Improved Models of Human Endometrial Organoids Based on Hydrogels from Decellularized Endometrium
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Organoid Development from Endometrial Biopsies
2.3. EndoECM-Hydrogel-Based Culture
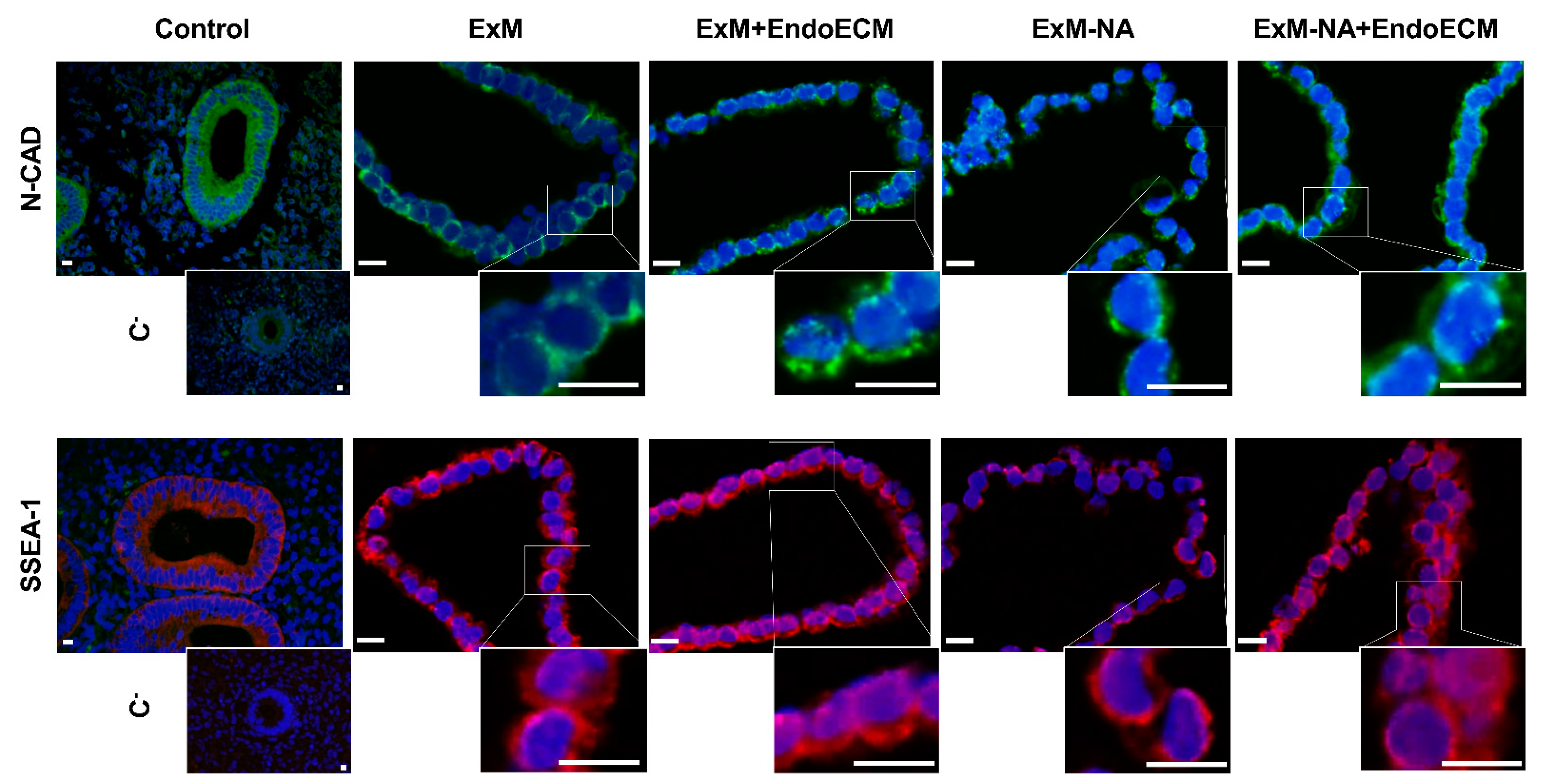
2.4. Histological and Immunohistochemical Characterization of Organoids
2.5. Chromosomal Stability
2.6. Comparative Proliferation Assays
2.7. Stemness Assessment
2.8. Statistical Analyses
3. Results
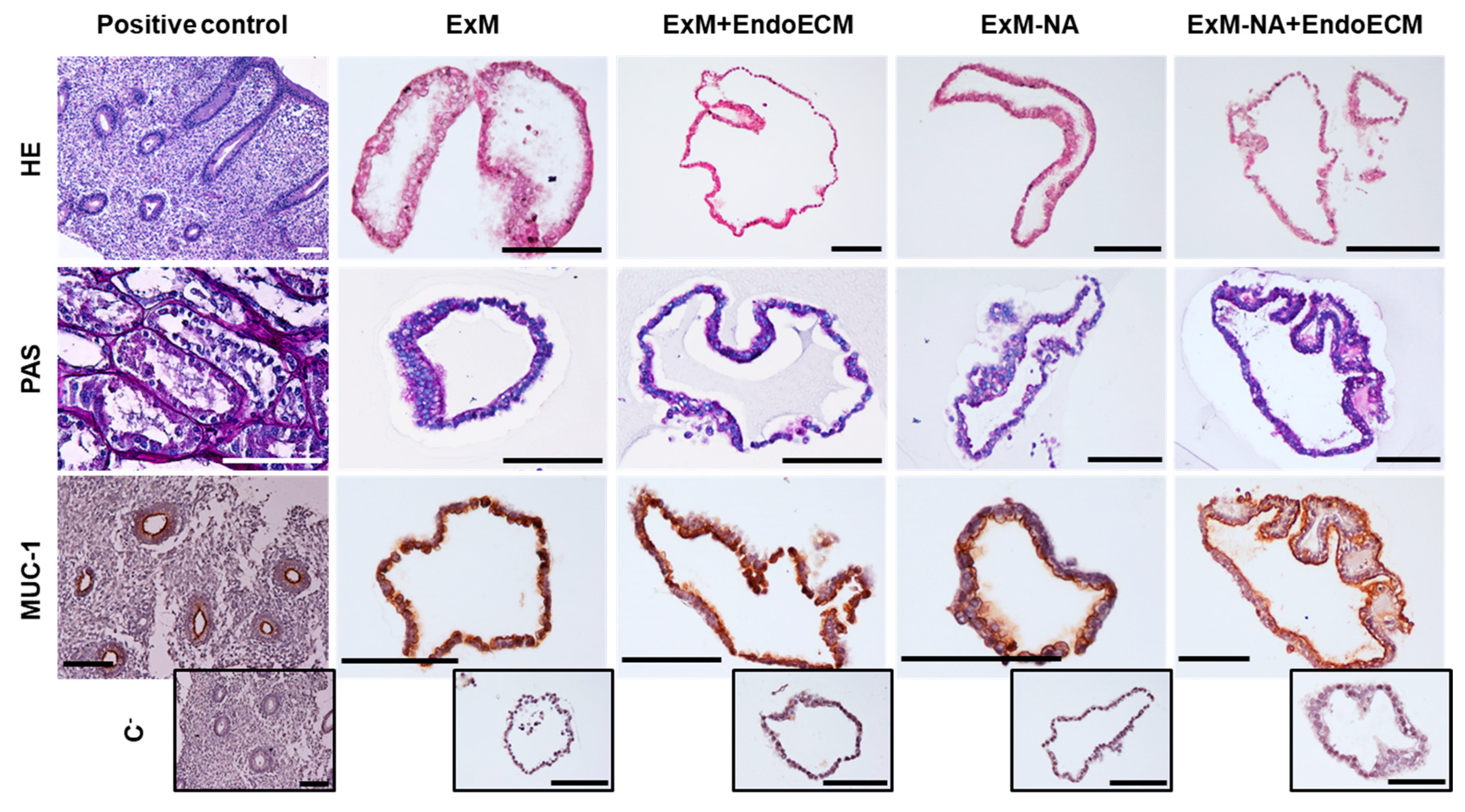
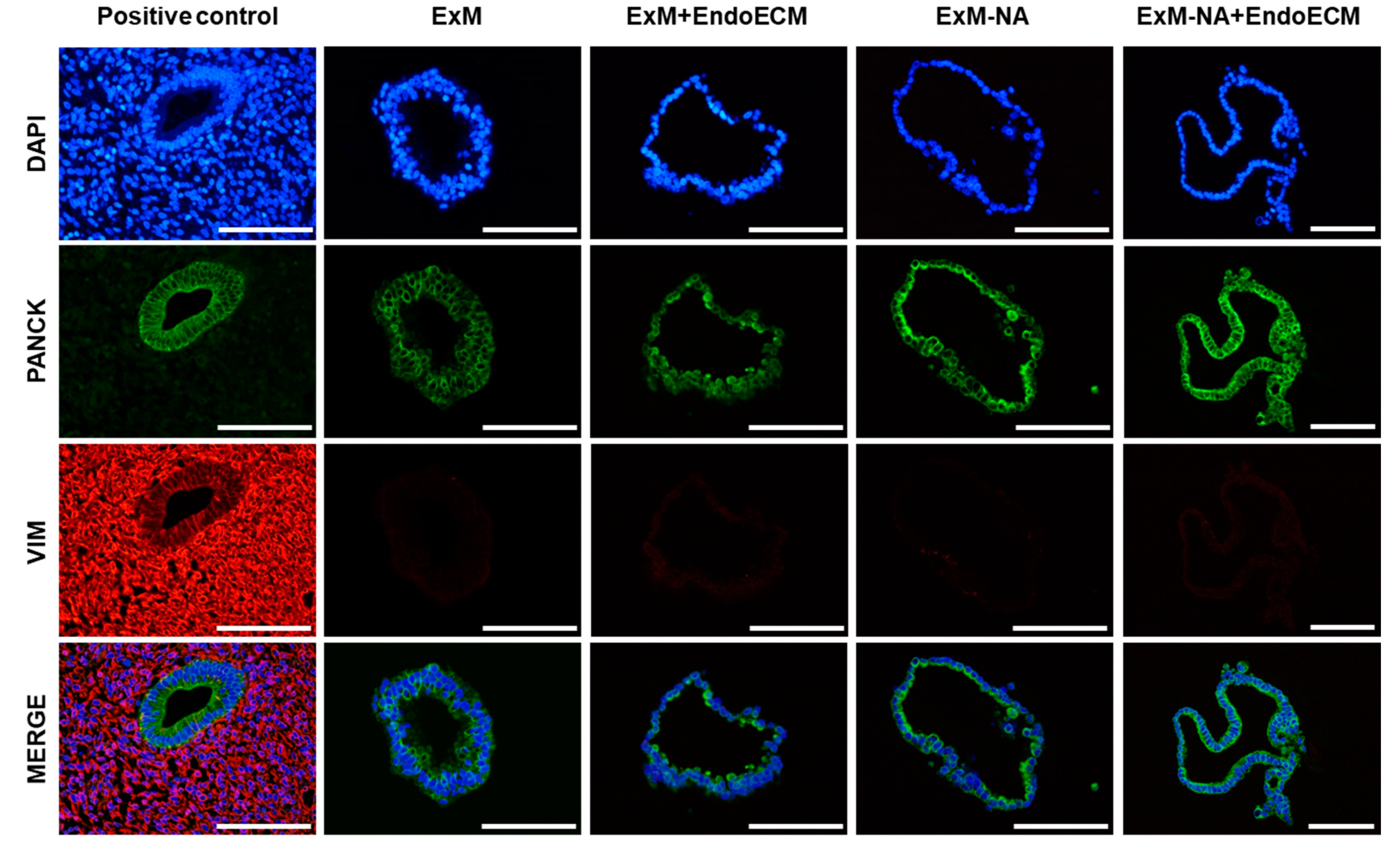
3.1. Human Endometrial Organoids Cultured with EndoECM Supplementation Recapitulate the In Vivo Phenotype of Endometrial Glands
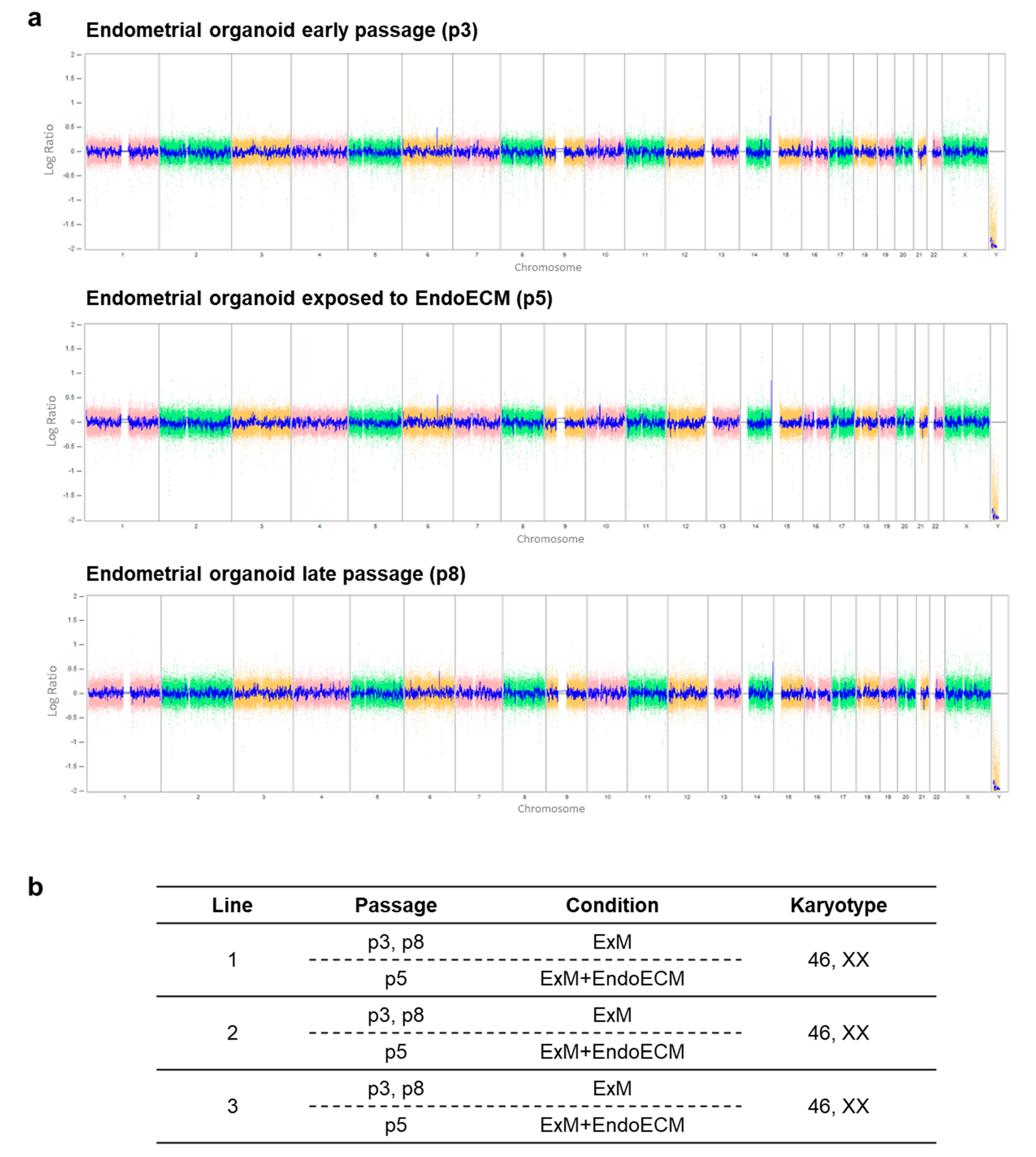
3.2. Human Endometrial Organoids Are Genetically Stable during Long-Term Culture Systems
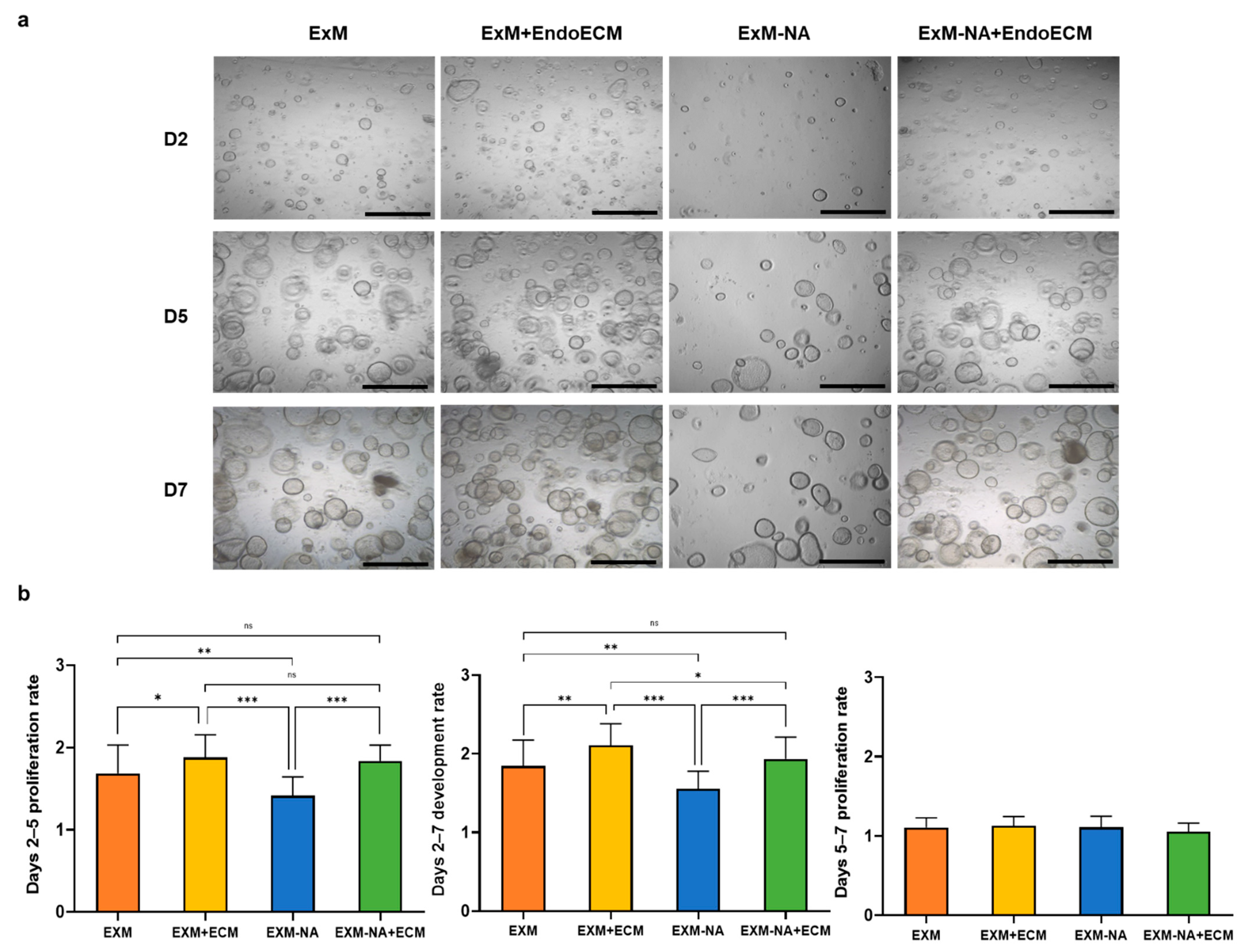
3.3. Tissue-Specific Extracellular Matrices Support Organoid Proliferation
3.3.1. Exposure of Human Endometrial Organoids to EndoECM Had a Positive Effect on Their Proliferation Rate
3.3.2. Human Endometrial Organoids Cultured under EndoECM Supplementation Show Higher Proliferation Capacity Based on Ki67 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lessey, B.A. The role of the endometrium during embryo implantation. Hum. Reprod. 2000, 15, 39–50. [Google Scholar] [PubMed]
- Strowitzki, T.; Germeyer, A.; Popovici, R.; Von Wolff, M. The human endometrium as a fertility-determining factor. Hum. Reprod. Update 2006, 12, 617–630. [Google Scholar] [CrossRef]
- Macer, M.L.; Taylor, H.S. Endometriosis and Infertility. Obstet. Gynecol. Clin. N. Am. 2012, 39, 535–549. [Google Scholar] [CrossRef]
- Salazar, C.A.; Isaacson, K.; Morris, S. A comprehensive review of Asherman’s syndrome: Causes, symptoms and treatment options. Curr. Opin. Obstet. Gynecol. 2017, 29, 249–256. [Google Scholar] [CrossRef]
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef]
- Bourdon, M.; Santulli, P.; Oliveira, J.; Marcellin, L.; Maignien, C.; Melka, L.; Bordonne, C.; Millisher, A.-E.; Plu-Bureau, G.; Cormier, J.; et al. Focal adenomyosis is associated with primary infertility. Fertil. Steril. 2020, 114, 1271–1277. [Google Scholar] [CrossRef]
- Yu, D.; Wong, Y.-M.; Cheong, Y.; Xia, E.; Li, T.C. Asherman syndrome—One century later. Fertil. Steril. 2008, 89, 759–779. [Google Scholar] [CrossRef] [PubMed]
- Senturk, L.M.; Erel, C.T. Thin endometrium in assisted reproductive technology. Curr. Opin. Obstet. Gynecol. 2008, 20, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.D.; Alstrup, A.K.O.; Duvald, C.S.; Mikkelsen, E.F.R.; Vendelbo, M.H.; Ovesen, P.G.; Pedersen, M. Animal Models of Fetal Medicine and Obstetrics. In Experimental Animal Models of Human Diseases—An Effective Therapeutic Strategy; IntechOpen: London, UK, 2018. [Google Scholar]
- Heidari-Khoei, H.; Esfandiari, F.; Hajari, M.A.; Ghorbaninejad, Z.; Piryaei, A.; Baharvand, H. Organoid technology in female reproductive biomedicine. Reprod. Biol. Endocrinol. 2020, 18, 1–19. [Google Scholar] [CrossRef]
- De Souza, N. Organoids. Nat. Methods 2018, 15, 23. [Google Scholar] [CrossRef]
- Serra, D.; Mayr, U.; Boni, A.; Lukonin, I.; Rempfler, M.; Meylan, L.C.; Stadler, M.B.; Strnad, P.; Papasaikas, P.; Vischi, D.; et al. Self-organization and symmetry breaking in intestinal organoid development. Nat. Cell Biol. 2019, 569, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Takasato, M.; Er, P.X.; Chiu, H.S.; Little, M.H. Generation of kidney organoids from human pluripotent stem cells. Nat. Protoc. 2016, 11, 1681–1692. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef]
- Arnold, J.T.; Kaufman, D.G.; Seppälä, M.; Lessey, B.A. Endometrial stromal cells regulate epithelial cell growth in vitro: A new co-culture model. Hum. Reprod. 2001, 16, 836–845. [Google Scholar] [CrossRef]
- Truskey, G.A. Human Microphysiological Systems and Organoids as in Vitro Models for Toxicological Studies. Front. Public Health 2018, 6, 185. [Google Scholar] [CrossRef]
- Yin, X.; Mead, B.E.; Safaee, H.; Langer, R.; Karp, J.M.; Levy, O. Engineering Stem Cell Organoids. Cell Stem Cell 2016, 18, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Cervelló, I.; Medrano, J.V.; Simón, C. Regenerative Medicine and Tissue Engineering in Reproductive Medicine. In Translating Regenerative Medicine to the Clinic; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 139–151. [Google Scholar]
- Li, D.; Lin, T.L.; Lipe, B.; Hopkins, R.A.; Shinogle, H.; Aljitawi, O.S. A novel extracellular matrix-based leukemia model supports leukemia cells with stem cell-like characteristics. Leuk. Res. 2018, 72, 105–112. [Google Scholar] [CrossRef]
- Lam, D.; Enright, H.A.; Cadena, J.; Peters, S.; Sales, A.P.; Osburn, J.J.; Soscia, D.A.; Kulp, K.S.; Wheeler, E.K.; Fischer, N.O. Tissue-specific extracellular matrix accelerates the formation of neural networks and communities in a neuron-glia co-culture on a multi-electrode array. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Ijima, H.; Nakamura, S.; Bual, R.P.; Yoshida, K. Liver-specific extracellular matrix hydrogel promotes liver-specific functions of hepatocytes in vitro and survival of transplanted hepatocytes in vivo. J. Biosci. Bioeng. 2019, 128, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Hellström, M.; Moreno-Moya, J.M.; Bandstein, S.; Bom, E.; Akouri, R.R.; Miyazaki, K.; Maruyama, T.; Brännström, M. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil. Steril. 2016, 106, 487–496.e1. [Google Scholar] [CrossRef]
- Miyazaki, K.; Maruyama, T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 2014, 35, 8791–8800. [Google Scholar] [CrossRef]
- Campo, H.; Garcia-Dominguez, X.; López-Martínez, S.; Faus, A.; Antón, J.S.V.; Marco-Jiménez, F.; Cervelló, I. Tissue-specific decellularized endometrial substratum mimicking different physiological conditions influences in vitro embryo development in a rabbit model. Acta Biomater. 2019, 89, 126–138. [Google Scholar] [CrossRef]
- Francés-Herrero, E.; De Miguel-Gómez, L.; López-Martínez, S.; Campo, H.; Garcia-Dominguez, X.; Diretto, G.; Faus, A.; Vicente, J.S.; Marco-Jiménez, F.; Cervelló, I. Development of Decellularized Oviductal Hydrogels as a Support for Rabbit Embryo Culture. Reprod. Sci. 2021, 28, 1644–1658. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, S.; Campo, H.; de Miguel-Gómez, L.; Faus, A.; Navarro, A.T.; Díaz, A.; Pellicer, A.; Ferrero, H.; Cervelló, I. A Natural Xenogeneic Endometrial Extracellular Matrix Hydrogel toward Improving Current Human in vitro Models and Future in vivo Applications. Front. Bioeng. Biotechnol. 2021, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Campo, H.; Baptista, P.M.; López-Pérez, N.; Faus, A.; Cervelló, I.; Simón, C. De- and recellularization of the pig uterus: A bioengineering pilot study. Biol. Reprod. 2016, 96, 34–45. [Google Scholar] [CrossRef]
- Freytes, D.O.; Martin, J.; Velankar, S.S.; Lee, A.S.; Badylak, S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 2008, 29, 1630–1637. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, H.C.; Schust, D.J.; Spencer, T. In vitro models of the human endometrium: Evolution and application for women’s health. Biol. Reprod. 2021, 104, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Laganà, A.S.; Vitale, S.G.; Salmeri, F.M.; Triolo, O.; Frangež, H.B.; Vrtačnik-Bokal, E.; Stojanovska, L.; Apostolopoulos, V.; Granese, R.; Sofo, V. Unus pro omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med. Hypotheses 2017, 103, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- O’Neill, J.D.; Anfang, R.; Anandappa, A.; Costa, J.; Javidfar, J.; Wobma, H.M.; Singh, G.; Freytes, D.O.; Bacchetta, M.D.; Sonett, J.R.; et al. Decellularization of Human and Porcine Lung Tissues for Pulmonary Tissue Engineering. Ann. Thorac. Surg. 2013, 96, 1046–1056. [Google Scholar] [CrossRef]
- Sullivan, D.C.; Mirmalek-Sani, S.-H.; Deegan, D.B.; Baptista, P.M.; Aboushwareb, T.; Atala, A.; Yoo, J.J. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 2012, 33, 7756–7764. [Google Scholar] [CrossRef]
- Baptista, P.M.; Vyas, D.; Moran, E.; Wang, Z.; Soker, S. Human Liver Bioengineering Using a Whole Liver Decellularized Bioscaffold. Adv. Struct. Saf. Stud. 2013, 1001, 289–298. [Google Scholar] [CrossRef]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.-K.; Black, L.D.; Kren, S.M.; Netoff, T.; Taylor, D. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Hellström, M.; El-Akouri, R.; Sihlbom, C.; Olsson, B.; Lengqvist, J.; Bäckdahl, H.; Johansson, B.; Olausson, M.; Sumitran-Holgersson, S.; Brännström, M. Towards the development of a bioengineered uterus: Comparison of different protocols for rat uterus decellularization. Acta Biomater. 2014, 10, 5034–5042. [Google Scholar] [CrossRef]
- DeQuach, J.A.; Mezzano, V.; Miglani, A.; Lange, S.; Keller, G.; Sheikh, F.; Christman, K.L. Simple and High Yielding Method for Preparing Tissue Specific Extracellular Matrix Coatings for Cell Culture. PLoS ONE 2010, 5, e13039. [Google Scholar] [CrossRef]
- French, K.M.; Boopathy, A.V.; DeQuach, J.A.; Chingozha, L.; Lu, H.; Christman, K.L.; Davis, M.E. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 2012, 8, 4357–4364. [Google Scholar] [CrossRef]
- Young, D.A.; Choi, Y.S.; Engler, A.J.; Christman, K.L. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 2013, 34, 8581–8588. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J. Direct comparison of different coating matrix on the hepatic differentiation from adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2015, 456, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Sackett, S.D.; Tremmel, D.M.; Ma, F.; Feeney, A.; Maguire, R.M.; Brown, M.E.; Zhou, Y.; Li, X.; O’Brien, C.; Li, L.; et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Su, J.; Satchell, S.C.; Shah, R.N.; Wertheim, J.A. Kidney decellularized extracellular matrix hydrogels: Rheological characterization and human glomerular endothelial cell response to encapsulation. J. Biomed. Mater. Res. Part A 2018, 106, 2448–2462. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jagannathan, N. Cytokeratin: A review on current concepts. Int. J. Orofac. Biol. 2018, 2, 6. [Google Scholar] [CrossRef]
- Haider, S.; Gamperl, M.; Burkard, T.R.; Kunihs, V.; Kaindl, U.; Junttila, S.; Fiala, C.; Schmidt, K.; Mendjan, S.; Knöfler, M.; et al. Estrogen Signaling Drives Ciliogenesis in Human Endometrial Organoids. Endocrinology 2019, 160, 2282–2297. [Google Scholar] [CrossRef]
- Cervelló, I.; Gil-Sanchis, C.; Mas, A.; Delgado-Rosas, F.; Martínez-Conejero, J.A.; Galán, A.; Martínez-Romero, A.; Martínez, S.; Navarro, I.; Ferro, J.; et al. Human Endometrial Side Population Cells Exhibit Genotypic, Phenotypic and Functional Features of Somatic Stem Cells. PLoS ONE 2010, 5, e10964. [Google Scholar] [CrossRef]
- Valentijn, A.J.; Palial, K.; Al-Lamee, H.; Tempest, N.; Drury, J.; Von Zglinicki, T.; Saretzki, G.; Murray, P.; Gargett, C.E.; Hapangama, D.K. SSEA-1 isolates human endometrial basal glandular epithelial cells: Phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum. Reprod. 2013, 28, 2695–2708. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.T.; Xiao, L.; Deane, J.A.; Tan, K.-S.; Cousins, F.L.; Masuda, H.; Sprung, C.N.; Rosamilia, A.; Gargett, C.E. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum. Reprod. 2017, 32, 2254–2268. [Google Scholar] [CrossRef]
- Giancotti, F.G. Integrin Signaling. Science 1999, 285, 1028–1033. [Google Scholar] [CrossRef]
- Tan, C.L.; Chin, T.; Tan, C.Y.R.; Rovito, H.A.; Quek, L.S.; Oblong, J.E.; Bellanger, S. Nicotinamide Metabolism Modulates the Proliferation/Differentiation Balance and Senescence of Human Primary Keratinocytes. J. Investig. Dermatol. 2019, 139, 1638–1647.e3. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology 2011, 141, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-Y.; Wang, J.-X.; Tong, X.-M.; Xu, W.-H.; Jiang, L.-Y.; Jing, X.-Y.; Yang, L.-Y.; Zhou, F.; Zhang, S.-Y. Three-dimensional cultures of human endometrial cells on Matrigel mimic in vivo morphology. Chin. Med. J. 2012, 125, 863–868. [Google Scholar]
- Dundon, M.; Madden, O.; Comizzoli, P. Three-dimensional culture of endometrial cells from domestic cats: A new in vitro platform for assessing plastic toxicity. PLoS ONE 2019, 14, e0217365. [Google Scholar] [CrossRef]
- Janzen, D.M.; Cheng, D.; Schafenacker, A.M.; Paik, D.Y.; Goldstein, A.; Witte, O.N.; Jaroszewicz, A.; Pellegrini, M.; Memarzadeh, S. Estrogen and progesterone together expand murine endometrial epithelial progenitor cells. Stem Cells 2013, 31, 808–822. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Výborný, K.; Vallová, J.; Kočí, Z.; Kekulová, K.; Jiráková, K.; Jendelová, P.; Hodan, J.; Kubinová, Š. Genipin and EDC crosslinking of extracellular matrix hydrogel derived from human umbilical cord for neural tissue repair. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Valdez, J.; Cook, C.D.; Ahrens, C.C.; Wang, A.J.; Brown, A.; Kumar, M.; Stockdale, L.; Rothenberg, D.; Renggli, K.; Gordon, E.; et al. On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial-stromal communication networks. Biomaterials 2017, 130, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Curley, C.J.; Dolan, E.B.; Otten, M.; Hinderer, S.; Duffy, G.P.; Murphy, B.P. An injectable alginate/extra cellular matrix (ECM) hydrogel towards acellular treatment of heart failure. Drug Deliv. Transl. Res. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Stzepourginski, I.; Nigro, G.; Jacob, J.-M.; Dulauroy, S.; Sansonetti, P.J.; Eberl, G.; Peduto, L. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc. Natl. Acad. Sci. USA 2017, 114, E506–E513. [Google Scholar] [CrossRef]
- Murphy, A.R.; Wiwatpanit, T.; Lu, Z.; Davaadelger, B.; Kim, J.J. Generation of Multicellular Human Primary Endometrial Organoids. J. Vis. Exp. 2019, e60384. [Google Scholar] [CrossRef]
- Wiwatpanit, T.; Murphy, A.R.; Lu, Z.; Urbanek, M.; Burdette, J.; Woodruff, T.K.; Kim, J.J. Scaffold-Free Endometrial Organoids Respond to Excess Androgens Associated With Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 769–780. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francés-Herrero, E.; Juárez-Barber, E.; Campo, H.; López-Martínez, S.; de Miguel-Gómez, L.; Faus, A.; Pellicer, A.; Ferrero, H.; Cervelló, I. Improved Models of Human Endometrial Organoids Based on Hydrogels from Decellularized Endometrium. J. Pers. Med. 2021, 11, 504. https://doi.org/10.3390/jpm11060504
Francés-Herrero E, Juárez-Barber E, Campo H, López-Martínez S, de Miguel-Gómez L, Faus A, Pellicer A, Ferrero H, Cervelló I. Improved Models of Human Endometrial Organoids Based on Hydrogels from Decellularized Endometrium. Journal of Personalized Medicine. 2021; 11(6):504. https://doi.org/10.3390/jpm11060504
Chicago/Turabian StyleFrancés-Herrero, Emilio, Elena Juárez-Barber, Hannes Campo, Sara López-Martínez, Lucía de Miguel-Gómez, Amparo Faus, Antonio Pellicer, Hortensia Ferrero, and Irene Cervelló. 2021. "Improved Models of Human Endometrial Organoids Based on Hydrogels from Decellularized Endometrium" Journal of Personalized Medicine 11, no. 6: 504. https://doi.org/10.3390/jpm11060504
APA StyleFrancés-Herrero, E., Juárez-Barber, E., Campo, H., López-Martínez, S., de Miguel-Gómez, L., Faus, A., Pellicer, A., Ferrero, H., & Cervelló, I. (2021). Improved Models of Human Endometrial Organoids Based on Hydrogels from Decellularized Endometrium. Journal of Personalized Medicine, 11(6), 504. https://doi.org/10.3390/jpm11060504





