Similar Features, Different Behaviors: A Comparative In Vitro Study of the Adipogenic Potential of Stem Cells from Human Follicle, Dental Pulp, and Periodontal Ligament
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Cell Culture
2.2. Cell Growth and Viability Assay
2.3. Immunocytochemical Analysis
2.4. In Vitro Differentiation Assays
2.5. Reverse Transcription PCR (RT-PCR) and Quantitative PCR
2.6. Statistical Analysis
3. Results
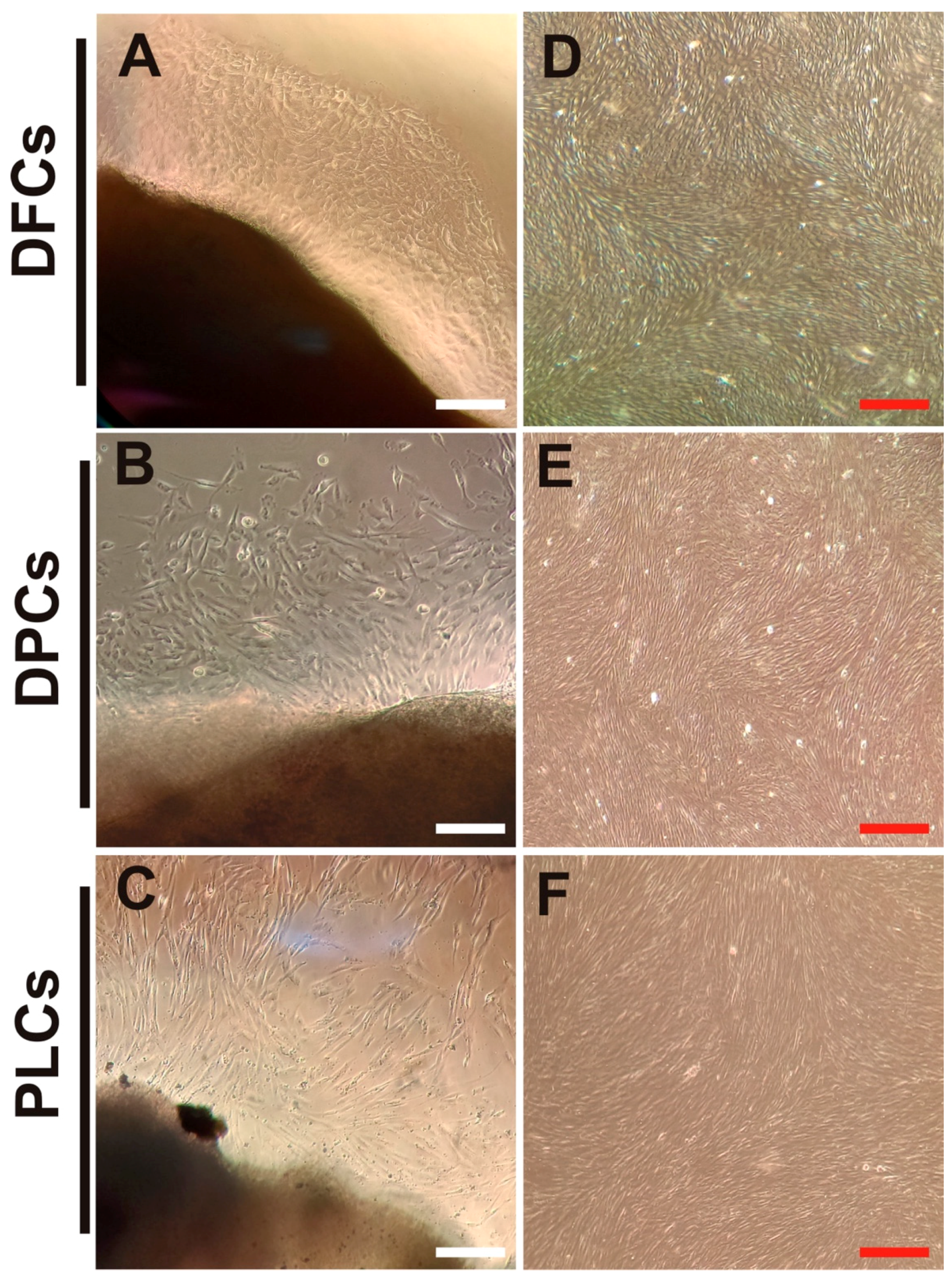
3.1. Isolation and Biological Features of DT-MSCs
3.2. Expression of Embryonic and Stem Cell Markers in Dental Stem Cells
3.3. Osteogenic Differentiation Assay
3.4. Effects of Adipogenic Induction Media on Dental Pulp Stem Cells
3.5. Adipogenic Differentiation of DFSCs, DPSCs, and PLSCs
3.6. Expression Patterns of Adipogenic Markers in Adipocytes Derived from Dental Stem Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASCs | adipose stem cells |
| AIM | adipogenic induction media |
| α-MEM | alpha-modified Eagle’s medium |
| BMSCs | bone marrow mesenchymal stem cells |
| BSA | bovine serum albumin |
| CFUs | colony-forming units |
| DAI | days after induction |
| DAPI | 4,6-diamidino-2-phenylindole |
| DEXA | Dexamethasone |
| DF | dental follicle |
| DFSCs | dental follicle stem cells |
| DP | dental pulp |
| DPSCs | dental pulp stem cells |
| DT-MSCs | dental tissue-derived mesenchymal stem cells |
| DMEM/F12 | Dulbecco’s modified Eagle’s medium |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GMSCs | gingival-derived mesenchymal stem cells |
| IBMX | isobutyl-methyl-xanthine |
| INDO | indomethacin |
| INS | insulin |
| LDs | lipid droplets |
| MPCs | human mesenchymal progenitor cells |
| MSCs | human mesenchymal stem cells |
| MSX2 | MSH-like homeobox |
| OROS | Oil Red O staining |
| PBS | phosphate buffer saline |
| PFA | paraformaldehyde |
| PL | periodontal ligament |
| PLSCs | periodontal ligament stem cells |
| PTEN | phosphatase and tensin homolog |
| RT | room temperature |
| SHED | human exfoliated deciduous teeth |
| TGPCs | tooth germ progenitor cells |
| UC-MSCs | umbilical cord MSCs |
| WNT10B | wingless-type member 10 |
References
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Liu, H.; Chen, M.; Ren, S.; Cheng, P.; Zhang, H. Mir-301b~miR-130b-PPARgamma axis underlies the adipogenic capacity of mesenchymal stem cells with different tissue origins. Sci. Rep. 2017, 7, 1160. [Google Scholar] [CrossRef] [Green Version]
- Tatebayashi, K.; Tanaka, Y.; Nakano-Doi, A.; Sakuma, R.; Kamachi, S.; Shirakawa, M.; Uchida, K.; Kageyama, H.; Takagi, T.; Yoshimura, S.; et al. Identification of Multipotent Stem Cells in Human Brain Tissue Following Stroke. Stem Cells Dev. 2017, 26, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Rodas-Junco, B.A.; Canul-Chan, M.; Herrera, R.A.R.; De-La-Peña, C.; Nic-Can, G.I. Stem Cells from Dental Pulp: What Epigenetics Can Do with Your Tooth. Front. Physiol. 2017, 8, 999. [Google Scholar] [CrossRef] [Green Version]
- Ferro, F.; Spelat, R.; Beltrami, A.P.; Cesselli, D.; Curcio, F. Isolation and Characterization of Human Dental Pulp Derived Stem Cells by Using Media Containing Low Human Serum Percentage as Clinical Grade Substitutes for Bovine Serum. PLoS ONE 2012, 7, e48945. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahfeldt, T.; Schinzel, R.T.; Lee, Y.-K.; Hendrickson, D.; Kaplan, A.; Lum, D.H.; Camahort, R.; Xia, F.; Shay, J.; Rhee, E.P.; et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat. Cell Biol. 2012, 14, 209–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, A.; Haakonsson, A.K.; Madsen, J.G.S.; Larsen, M.; Forss, I.; Madsen, M.R.; Van Hauwaert, E.L.; Wiwie, C.; Jespersen, N.Z.; Tencerova, M.; et al. Osteogenesis depends on commissioning of a network of stem cell transcription factors that act as repressors of adipogenesis. Nat. Genet. 2019, 51, 716–727. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Shook, B.; Gonzalez, G.C.R.; Ebmeier, S.; Grisotti, G.; Zwick, R.; Horsley, V. The Role of Adipocytes in Tissue Regeneration and Stem Cell Niches. Annu. Rev. Cell Dev. Biol. 2016, 32, 609–631. [Google Scholar] [CrossRef] [Green Version]
- Lemos, D.R.; Paylor, B.; Chang, C.; Sampaio, A.; Underhill, T.M.; Rossi, F.M.V. Functionally Convergent White Adipogenic Progenitors of Different Lineages Participate in a Diffused System Supporting Tissue Regeneration. Stem Cells 2012, 30, 1152–1162. [Google Scholar] [CrossRef]
- Xiao, L.; Tsutsui, T. Human dental mesenchymal stem cells and neural regeneration. Hum. Cell 2013, 26, 91–96. [Google Scholar] [CrossRef]
- Yu, T.; Volponi, A.A.; Babb, R.; An, Z.; Sharpe, P.T. Stem Cells in Tooth Development, Growth, Repair, and Regeneration. Curr. Top. Dev. Biol. 2015, 115, 187–212. [Google Scholar] [CrossRef]
- Di Vito, A.; Giudice, A.; Chiarella, E.; Malara, N.; Bennardo, F.; Fortunato, L. In Vitro Long-Term Expansion and High Osteogenic Potential of Periodontal Ligament Stem Cells: More Than a Mirage. Cell Transplant. 2018, 28, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Pisciotta, A.; Riccio, M.; Carnevale, G.; Beretti, F.; Gibellini, L.; Maraldi, T.; Cavallini, G.M.; Ferrari, A.; Bruzzesi, G.; De Pol, A. Human Serum Promotes Osteogenic Differentiation of Human Dental Pulp Stem Cells In Vitro and In Vivo. PLoS ONE 2012, 7, e50542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarle, S.; Shi, S.; Kaigler, D. Development of a serum-free system to expand dental-derived stem cells: PDLSCs and SHEDs. J. Cell. Physiol. 2010, 226, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Ma, L.; Makino, Y.; Yamaza, H.; Akiyama, K.; Hoshino, Y.; Song, G.; Kukita, T.; Nonaka, K.; Shi, S.; Yamaza, T. Cryopreserved Dental Pulp Tissues of Exfoliated Deciduous Teeth Is a Feasible Stem Cell Resource for Regenerative Medicine. PLoS ONE 2012, 7, e51777. [Google Scholar] [CrossRef] [Green Version]
- Gazarian, K.G.; Ramírez-García, L.R. Human Deciduous Teeth Stem Cells (SHED) Display Neural Crest Signature Characters. PLoS ONE 2017, 12, e0170321. [Google Scholar] [CrossRef]
- Gopinathan, G.; Kolokythas, A.; Luan, X.; Diekwisch, T.G. Epigenetic Marks Define the Lineage and Differentiation Potential of Two Distinct Neural Crest-Derived Intermediate Odontogenic Progenitor Populations. Stem Cells Dev. 2013, 22, 1763–1778. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Monterubbianesi, R.; Bencun, M.; Pagella, P.; Woloszyk, A.; Orsini, G.; Mitsiadis, T.A. A comparative in vitro study of the osteogenic and adipogenic potential of human dental pulp stem cells, gingival fibroblasts and foreskin fibroblasts. Sci. Rep. 2019, 9, 1761. [Google Scholar] [CrossRef]
- Fracaro, L.; Senegaglia, A.C.; Herai, R.H.; Leitolis, A.; Boldrini-Leite, L.M.; Rebelatto, C.L.K.; Travers, P.J.; Brofman, P.R.S.; Correa, A. The Expression Profile of Dental Pulp-Derived Stromal Cells Supports Their Limited Capacity to Differentiate into Adipogenic Cells. Int. J. Mol. Sci. 2020, 21, 2753. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Li, J.; Qiao, X.; Yu, M.; Tang, W.; Wang, H.; Guo, W.; Tian, W. Comparison of Odontogenic Differentiation of Human Dental Follicle Cells and Human Dental Papilla Cells. PLoS ONE 2013, 8, e62332. [Google Scholar] [CrossRef] [Green Version]
- Styner, M.; Sen, B.; Xie, Z.; Case, N.; Rubin, J. Indomethacin promotes adipogenesis of mesenchymal stem cells through a cyclooxygenase independent mechanism. J. Cell. Biochem. 2010, 111, 1042–1050. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W. Current Methods of Adipogenic Differentiation of Mesenchymal Stem Cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From Stem Cell to Adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef] [Green Version]
- Yalvac, M.; Ramazanoglu, M.; Tekguc, M.; Bayrak, O.F.; Shafigullina, A.K.; Salafutdinov, I.; Blatt, N.; Kiyasov, A.P.; Sahin, F.; Palotas, A.; et al. Human Tooth Germ Stem Cells Preserve Neuro-Protective Effects after Long-Term Cryo-Preservation. Curr. Neurovascular Res. 2010, 7, 49–58. [Google Scholar] [CrossRef]
- Wang, X.; You, L.; Cui, X.; Li, Y.; Wang, X.; Xu, P.; Zhu, L.; Wen, J.; Pang, L.; Guo, X.; et al. Evaluation and optimization of differentiation conditions for human primary brown adipocytes. Sci. Rep. 2018, 8, 5304. [Google Scholar] [CrossRef] [Green Version]
- Yalvac, M.; Ramazanoglu, M.; Rizvanov, A.; Sahin, F.; Bayrak, O.F.; Salli, U.; Palotás, A.; Köse, G.T. Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: Implications in neo-vascularization, osteo-, adipo- and neurogenesis. Pharmacogenomics J. 2009, 10, 105–113. [Google Scholar] [CrossRef]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.K.; Itte, V.N.; Kingham, P.J.; Novikov, L.; Wiberg, M.; Kelk, P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci. Rep. 2017, 7, 12605. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, F.; Sun, Y.; Jiang, B.; Zhang, W.; Yang, J.; Xu, G.-T.; Liang, A.; Liu, S. Concise Reviews: Characteristics and Potential Applications of Human Dental Tissue-Derived Mesenchymal Stem Cells. Stem Cells 2014, 33, 627–638. [Google Scholar] [CrossRef]
- Eslaminejad, B.; Vahabi, S.; Shariati, M.; Nazarian, H. In vitro Growth and Characterization of Stem Cells from Human Dental Pulp of Deciduous Versus Permanent Teeth. J. Dent. (Tehran Iran) 2010, 7, 185–195. [Google Scholar]
- Huang, C.-E.; Hu, F.-W.; Yu, C.-H.; Tsai, L.-L.; Lee, T.-H.; Chou, M.-Y.; Yu, C.-C. Concurrent Expression of Oct4 and Nanog Maintains Mesenchymal Stem-Like Property of Human Dental Pulp Cells. Int. J. Mol. Sci. 2014, 15, 18623–18639. [Google Scholar] [CrossRef] [Green Version]
- Ferro, F.; Spelat, R.; D′Aurizio, F.; Puppato, E.; Pandolfi, M.; Beltrami, A.P.; Cesselli, D.; Falini, G.; Beltrami, C.A.; Curcio, F. Dental Pulp Stem Cells Differentiation Reveals New Insights in Oct4A Dynamics. PLoS ONE 2012, 7, e41774. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.L.; Holanda-Afonso, R.C.; Moura-Neto, V.; Bolognese, A.M.; DosSantos, M.; Souza, M.M. Human dental follicle cells express embryonic, mesenchymal and neural stem cells markers. Arch. Oral Biol. 2017, 73, 121–128. [Google Scholar] [CrossRef]
- Teti, G.; Salvatore, V.; Focaroli, S.; Durante, S.; Mazzotti, A.; Dicarlo, M.; Mattioli-Belmonte, M.; Orsini, G. In vitro osteogenic and odontogenic differentiation of human dental pulp stem cells seeded on carboxymethyl cellulose-hydroxyapatite hybrid hydrogel. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Chosa, N.; Hasegawa, T.; Nishihira, S.; Okubo, N.; Takahashi, M.; Sugiyama, Y.; Tanaka, M.; Ishisaki, A. Dental pulp cells derived from permanent teeth express higher levels of R-cadherin than do deciduous teeth: Implications of the correlation between R-cadherin expression and restriction of multipotency in mesenchymal stem cells. Arch. Oral Biol. 2012, 57, 44–51. [Google Scholar] [CrossRef]
- Karaöz, E.; Doğan, B.N.; Aksoy, A.; Gacar, G.; Akyüz, S.; Ayhan, S.; Genç, Z.S.; Yürüker, S.; Duruksu, G.; Demircan, P.; et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem. Cell Biol. 2009, 133, 95–112. [Google Scholar] [CrossRef]
- Wang, X.; Sha, X.-J.; Li, G.-H.; Yang, F.-S.; Ji, K.; Wen, L.-Y.; Liu, S.-Y.; Chen, L.; Ding, Y.; Xuan, K. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch. Oral Biol. 2012, 57, 1231–1240. [Google Scholar] [CrossRef]
- Ponnaiyan, D.; Jegadeesan, V. Comparison of phenotype and differentiation marker gene expression profiles in human dental pulp and bone marrow mesenchymal stem cells. Eur. J. Dent. 2014, 8, 307–313. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, H.; Liu, X.; Sheng, Y.; Liu, X.; Jiang, C. Dental Follicle Stem Cells: Tissue Engineering and Immunomodulation. Stem Cells Dev. 2019, 28, 986–994. [Google Scholar] [CrossRef]
- Winning, L.; El Karim, I.; Lundy, F.T. A Comparative Analysis of the Osteogenic Potential of Dental Mesenchymal Stem Cells. Stem Cells Dev. 2019, 28, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L. Dental pulp stem cells: Function, isolation and applications in regenerative medicine. J. Tissue Eng. Regen. Med. 2014, 9, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Dangaria, S.J.; Ito, Y.; Luan, X.; Diekwisch, T.G. Differentiation of Neural-Crest-Derived Intermediate Pluripotent Progenitors into Committed Periodontal Populations Involves Unique Molecular Signature Changes, Cohort Shifts, and Epigenetic Modifications. Stem Cells Dev. 2011, 20, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Hilkens, P.; Gervois, P.; Fanton, Y.; Vanormelingen, J.; Martens, W.; Struys, T.; Politis, C.; Lambrichts, I.; Bronckaers, A. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013, 353, 65–78. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [Green Version]
- Sokolik, C.; Liu, Y.; Bauer, D.; McPherson, J.; Broeker, M.; Heimberg, G.; Qi, L.S.; Sivak, D.; Thomson, M. Transcription Factor Competition Allows Embryonic Stem Cells to Distinguish Authentic Signals from Noise. Cell Syst. 2015, 1, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Karahuseyinoglu, S.; Kocaefe, C.; Balci, D.; Erdemli, E.; Can, A. Functional Structure of Adipocytes Differentiated from Human Umbilical Cord Stroma-Derived Stem Cells. Stem Cells 2008, 26, 682–691. [Google Scholar] [CrossRef]
- Akpınar, G.; Kasap, M.; Aksoy, A.; Duruksu, G.; Gacar, G.; Karaoz, E. Phenotypic and Proteomic Characteristics of Human Dental Pulp Derived Mesenchymal Stem Cells from a Natal, an Exfoliated Deciduous, and an Impacted Third Molar Tooth. Stem Cells Int. 2014, 2014, 1–19. [Google Scholar] [CrossRef]
- Lei, M.; Li, K.; Li, B.; Gao, L.-N.; Chen, F.-M.; Jin, Y. Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials 2014, 35, 6332–6343. [Google Scholar] [CrossRef]
- Kang, R.; Zhou, Y.; Tan, S.; Zhou, G.; Aagaard, L.; Xie, L.; Bünger, C.; Bolund, L.; Luo, Y. Mesenchymal stem cells derived from human induced pluripotent stem cells retain adequate osteogenicity and chondrogenicity but less adipogenicity. Stem Cell Res. Ther. 2015, 6, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafner, A.-L.; Contet, J.; Ravaud, C.; Yao, X.; Villageois, P.; Suknuntha, K.; Annab, K.; Peraldi, P.; Binetruy, B.; Slukvin, I.I.; et al. Brown-like adipose progenitors derived from human induced pluripotent stem cells: Identification of critical pathways governing their adipogenic capacity. Sci. Rep. 2016, 6, 32490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siersbaek, R.; Nielsen, R.; John, S.; Sung, M.-H.; Baek, S.; Loft, A.; Hager, G.L.; Mandrup, S. Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. EMBO J. 2011, 30, 1459–1472. [Google Scholar] [CrossRef] [Green Version]
- Boquest, A.C.; Noer, A.; Sørensen, A.L.; Vekterud, K.; Collas, P. CpG Methylation Profiles of Endothelial Cell-Specific Gene Promoter Regions in Adipose Tissue Stem Cells Suggest Limited Differentiation Potential Toward the Endothelial Cell Lineage. Stem Cells 2007, 25, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, S.; Lee, J.E.; Baldridge, A.; Grullon, S.; Peng, W.; Ge, K. Histone H3K9 methyltransferase G9a represses PPARgamma expression and adipogenesis. EMBO J. 2013, 32, 45–59. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K. Adipocytes. Curr. Biol. 2014, 24, R988–R993. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.-C.; Lai, Y.-C.; Li, L.-H.; Liao, K.; Lai, H.-C.; Kao, S.-Y.; Wang, J.; Chuong, C.-M.; Hung, S.-C. Methylation and PTEN activation in dental pulp mesenchymal stem cells promotes osteogenesis and reduces oncogenesis. Nat. Commun. 2019, 10, 2226. [Google Scholar] [CrossRef]
- Khanna-Jain, R.; Vanhatupa, S.; Vuorinen, A.; Sandor, G.K.B.; Suuronen, R.; Mannerstrom, B.; Miettinen, S. Growth and Differentiation of Human Dental Pulp Stem Cells Maintained in Fetal Bovine Serum, Human Serum and Serum-free/Xeno-free Culture Media. J. Stem Cell Res. Ther. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Han, S.-M.; Han, S.-H.; Coh, Y.-R.; Jang, G.; Ra, J.C.; Kang, S.-K.; Lee, H.-W.; Youn, H.-Y. Enhanced proliferation and differentiation of Oct4- and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp. Mol. Med. 2014, 46, e101. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Seo, K.W.; So, A.Y.; Seo, M.S.; Yu, K.R.; Kang, S.K.; Kang, K.S. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ. 2011, 19, 534–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Differentiation Cocktail | Treatments | ||||||
|---|---|---|---|---|---|---|---|
| Basic Conditions | Culture Medium | α-MEM | α-MEM | α-MEM | DMEM/F12 | DMEM/F12 | α-MEM or DMEM/F12 |
| FBS (v/v) | 10% | 10% | 10% | 10% | 10% | 10% | |
| Basic chemical factors | INS | 10 μM | 1 μM | 1.7 μM | 1.7 μM | 1.7 μM | - |
| IBMX | 500 μM | 500 μM | 500 μM | 500 μM | 500 μM | - | |
| DEXA | 1 μM | 10 μM | 1 μM | 1 μM | 1 μM | - | |
| INDO | 200 μM | 200 μM | 60 μM | 60 μM | 100 μM | - | |
| Induction time (days) | 21 | 21 | 21 | 21 | 21 | 21 | |
| Adipogenic induction medium (AIM) | AIM-1 | AIM-2 | AIM-3 | AIM-4 | AIM-5 | Control | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercado-Rubio, M.D.; Pérez-Argueta, E.; Zepeda-Pedreguera, A.; Aguilar-Ayala, F.J.; Peñaloza-Cuevas, R.; Kú-González, A.; Rojas-Herrera, R.A.; Rodas-Junco, B.A.; Nic-Can, G.I. Similar Features, Different Behaviors: A Comparative In Vitro Study of the Adipogenic Potential of Stem Cells from Human Follicle, Dental Pulp, and Periodontal Ligament. J. Pers. Med. 2021, 11, 738. https://doi.org/10.3390/jpm11080738
Mercado-Rubio MD, Pérez-Argueta E, Zepeda-Pedreguera A, Aguilar-Ayala FJ, Peñaloza-Cuevas R, Kú-González A, Rojas-Herrera RA, Rodas-Junco BA, Nic-Can GI. Similar Features, Different Behaviors: A Comparative In Vitro Study of the Adipogenic Potential of Stem Cells from Human Follicle, Dental Pulp, and Periodontal Ligament. Journal of Personalized Medicine. 2021; 11(8):738. https://doi.org/10.3390/jpm11080738
Chicago/Turabian StyleMercado-Rubio, Melissa D., Erick Pérez-Argueta, Alejandro Zepeda-Pedreguera, Fernando J. Aguilar-Ayala, Ricardo Peñaloza-Cuevas, Angela Kú-González, Rafael A. Rojas-Herrera, Beatriz A. Rodas-Junco, and Geovanny I. Nic-Can. 2021. "Similar Features, Different Behaviors: A Comparative In Vitro Study of the Adipogenic Potential of Stem Cells from Human Follicle, Dental Pulp, and Periodontal Ligament" Journal of Personalized Medicine 11, no. 8: 738. https://doi.org/10.3390/jpm11080738








