SAGE Open Med. 2020, 8, 205031212096645. [Google Scholar] [CrossRef] [PubMed]
- Olivares, E.L.; Ribeiro, V.P.; Werneck de Castro, J.P.S.; Ribeiro, K.C.; Mattos, E.C.; Goldenberg, R.C.S.; Mill, J.G.; Dohmann, H.F.; dos Santos, R.R.; de Carvalho, A.C.C.; et al. Bone marrow stromal cells improve cardiac performance in healed infarcted rat hearts. Am. J. Physiol. Circ. Physiol. 2004, 287, H464–H470. [Google Scholar] [CrossRef] [Green Version]
- Irion, C.I.; Martins, E.L.; Christie, M.L.A.; de Andrade, C.B.V.; de Moraes, A.C.N.; Ferreira, R.P.; Pimentel, C.F.; Suhett, G.D.; de Carvalho, A.C.C.; Lindoso, R.S.; et al. Acute Myocardial Infarction Reduces Respiration in Rat Cardiac Fibers, despite Adipose Tissue Mesenchymal Stromal Cell Transplant. Stem Cells Int. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- Fidelis-De-Oliveira, P.; Werneck-De-Castro, J.P.S.; Pinho-Ribeiro, V.; Shalom, B.C.M.; Nascimento-Silva, J.H.; Souza, R.H.C.E.; Cruz, I.S.; Rangel, R.R.; Goldenberg, R.C.S.; Campos-De-Carvalho, A.C. Soluble Factors from Multipotent Mesenchymal Stromal Cells have Antinecrotic Effect on Cardiomyocytes in Vitro and Improve Cardiac Function in Infarcted Rat Hearts. Cell Transplant. 2012, 21, 1011–1021. [Google Scholar] [CrossRef] [Green Version]
- Bartunek, J.; Terzic, A.; Davison, B.A.; Filippatos, G.S.; Radovanovic, S.; Beleslin, B.; Merkely, B.; Musialek, P.; Wojakowski, W.; Andreka, P.; et al. Cardiopoietic cell therapy for advanced ischemic heart failure: Results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur. Heart J. 2016, 38, ehw543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, K.R.; Frisén, J.; Fritsche-Danielson, R.; Melton, D.A.; Murry, C.E.; Weissman, I.L. Regenerating the field of cardiovascular cell therapy. Nat. Biotechnol. 2019, 37, 232–237. [Google Scholar] [CrossRef]
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling—concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Giacca, M. Cardiac Regeneration After Myocardial Infarction: An Approachable Goal. Curr. Cardiol. Rep. 2020, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Engel, F.B. Advances in heart regeneration based on cardiomyocyte proliferation and regenerative potential of binucleated cardiomyocytes and polyploidization. Clin. Sci. 2019, 133, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, K.; Li, T.-S.; Luthringer, D.; Terrovitis, J.; Cheng, K.; Chakravarty, T.; Galang, G.; Zhang, Y.; Schoenhoff, F.; Van Eyk, J.; et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 2012, 125, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Samak, M.; Hinkel, R. Stem Cells in Cardiovascular Medicine: Historical Overview and Future Prospects. Cells 2019, 8, 1530. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Huang, W.; Jiang, L.; Paul, C.; Li, X.; Wang, Y. Concise Review: Reduction of Adverse Cardiac Scarring Facilitates Pluripotent Stem Cell-Based Therapy for Myocardial Infarction. Stem Cells 2019, 37, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Jackson, A.O.; Rahman, G.A.; Yin, K.; Long, S. Enhancing Matured Stem-Cardiac Cell Generation and Transplantation: A Novel Strategy for Heart Failure Therapy. J. Cardiovasc. Transl. Res. 2020, 1–17. [Google Scholar]
- Fan, C.; Fast, V.G.; Tang, Y.; Zhao, M.; Turner, J.F.; Krishnamurthy, P.; Rogers, J.M.; Valarmathi, M.T.; Yang, J.; Zhu, W.; et al. Cardiomyocytes from CCND2-overexpressing human induced-pluripotent stem cells repopulate the myocardial scar in mice: A 6-month study. J. Mol. Cell. Cardiol. 2019, 137, 25–33. [Google Scholar] [CrossRef]
- Saludas, L.; Garbayo, E.; Mazo, M.; Pelacho, B.; Abizanda, G.; Iglesias-Garcia, O.; Raya, A.; Prósper, F.; Blanco-Prieto, M.J. Long-term engraftment of human cardiomyocytes combined with biodegradable microparticles induces heart repair. J. Pharmacol. Exp. Ther. 2019, 370, 761–771. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The biological basis for cardiac repair after myocardial infarction. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Ferrini, A.; Stevens, M.M.; Sattler, S.; Rosenthal, N. Toward Regeneration of the Heart: Bioengineering Strategies for Immunomodulation. Front. Cardiovasc. Med. 2019, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Liehn, E.A.; Postea, O.; Curaj, A.; Marx, N. Repair after myocardial infarction, between fantasy and reality: The role of chemokines. J. Am. Coll. Cardiol. 2011, 58, 2357–2362. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Fan, C.; Ernst, P.J.; Tang, Y.; Zhu, H.; Mattapally, S.; Oduk, Y.; Borovjagin, A.V.; Zhou, L.; Zhang, J.; et al. Y-27632 preconditioning enhances transplantation of human-induced pluripotent stem cell-derived cardiomyocytes in myocardial infarction mice. Cardiovasc. Res. 2019, 115, 343–356. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Z.; Dong, M. Cardiac repair in a murine model of myocardial infarction with human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2020, 11, 297. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Ting, S.; Zhen, Z.; Luo, F.; Zhu, Z.; Jiang, Y.; Sun, S.; Lai, W.H.; Lian, Q.; et al. Potent immunomodulation and angiogenic effects of mesenchymal stem cells versus cardiomyocytes derived from pluripotent stem cells for treatment of heart failure. Stem Cell Res. Ther. 2019, 10, 78. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.W. Cardiovascular Toxicities of Immunosuppressive Agents. Am. J. Transplant. 2002, 2, 807–818. [Google Scholar] [CrossRef]
- Tavares, P.; Reis, F.; Ribeiro, C.A.F.; Teixeira, F. Cardiovascular effects of cyclosporin treatment in an experimental model. Rev. Port. Cardiol. 2002, 21, 141–155. [Google Scholar] [PubMed]
- Zhu, K.; Wu, Q.; Ni, C.; Zhang, P.; Zhong, Z.; Wu, Y.; Wang, Y.; Xu, Y.; Kong, M.; Cheng, H.; et al. Lack of Remuscularization Following Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells in Infarcted Nonhuman Primates. Circ. Res. 2018, 122, 958–969. [Google Scholar] [CrossRef]
- Fang, Y.H.; Wang, S.P.H.; Chang, H.Y.; Yang, P.J.; Liu, P.Y.; Liu, Y.W. Immunogenicity in stem cell therapy for cardiac regeneration. Acta Cardiol. Sin. 2020, 36, 588–594. [Google Scholar]
- Antonio, E.L.; Dos Santos, A.A.; Araujo, S.R.R.; Bocalini, D.S.; dos Santos, L.; Fenelon, G.; Franco, M.F.; Tucci, P.J.F. Left Ventricle Radio-frequency Ablation in the Rat: A New Model of Heart Failure due to Myocardial Infarction Homogeneous in Size and Low in Mortality. J. Card. Fail. 2009, 15, 540–548. [Google Scholar] [CrossRef]
- Dow, J.S.; Bhandari, A.; Hale, S.L.; Kloner, R.A. Does sex influence the incidence or severity of reperfusion-induced cardiac arrhythmias? Springerplus 2015, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, M.L.; Bolli, R.; Canty, J.M.; Du, X.J.; Frangogiannis, N.G.; Frantz, S.; Gourdie, R.G.; Holmes, J.W.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol. Hear. Circ. Physiol. 2018, 314, H812–H838. [Google Scholar] [CrossRef] [PubMed]
- Asleh, R.; Manemann, S.M.; Weston, S.A.; Bielinski, S.J.; Chamberlain, A.M.; Jiang, R.; Gerber, Y.; Roger, V.L. Sex Differences in Outcomes After Myocardial Infarction in the Community. Am. J. Med. 2021, 134, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.L.; Meyer, P.M.D.; Marwick, T.H.; Lam, C.S.P.; Kaye, D.M. Sex differences in cardiovascular pathophysiology why women are overrepresented in heart failure with preserved ejection fraction. Circulation 2018, 138, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L. Gender differences in the pathophysiology, clinical presentation, and outcomes of ischemic heart failure. Curr. Heart Fail. Rep. 2012, 9, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Albrektsen, G.; Heuch, I.; Løchen, M.L.; Thelle, D.S.; Wilsgaard, T.; Njølstad, I.; Bønaa, K.H. Lifelong gender gap in risk of incident myocardial infarction: The Tromsø study. JAMA Intern. Med. 2016, 176, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Koehler, J.; Kuehnel, T.; Kees, F.; Hoecherl, K.; Grobecker, H.F. Comparison of bioavailability and metabolism with two commercial formulations of cyclosporine a in rats. Drug Metab. Dispos. 2002, 30, 658–662. [Google Scholar] [CrossRef] [Green Version]
- Diehl, R.; Ferrara, F.; Müller, C.; Dreyer, A.Y.; McLeod, D.D.; Fricke, S.; Boltze, J. Immunosuppression for in vivo research: State-of-the-art protocols and experimental approaches. Cell. Mol. Immunol. 2017, 14, 146–179. [Google Scholar] [CrossRef] [Green Version]
- Midha, R.; Mackinnon, S.E.; Evans, P.J.; Best, T.J.; Wong, P.Y. Subcutaneous injection of oral cyclosporin A solution. Microsurgery 1992, 13, 92–94. [Google Scholar] [CrossRef]
- Sato, Y.; Araki, H.; Kato, J.; Nakamura, K.; Kawano, Y.; Kobune, M.; Sato, T.; Miyanishi, K.; Takayama, T.; Takahashi, M.; et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood 2005, 106, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Jansen Of Lorkeers, S.J.; Hart, E.; Tang, X.L.; Chamuleau, M.E.D.; Doevendans, P.A.; Bolli, R.; Chamuleau, S.A.J. Cyclosporin in cell therapy for cardiac regeneration. J. Cardiovasc. Transl. Res. 2014, 7, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiograph. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef]



| Status | Phase | N#/ Phase | % (From Total) | Cause | Group | N#/ Group | % (From Total) |
|---|---|---|---|---|---|---|---|
| Dead | MI induction | 1 | 2.4% | Irreversible fibrillation | MI | 1 | 2.4% |
| 1–6 days after MI induction (pre-echocardiography) | 2 | 4.8% | Anesthetic overload | MI | 1 | 2.4% | |
| Sudden death * | MI | 1 | 2.4% | ||||
| Baseline echocardiogram (day 6) | 2 | 4.8% | Anesthetic overload | MI | 2 | 4.8% | |
| Injections procedure | 6 | 14.3% | Cardiorespiratory arrest | PSC | 3 | 7.1% | |
| CELL | 3 | 7.1% | |||||
| 1–30 days after injection (pre-final echocardiography) | 6 | 14.3% | Sudden death * | SHAM | 1 | 2.4% | |
| PSC | 3 | 7.1% | |||||
| CELL | 2 | 4.8% | |||||
| Final echocardiogram (day 37) | 0 | 0.0% | - | 0 | 0.0% | ||
| Live | LVEF cutoff (based on CTRLs) | 4 ** | 9.5% | 20% impairment vs. CTRL animals at baseline | PSC | 2 | 4.8% |
| CELL | 2 | 4.8% | |||||
| Completed follow-up and used for further analysis | 21 | 50.0% | - | CTRL | 4 | 9.5% | |
| - | SHAM | 4 | 9.5% | ||||
| - | PSC | 6 | 14.2% | ||||
| - | CELL | 7 | 16.7% | ||||
| Total | 42 | 100% | 42 | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biagi, D.; Fantozzi, E.T.; Campos-Oliveira, J.C.; Naghetini, M.V.; Ribeiro, A.F., Jr.; Rodrigues, S.; Ogusuku, I.; Vanderlinde, R.; Christie, M.L.A.; Mello, D.B.; et al. In Situ Maturated Early-Stage Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Improve Cardiac Function by Enhancing Segmental Contraction in Infarcted Rats. J. Pers. Med. 2021, 11, 374. https://doi.org/10.3390/jpm11050374
Biagi D, Fantozzi ET, Campos-Oliveira JC, Naghetini MV, Ribeiro AF Jr., Rodrigues S, Ogusuku I, Vanderlinde R, Christie MLA, Mello DB, et al. In Situ Maturated Early-Stage Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Improve Cardiac Function by Enhancing Segmental Contraction in Infarcted Rats. Journal of Personalized Medicine. 2021; 11(5):374. https://doi.org/10.3390/jpm11050374
Chicago/Turabian StyleBiagi, Diogo, Evelyn Thais Fantozzi, Julliana Carvalho Campos-Oliveira, Marcus Vinicius Naghetini, Antonio Fernando Ribeiro, Jr., Sirlene Rodrigues, Isabella Ogusuku, Rubia Vanderlinde, Michelle Lopes Araújo Christie, Debora Bastos Mello, and et al. 2021. "In Situ Maturated Early-Stage Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Improve Cardiac Function by Enhancing Segmental Contraction in Infarcted Rats" Journal of Personalized Medicine 11, no. 5: 374. https://doi.org/10.3390/jpm11050374
APA StyleBiagi, D., Fantozzi, E. T., Campos-Oliveira, J. C., Naghetini, M. V., Ribeiro, A. F., Jr., Rodrigues, S., Ogusuku, I., Vanderlinde, R., Christie, M. L. A., Mello, D. B., de Carvalho, A. C. C., Valadares, M., Cruvinel, E., & Dariolli, R. (2021). In Situ Maturated Early-Stage Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Improve Cardiac Function by Enhancing Segmental Contraction in Infarcted Rats. Journal of Personalized Medicine, 11(5), 374. https://doi.org/10.3390/jpm11050374