Association of Matrix Metalloproteinases with Coronary Artery Calcification in Patients with CHD
Abstract
1. Introduction
2. Materials and Methods
- −
- Panel Milliplex Catalog ID. HMMP1MAG-55K-03, including the determination of matrix metalloproteinase 3 (MMP-3), matrix metalloproteinase 12 (MMP-12) and matrix metalloproteinase 13 (MMP-13);
- −
- Panel Milliplex Catalog ID. HMMP2MAG-55K-05, including the determination of matrix metalloproteinase 1 (MMP-1), matrix metalloproteinase 2 (MMP-2), matrix metalloproteinase 7 (MMP-7), matrix metalloproteinase 9 (MMP-9) and matrix metalloproteinase 10 (MMP-10).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Lee, J.S.; Basalyga, D.M.; Simionescu, A.; Isenburg, J.C.; Simionescu, D.T.; Vyavahare, N.R. Elastin calcification in the rat subdermal model is accompanied by up-regulation of degradative and osteogenic cellular responses. Am. J. Pathol. 2006, 168, 490–498. [Google Scholar] [CrossRef]
- Beaudeux, J.-L.; Giral, P.; Bruckert, E.; Foglietti, M.-J.; Chapman, M.J. Matrix metalloproteinases, inflammation and atherosclerosis: Therapeutic perspectives. Clin. Chem. Lab. Med. 2004, 42, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Daskalopoulou, S.S.; Perrea, D.; Liapis, C. Matrix Metalloproteinases and Diabetic Vascular Complications. Angiology 2005, 56, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Uzui, H.; Harpf, A.; Liu, M.; Doherty, T.M.; Shukla, A.; Chai, N.N.; Tripathi, P.V.; Jovinge, S.; Wilkin, D.J.; Asotra, K.; et al. Increased expression of membrane type 3-matrix metalloproteinase in human atherosclerotic plaque: Role of activated macrophages and inflammatory cytokines. Circulation 2002, 106, 3024–3030. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Matrix metalloproteinases: Influence on smooth muscle cells and atherosclerotic plaque stability. Expert Rev. Cardiovasc. Ther. 2007, 5, 265–282. [Google Scholar] [CrossRef]
- Hopps, E.; Caimi, G. Matrix metalloproteases as a pharmacological target in cardiovascular diseases. Eur. Rev. Med. Pharm. Sci. 2015, 19, 2583–2589. [Google Scholar]
- Cuvelliez, M.; Vandewalle, V.; Brunin, M.; Beseme, O.; Hulot, A.; De Groote, P.; Amouyel, P.; Bauters, C.; Marot, G.; Pinet, F. Circulating proteomic signature of early death in heart failure patients with reduced ejection fraction. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Vacek, T.; Rehman, S.; Yu, S.; Neamtu, D.; Givimani, S.; Tyagi, S. Matrix metalloproteinases in atherosclerosis: Role of nitric oxide, hydrogen sulfide, homocysteine, and polymorphisms. Vasc. Health Risk Manag. 2015, 11, 173–183. [Google Scholar] [CrossRef]
- Gaubatz, J.W.; Ballantyne, C.M.; Wasserman, B.A.; He, M.; Chambless, L.E.; Boerwinkle, E.; Hoogeveen, R.C. Association of Circulating Matrix Metalloproteinases With Carotid Artery Characteristics: The Atherosclerosis Risk in Communities Carotid MRI Study. Arter. Thromb. Vasc. Biol. 2010, 30, 1034–1042. [Google Scholar] [CrossRef]
- Sasaki, T.; Nakamura, K.; Sasada, K.; Okada, S.; Cheng, X.W.; Suzuki, T.; Murohara, T.; Sato, K.; Kuzuya, M. Matrix metalloproteinase-2 deficiency impairs aortic atherosclerotic calcification in ApoE-deficient mice. Atherosclerosis 2013, 227, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-G.; Meng, F.-X.; Li, B.-W.; Sheng, Y.-M.; Liu, M.-M.; Wang, B.; Li, H.-W.; Xiu, R.-J. Gelatinases promote calcification of vascular smooth muscle cells by up-regulating bone morphogenetic protein-2. Biochem. Biophys. Res. Commun. 2016, 470, 287–293. [Google Scholar] [CrossRef]
- Kondapalli, M.S.; Galimudi, R.K.; Gundapaneni, K.K.; Padala, C.; Cingeetham, A.; Gantala, S.; Ali, A.; Shyamala, N.; Sahu, S.K.; Nallari, P.; et al. MMP 1 circulating levels and promoter polymorphism in risk prediction of coronary artery disease in asymptomatic first degree relatives. Gene 2016, 595, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Greif, M.; Broedl, U.C.; Lebherz, C.; Laubender, R.P.; Becker, A.; Von Ziegler, F.; Tittus, J.; Reiser, M.; Becker, C.; et al. MMP-1 serum levels predict coronary atherosclerosis in humans. Cardiovasc. Diabetol. 2009, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, E.; Marmur, J.D.; Hegde, S.; Yanamadala, S.; Batuman, O.A.; Chopra, V.; Ay, G.; Eng, C. Relation of baseline plasma MMP-1 levels to long-term all-cause mortality in patients with known or suspected coronary artery disease referred for coronary angiography. Atherosclerosis 2015, 239, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Aloui, S.; Zidi, W.; Ouali, S.; Guizani, I.; Hadj-Taieb, S.; Mourali, M.S.; Feki, M.; Allal-Elasmi, M. Association of matrix metalloproteinase 3 and endogenous inhibitors with inflammatory markers in mitral valve disease and calcification. Mol. Biol. Rep. 2018, 45, 2135–2143. [Google Scholar] [CrossRef]
- Fedarko, N.S.; Jain, A.; Karadag, A.; Fisher, L.W. Three small integrin-binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB J. 2004, 18, 734–736. [Google Scholar] [CrossRef]
- Goncalves, I.; Bengtsson, E.; Colhoun, H.M.; Shore, A.C.; Palombo, C.; Natali, A.; Edsfeldt, A.; Dunér, P.; Fredrikson, G.N.; Björkbacka, H.; et al. Elevated Plasma Levels of MMP-12 Are Associated With Atherosclerotic Burden and Symptomatic Cardiovascular Disease in Subjects With Type 2 Diabetes. Arter. Thromb. Vasc. Biol. 2015, 35, 1723–1731. [Google Scholar] [CrossRef]
- Abbas, A.; Aukrust, P.; Russell, D.; Krohg-Sørensen, K.; Almås, T.; Bundgaard, D.; Bjerkeli, V.; Sagen, E.L.; Michelsen, A.E.; Dahl, T.B.; et al. Matrix Metalloproteinase 7 Is Associated with Symptomatic Lesions and Adverse Events in Patients with Carotid Atherosclerosis. PLoS ONE 2014, 9, e84935. [Google Scholar] [CrossRef]
- Ben Braiek, A.; Chahed, H.; Dumont, F.; Abdelhak, F.; Hichem, D.; Gamra, H.; Baudin, B. Identification of biomarker panels as predictors of severity in coronary artery disease. J. Cell. Mol. Med. 2021, 25, 1518–1530. [Google Scholar] [CrossRef]
- Wu, H.-D.; Bai, X.; Chen, D.-M.; Cao, H.-Y.; Qin, L. Association of Genetic Polymorphisms in Matrix Metalloproteinase-9 and Coronary Artery Disease in the Chinese Han Population: A Case–Control Study. Genet. Test. Mol. Biomarkers 2013, 17, 707–712. [Google Scholar] [CrossRef]
- Moradi, N.; Fadaei, R.; Ahmadi, R.; Mohammad, M.H.; Shahmohamadnejad, S.; Tavakoli-Yaraki, M.; Aghajani, H.; Fallah, S. Role of serum MMP-9 levels and vitamin D receptor polymorphisms in the susceptibility to coronary artery disease: An association study in Iranian population. Gene 2017, 628, 295–300. [Google Scholar] [CrossRef]
- Chen, Y.; Waqar, A.B.; Nishijima, K.; Ning, B.; Kitajima, S.; Matsuhisa, F.; Chen, L.; Liu, E.; Koike, T.; Yu, Y.; et al. Macrophage-derived MMP-9 enhances the progression of atherosclerotic lesions and vascular calcification in transgenic rabbits. J. Cell. Mol. Med. 2020, 24, 4261–4274. [Google Scholar] [CrossRef] [PubMed]
- Hecht, E.; Freise, C.; Websky, K.V.; Nasser, H.; Kretzschmar, N.; Stawowy, P.; Hocher, B.; Querfeld, U. The matrix metalloproteinases 2 and 9 initiate uraemic vascular calcifications. Nephrol. Dial. Transplant. 2016, 31, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Freise, C.; Kretzschmar, N.; Querfeld, U. Wnt signaling contributes to vascular calcification by induction of matrix metalloproteinases. BMC Cardiovasc. Disord. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, E.; Yu, Y.; Kitajima, S.; Koike, T.; Jin, Y.; Morimoto, M.; Hatakeyama, K.; Asada, Y.; Watanabe, T.; et al. Macrophage Metalloelastase Accelerates the Progression of Atherosclerosis in Transgenic Rabbits. Circulation 2006, 113, 1993–2001. [Google Scholar] [CrossRef] [PubMed]

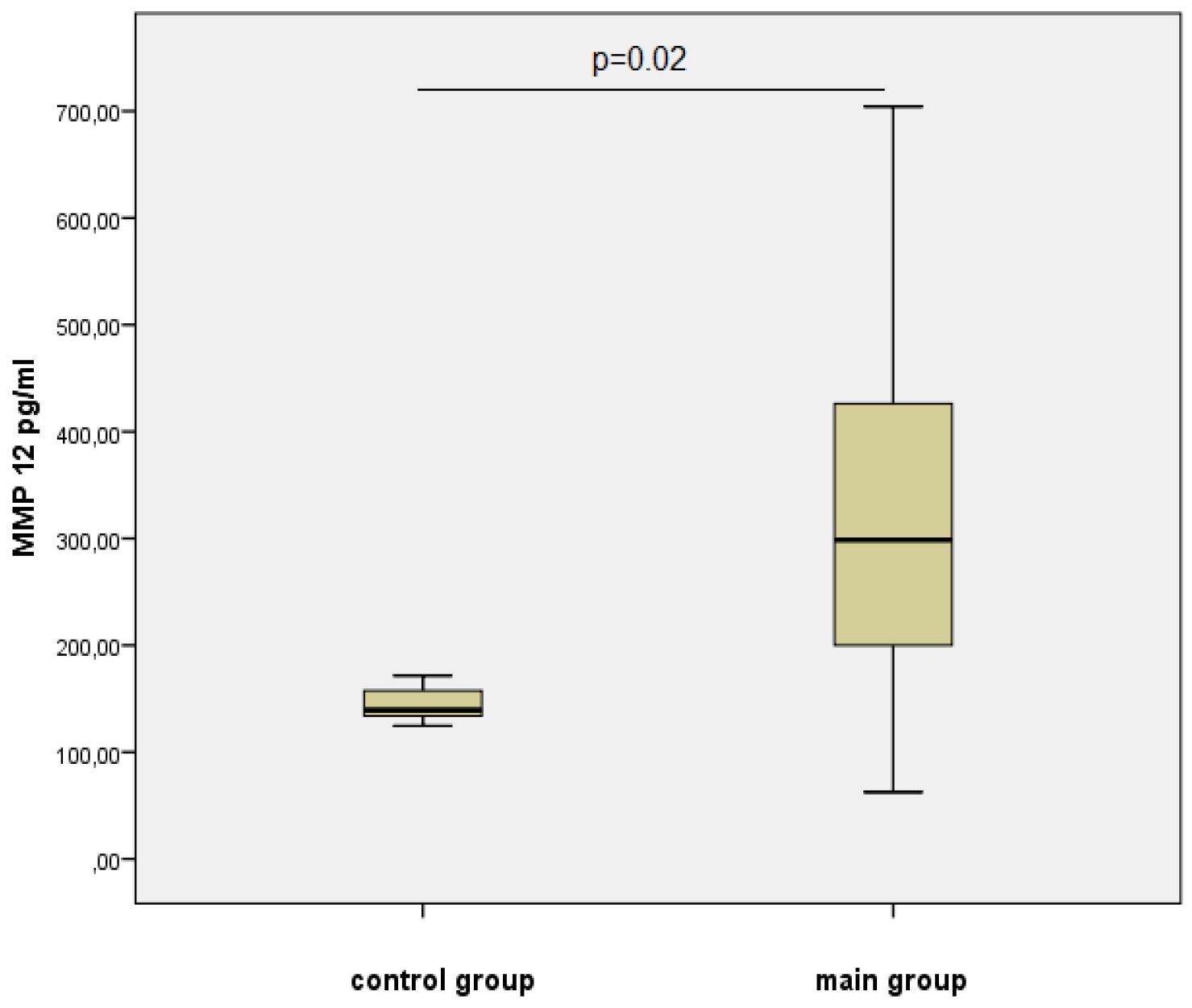
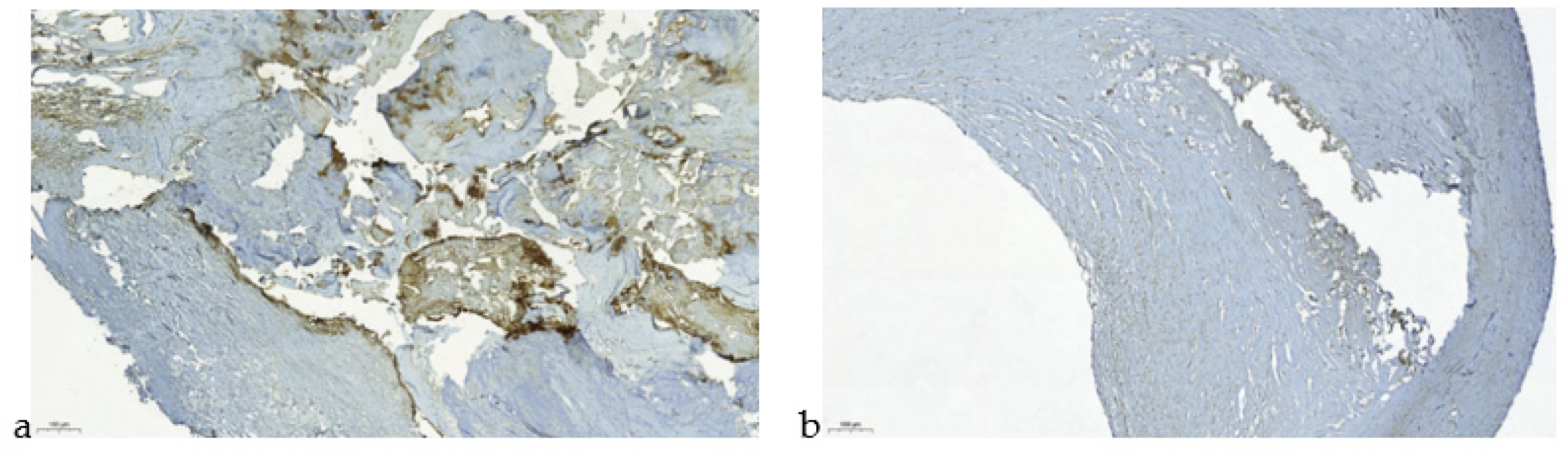
| Parameters | Meaning |
|---|---|
| Clinical and anamnestic characteristics | |
| Age, years (M ± SD) | 60.4 ± 6.3 |
| Body mass index, kg/m2 (M ± SD) | 29.3 ± 4.7 |
| Systolic pressure, mmHg (M ± SD) | 137.7 ± 12.8 |
| Diastolic pressure, mmHg (M ± SD) | 84.9 ± 7.3 |
| Heart rate, beats per minute (M ± SD) | 69.7 ± 6.81 |
| The history of heart attack (absolute in %) | 69.2 |
| The history of type II diabetes (absolute in %) | 11.5 |
| The family history of CHD (absolute in %) | 41.3 |
| Smoking (absolute in %) | 15.4 |
| Angina pectoris | |
| Functional Class I | 0% |
| Functional Class II | 10.3% |
| Functional Class III | 83.3% |
| Functional Class IV | 6.4% |
| Multivessel atherosclerotic lesion | |
| of coronary arteries (more than two vessels) | 92.3% |
| Biochemical parameters, Me (25%; 75%) | |
| Calcitonin, (pg/mL) | 1.86 (0.02; 2.98) |
| Osteoprotegerin, (pg/mL) | 52.99 (35.43; 79.95) |
| Osteopontin, (ng/mL) | 27.05 (17.62; 39.61) |
| Osteocalcin, (ng/mL) | 13.26 (8.46; 16.63) |
| Osteonectin, (µg/mL) | 8.96 (7.74; 10.72) |
| Ca (mol/L) | 2.3 (2.22; 2.43) |
| Parameters | Control n = 36 | Group with CHD n = 78 | p |
|---|---|---|---|
| MMP-2 (ng/mL) | 111.68 (89.14; 126.89) | 104.28 (78.71; 120.04) | 0.422 |
| MMP-3 (ng/mL) | 47.1 (28.4; 66.5) | 33.44 (21.11; 63.65) | 0.311 |
| MMP-9 (ng/mL) | 228.0 (130.18; 354.96) | 276.01 (151.31; 327.58) | 0.342 |
| MMP-10 (ng/mL) | 0.62 (0.52; 0.82) | 0.68 (0.51; 0.82) | 0.785 |
| MMP-13 (pg/mL) | 30.35 (17.56; 71.9) | 36.37 (17.28; 58.16) | 0.739 |
| Parameters | MMP-1 (ng/mL) | MMP-7 (ng/mL) | MMP-12 (pg/mL) | ||||
|---|---|---|---|---|---|---|---|
| p | p | p | |||||
| BMI | <25 | 7.96 (4.61; 15.78) | 0.97 | 9.36 (8.25; 12.31) | 0.65 | 218.7 (116.9; 338.5) | 0.10 |
| >25 | 7.74 (5.49; 11.84) | 11.07 (9.36; 15.86) | 233.6 (149.6; 382.8) | ||||
| Smoking | no | 9.22 (6.18; 18.37) | 0.52 | 11.48 (9.36;15.09) | 0.08 | 299.5 (208.6; 513.6) | 0.67 |
| yes | 8.37 (2.11; 20.29) | 15.09 (8.92; 17.36) | 200.8 (101.2; 354.5) | ||||
| Family history of CHD | no | 10.71 (7.64; 22.78) | 0.04 | 12.72 (9.36; 16.05) | 0.53 | 298.7 (205.3; 554.9) | 0.19 |
| yes | 7.41 (5.36; 13.26) | 10.22 (9.36; 15.09) | 301.4 (174.0; 393.2) | ||||
| Parameters | With Calcification n = 103 | Without Calcification n = 53 | p |
|---|---|---|---|
| MMP-9 (ng/mg of protein) | 3.61 (1.62; 5.08) | 2.24 (1.22; 4.16) | 0.017 |
| MMP-3 (ng/mg of protein) | 2.05 (1.52; 3.3) | 2.00 (1.24; 4.38) | 0.881 |
| MMP-7 (ng/mg of protein) | 0.78 (0.27; 2.41) | 0.62 (0.29; 1.56) | 0.6 |
| MMP-1 (ng/mg of protein) | 71.37 (21.09;154.99) | 49.21 (6.05;299.3) | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polonskaya, Y.V.; Kashtanova, E.V.; Murashov, I.S.; Striukova, E.V.; Kurguzov, A.V.; Stakhneva, E.M.; Shramko, V.S.; Maslatsov, N.A.; Chernyavsky, A.M.; Ragino, Y.I. Association of Matrix Metalloproteinases with Coronary Artery Calcification in Patients with CHD. J. Pers. Med. 2021, 11, 506. https://doi.org/10.3390/jpm11060506
Polonskaya YV, Kashtanova EV, Murashov IS, Striukova EV, Kurguzov AV, Stakhneva EM, Shramko VS, Maslatsov NA, Chernyavsky AM, Ragino YI. Association of Matrix Metalloproteinases with Coronary Artery Calcification in Patients with CHD. Journal of Personalized Medicine. 2021; 11(6):506. https://doi.org/10.3390/jpm11060506
Chicago/Turabian StylePolonskaya, Yana V., Elena V. Kashtanova, Ivan S. Murashov, Evgenia V. Striukova, Alexey V. Kurguzov, Ekaterina M. Stakhneva, Viktoria S. Shramko, Nikolay A. Maslatsov, Aleksandr M. Chernyavsky, and Yulia I. Ragino. 2021. "Association of Matrix Metalloproteinases with Coronary Artery Calcification in Patients with CHD" Journal of Personalized Medicine 11, no. 6: 506. https://doi.org/10.3390/jpm11060506
APA StylePolonskaya, Y. V., Kashtanova, E. V., Murashov, I. S., Striukova, E. V., Kurguzov, A. V., Stakhneva, E. M., Shramko, V. S., Maslatsov, N. A., Chernyavsky, A. M., & Ragino, Y. I. (2021). Association of Matrix Metalloproteinases with Coronary Artery Calcification in Patients with CHD. Journal of Personalized Medicine, 11(6), 506. https://doi.org/10.3390/jpm11060506





.JPG)


