Preliminary Results of the “Capasquelet” Technique for Managing Femoral Bone Defects—Combining a Masquelet Induced Membrane and Capanna Vascularized Fibula with an Allograft
Abstract
:1. Introduction
2. Method
2.1. Surgical Technique
2.1.1. The First Stage
2.1.2. Interstage Planification
2.1.3. The Second Stage
2.1.4. Postoperative Management
2.2. Data Collection and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zekry, K.M.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Alkhooly, A.Z.A.; Abd-Elfattah, A.S.; Elsaid, A.N.S.; Ahmed, A.R.; Tsuchiya, H. Reconstruction of Intercalary Bone Defect after Resection of Malignant Bone Tumor. J. Orthop. Surg. (Hong Kong) 2019, 27. [Google Scholar] [CrossRef] [Green Version]
- Rajasekaran, R.B.; Jayaramaraju, D.; Venkataramani, H.; Agraharam, D.; Shanmuganathan, R.S.; Shanmuganathan, R. Successful Reconstruction of a Post-Traumatic Defect of 16 cm of the Distal Femur by Modified Capanna’s Technique (Vascularised Free Fibula Combined with Allograft)—A Case Report and Technical Note. Trauma Case Rep. 2018, 17, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Ossendorf, C.; Leerapun, T.; Sim, F.H. Intercalary Segmental Reconstruction after Bone Tumor Resection. Eur. J. Surg. Oncol. 2008, 34, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, G.N.; Mavrogenis, A.F.; Mauffrey, C.; Lesenský, J.; Angelini, A.; Megaloikonomos, P.D.; Igoumenou, V.G.; Papanastassiou, J.; Savvidou, O.; Ruggieri, P.; et al. Intercalary Reconstructions after Bone Tumor Resections: A Review of Treatments. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Vidal, L.; Kampleitner, C.; Brennan, M.Á.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef]
- Brunet, O.; Anract, P.; Bouabid, S.; Babinet, A.; Dumaine, V.; Toméno, B.; Biau, D. Intercalary Defects Reconstruction of the Femur and Tibia after Primary Malignant Bone Tumour Resection. A Series of 13 Cases. Orthop. Traumatol. Surg. Res. 2011, 97, 512–519. [Google Scholar] [CrossRef] [Green Version]
- San Julian Aranguren, M.; Leyes, M.; Mora, G.; Cañadell, J. Consolidation of Massive Bone Allografts in Limb-Preserving Operations for Bone Tumours. Int. Orthop. 1995, 19, 377–382. [Google Scholar] [CrossRef]
- Frisoni, T.; Cevolani, L.; Giorgini, A.; Dozza, B.; Donati, D.M. Factors Affecting Outcome of Massive Intercalary Bone Allografts in the Treatment of Tumours of the Femur. J. Bone Joint Surg. Br. 2012, 94-B, 836–841. [Google Scholar] [CrossRef]
- Hornicek, F.J.; Gebhardt, M.C.; Tomford, W.W.; Sorger, J.I.; Zavatta, M.; Menzner, J.P.; Mankin, H.J. Factors Affecting Nonunion of the Allograft-Host Junction. Clin. Orthop Relat Res. 2001, 382, 87–98. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Lu, Y.; Zhu, H.; Ji, C.; Wang, Z. Factors Influencing Osseous Union Following Surgical Treatment of Bone Tumors with Use of the Capanna Technique. J. Bone Joint Surg. 2019, 101, 2036–2043. [Google Scholar] [CrossRef]
- Capanna, R.; Campanacci, D.A.; Belot, N.; Beltrami, G.; Manfrini, M.; Innocenti, M.; Ceruso, M. A New Reconstructive Technique for Intercalary Defects of Long Bones: The Association of Massive Allograft with Vascularized Fibular Autograft. Long-Term Results and Comparison with Alternative Techniques. Orthop. Clin. N. Am. 2007, 38, 51–60. [Google Scholar] [CrossRef]
- Karger, C.; Kishi, T.; Schneider, L.; Fitoussi, F.; Masquelet, A.-C. French Society of Orthopaedic Surgery and Traumatology (SoFCOT) Treatment of Posttraumatic Bone Defects by the Induced Membrane Technique. Orthop. Traumatol. Surg. Res. 2012, 98, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masquelet, A.C.; Fitoussi, F.; Begue, T.; Muller, G.P. [Reconstruction of the long bones by the induced membrane and spongy autograft]. Ann. Chir. Plast. Esthet. 2000, 45, 346–353. [Google Scholar]
- Masquelet, A.C. Muscle Reconstruction in Reconstructive Surgery: Soft Tissue Repair and Long Bone Reconstruction. Langenbeck’s Arch. Surg. 2003, 388, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Pelissier, P.; Masquelet, A.C.; Bareille, R.; Pelissier, S.M.; Amedee, J. Induced Membranes Secrete Growth Factors Including Vascular and Osteoinductive Factors and Could Stimulate Bone Regeneration. J. Orthop. Res. 2004, 22, 73–79. [Google Scholar] [CrossRef]
- Cuthbert, R.J.; Churchman, S.M.; Tan, H.B.; McGonagle, D.; Jones, E.; Giannoudis, P.V. Induced Periosteum a Complex Cellular Scaffold for the Treatment of Large Bone Defects. Bone 2013, 57, 484–492. [Google Scholar] [CrossRef]
- Masquelet, A.C. La technique de la membrane induite dans les reconstructions osseuses segmentaires: Développement et perspectives. Bull. Acad. Natl. Méd. 2017, 201, 439–453. [Google Scholar] [CrossRef]
- Masson, E. Fibula Vascularisée. Techniques, Indications en Orthopédie et Traumatologie. Available online: https://www.em-consulte.com/article/20991/fibula-vascularisee-techniques-indications-en-orth (accessed on 30 January 2021).
- Dréano, T.P.; Lecestre, P.; Levadoux, M.; Masquelet, A.C.; Poitout, D.; Polle, G.; Rigal, F.; Roussignol, X.; Tripon, P. Traumatic Bone Loss of the Diaphysis, Table Ronde Sous la Direction de Xavier ROUSSIGNOL. 2005. Available online: https://soo.com.fr/download/media/f0e/13d/156-com.pdf (accessed on 30 June 2021).
- Ganel, A.; Yaffe, B. Ankle Instability of the Donor Site Following Removal of Vascularized Fibula Bone Graft. Ann. Plast. Surg. 1990, 24, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A. Surgical Technique. Vascularized Transfer of the the Fibular Shaft. J. Microsurg. 1979, 1, 100. [Google Scholar]
- Bumbasirevic, M.; Stevanovic, M.; Bumbasirevic, V.; Lesic, A.; Atkinson, H.D.E. Free Vascularised Fibular Grafts in Orthopaedics. Int. Orthop. 2014, 38, 1277–1282. [Google Scholar] [CrossRef] [Green Version]
- Yajima, H.; Tamai, S.; Mizumoto, S.; Ono, H. Vascularised Fibular Grafts for Reconstruction of the Femur. J. Bone Joint Surg. Br. 1993, 75, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Sanders, P.T.J.; Spierings, J.F.; Albergo, J.I.; Bus, M.P.A.; Fiocco, M.; Farfalli, G.L.; van de Sande, M.A.J.; Aponte-Tinao, L.A.; Dijkstra, P.D.S. Long-Term Clinical Outcomes of Intercalary Allograft Reconstruction for Lower-Extremity Bone Tumors. JBJS 2020, 102, 1042–1049. [Google Scholar] [CrossRef]
- Henderson, E.R.; Groundland, J.S.; Pala, E.; Dennis, J.A.; Wooten, R.; Cheong, D.; Windhager, R.; Kotz, R.I.; Mercuri, M.; Funovics, P.T.; et al. Failure Mode Classification for Tumor Endoprostheses: Retrospective Review of Five Institutions and a Literature Review. J. Bone Joint Surg. 2011, 93, 418–429. [Google Scholar] [CrossRef]
- Ahmed, A.; Manabe, J.; Kawaguchi, N.; Matsumoto, S.; Matsushita, Y. Radiographic Analysis of Pasteurized Autologous Bone Graft. Skeletal Radiol. 2003, 32, 454–461. [Google Scholar] [CrossRef]
- Devlin, N.J.; Brooks, R. EQ-5D and the EuroQol Group: Past, Present and Future. Appl. Health Econ. Health Policy 2017, 15, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campanacci, D.A.; Totti, F.; Puccini, S.; Beltrami, G.; Scoccianti, G.; Delcroix, L.; Innocenti, M.; Capanna, R. Intercalary Reconstruction of Femur after Tumour Resection: Is a Vascularized Fibular Autograft plus Allograft a Long-Lasting Solution? Bone Joint J. 2018, 100-B, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Ogura, K.; Miyamoto, S.; Sakuraba, M.; Fujiwara, T.; Chuman, H.; Kawai, A. Intercalary Reconstruction after Wide Resection of Malignant Bone Tumors of the Lower Extremity Using a Composite Graft with a Devitalized Autograft and a Vascularized Fibula. Sarcoma 2015, 2015, 861575. [Google Scholar] [CrossRef]
- Masquelet, A.; Kanakaris, N.K.; Obert, L.; Stafford, P.; Giannoudis, P.V. Bone Repair Using the Masquelet Technique. JBJS 2019, 101, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Errani, C.; Ceruso, M.; Donati, D.M.; Manfrini, M. Microsurgical Reconstruction with Vascularized Fibula and Massive Bone Allograft for Bone Tumors. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Muratori, F.; Totti, F.; D’Arienzo, A.; Scorianz, M.; Scoccianti, G.; Beltrami, G.; Campo, F.R.; Citarelli, C.; Capanna, R.; Campanacci, D.A. Biological Intercalary Reconstruction with Bone Grafts After Joint-Sparing Resection of the Lower Limb: Is This an Effective and Durable Solution for Joint Preservation? Surg. Technol. Int. 2018, 32, 345–346. [Google Scholar]
- Bakri, K.; Stans, A.A.; Mardini, S.; Moran, S.L. Combined Massive Allograft and Intramedullary Vascularized Fibula Transfer: The Capanna Technique for Lower-Limb Reconstruction. Semin. Plast Surg. 2008, 22, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazourek, L.; Tomáš, T.; Mahdal, M.; Janíček, P.; Černý, J.; Ondrůšek, Š. [Use of Solid Intercalary Allografts for Reconstruction Following the Resection of Primary Bone Tumors]. Acta Chir. Orthop. Traumatol. Cech. 2018, 85, 171–178. [Google Scholar] [PubMed]
- Houdek, M.T.; Wagner, E.R.; Stans, A.A.; Shin, A.Y.; Bishop, A.T.; Sim, F.H.; Moran, S.L. What Is the Outcome of Allograft and Intramedullary Free Fibula (Capanna Technique) in Pediatric and Adolescent Patients With Bone Tumors? Clin. Orthop. Relat. Res. 2016, 474, 660–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Q.; Sun, Z.; Gu, S. [Progress of Masquelet technique to repair bone defect]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2013, 27, 1273–1276. [Google Scholar] [PubMed]
- Costa, S.; Reagan, M.R. Therapeutic Irradiation: Consequences for Bone and Bone Marrow Adipose Tissue. Front. Endocrinol. 2019, 10, 587. [Google Scholar] [CrossRef] [Green Version]
- Ling, X.F.; Peng, X. What Is the Price to Pay for a Free Fibula Flap? A Systematic Review of Donor-Site Morbidity Following Free Fibula Flap Surgery. Plast. Reconstr. Surg. 2012, 129, 657–674. [Google Scholar] [CrossRef]
- Jones, C.W.; Shatrov, J.; Jagiello, J.M.; Millington, S.; Hong, A.; Boyle, R.; Stalley, P.D. Clinical, Functional and Radiological Outcomes of Extracorporeal Irradiation in Limb Salvage Surgery for Bone Tumours. Bone Joint J. 2017, 99, 1681–1688. [Google Scholar] [CrossRef]
- Liu, Q.; He, H.; Duan, Z.; Zeng, H.; Yuan, Y.; Wang, Z.; Luo, W. Intercalary Allograft to Reconstruct Large-Segment Diaphysis Defects After Resection of Lower Extremity Malignant Bone Tumor. Cancer Manag. Res. 2020, 12, 4299–4308. [Google Scholar] [CrossRef]
- Jayaramaraju, D.; Venkataramani, H.; Rajasekaran, R.B.; Agraharam, D.; Sabapathy, S.R.; Rajasekaran, S. Modified Capanna’s Technique (Vascularized Free Fibula Combined with Allograft) as a Single-Stage Procedure in Post-Traumatic Long-Segment Defects of the Lower End of the Femur: Outcome Analysis of a Series of 19 Patients with an Average Gap of 14 Cm. Indian J. Plast. Surg. 2019, 52, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Zaretski, A.; Amir, A.; Meller, I.; Leshem, D.; Kollender, Y.; Barnea, Y.; Bickels, J.; Shpitzer, T.; Ad-El, D.; Gur, E. Free Fibula Long Bone Reconstruction in Orthopedic Oncology: A Surgical Algorithm for Reconstructive Options. Plast. Reconstr. Surg. 2004, 113, 1989–2000. [Google Scholar] [CrossRef]
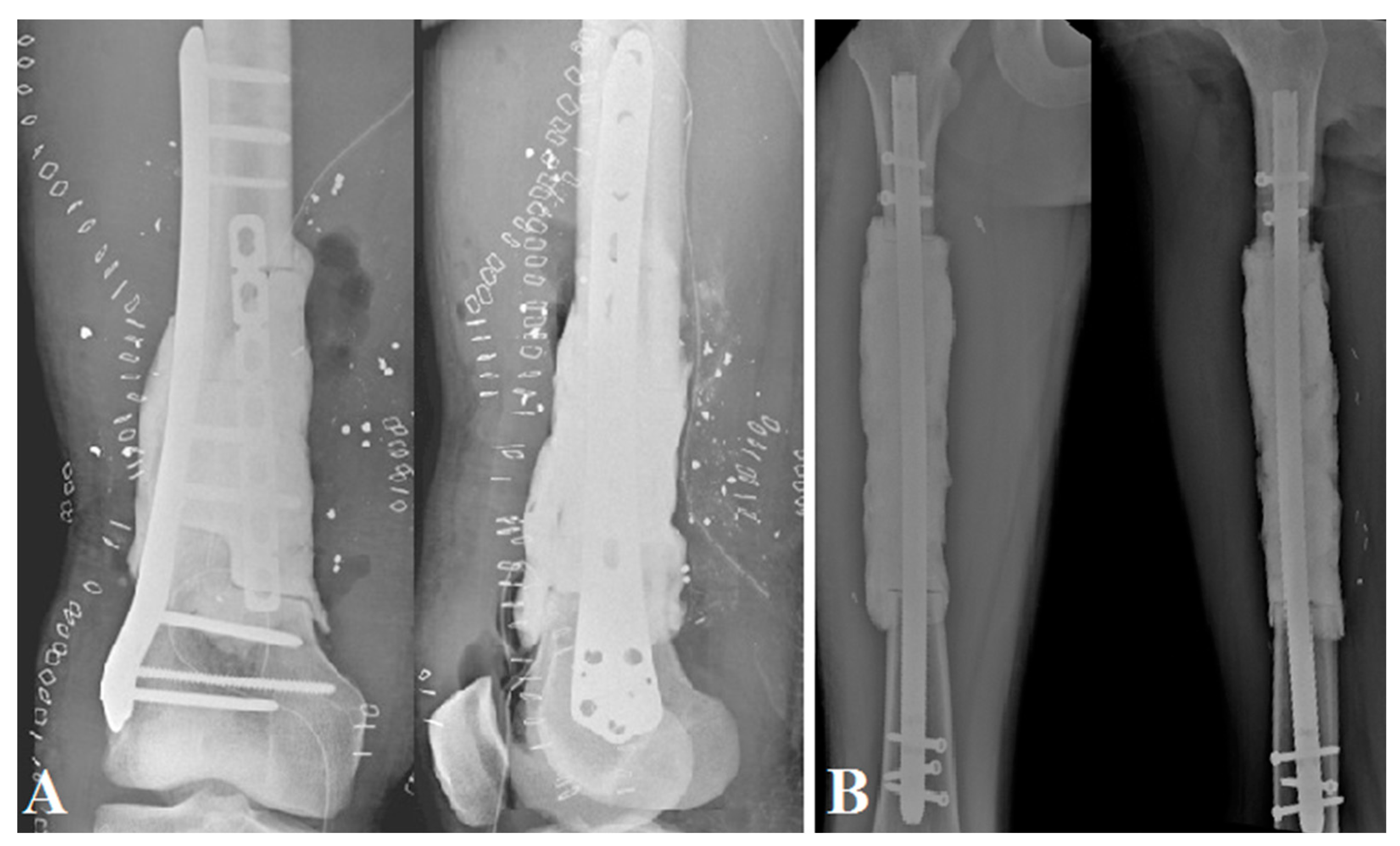




| Patient No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Epidemiology data | ||||
| Gender | Female | Male | Male | Male |
| Age (years) | 44 | 28 | 18 | 24 |
| BMI (kg/m2) | 24.5 | 22.3 | 20.5 | 24.9 |
| Etiology | Traumatic (RTA) | Osteosarcoma | Ewing Tumor | Traumatic (Ballistic) |
| Length Bone Loss (mm) | 100 | 220 | 180 | 110 (Step-cut) |
| Follow up (months) | 36 | 22 | 14 | 12 |
| Surgical data | ||||
| First-stage associated surgery | Preparation | Resection R0 | Resection R0 | Sepsis treatment |
| Spacer stabilization | IMN | IMN | IMN | Plate |
| Total operative time (T1; T2) (minutes) | 716 (136; 580) | 774 (295; 479) | 656 (192; 464) | 793 (235; 558) |
| Fibula graft length (mm) | 150 | 280 | 220 | 160 |
| Graft stabilization | Plate | Plate | Plate | Platex2 |
| Delay T1 and T2 (weeks) | 8 | 22 | 24 | 12 |
| Postoperative data | ||||
| Complications | Hematoma | No | Material failure | No |
| Delay surgical revision (weeks) | 3 (after T2) | 28 (after T2) | ||
| Type of surgery | Hematoma evacuation | Fixation revision | ||
| Time for consolidation (months) | 4 | 7 | DOD | No X-ray * |
| ISOLS score at three months (%) | 86.7 | 73.3 | 75.6 | |
| ISOLS score at six months (%) | 95.6 | 95.6 | 68.9 | |
| Functional results | ||||
| Full weight-bearing (weeks) | 12 | 12 | 12 | 8 |
| EQ5D | 70 | 75 | DOD | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Combal, A.; Thuau, F.; Fouasson-Chailloux, A.; Arrigoni, P.-P.; Baud’huin, M.; Duteille, F.; Crenn, V. Preliminary Results of the “Capasquelet” Technique for Managing Femoral Bone Defects—Combining a Masquelet Induced Membrane and Capanna Vascularized Fibula with an Allograft. J. Pers. Med. 2021, 11, 774. https://doi.org/10.3390/jpm11080774
Combal A, Thuau F, Fouasson-Chailloux A, Arrigoni P-P, Baud’huin M, Duteille F, Crenn V. Preliminary Results of the “Capasquelet” Technique for Managing Femoral Bone Defects—Combining a Masquelet Induced Membrane and Capanna Vascularized Fibula with an Allograft. Journal of Personalized Medicine. 2021; 11(8):774. https://doi.org/10.3390/jpm11080774
Chicago/Turabian StyleCombal, Alexis, François Thuau, Alban Fouasson-Chailloux, Pierre-Paul Arrigoni, Marc Baud’huin, Franck Duteille, and Vincent Crenn. 2021. "Preliminary Results of the “Capasquelet” Technique for Managing Femoral Bone Defects—Combining a Masquelet Induced Membrane and Capanna Vascularized Fibula with an Allograft" Journal of Personalized Medicine 11, no. 8: 774. https://doi.org/10.3390/jpm11080774
APA StyleCombal, A., Thuau, F., Fouasson-Chailloux, A., Arrigoni, P.-P., Baud’huin, M., Duteille, F., & Crenn, V. (2021). Preliminary Results of the “Capasquelet” Technique for Managing Femoral Bone Defects—Combining a Masquelet Induced Membrane and Capanna Vascularized Fibula with an Allograft. Journal of Personalized Medicine, 11(8), 774. https://doi.org/10.3390/jpm11080774








