Automatic Diagnosis of Bipolar Disorder Using Optical Coherence Tomography Data and Artificial Intelligence
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Correlations between the Most Discriminating Variables
3.2. Correlation with Disease Duration, Number of Hospitalizations and Manic Episodes
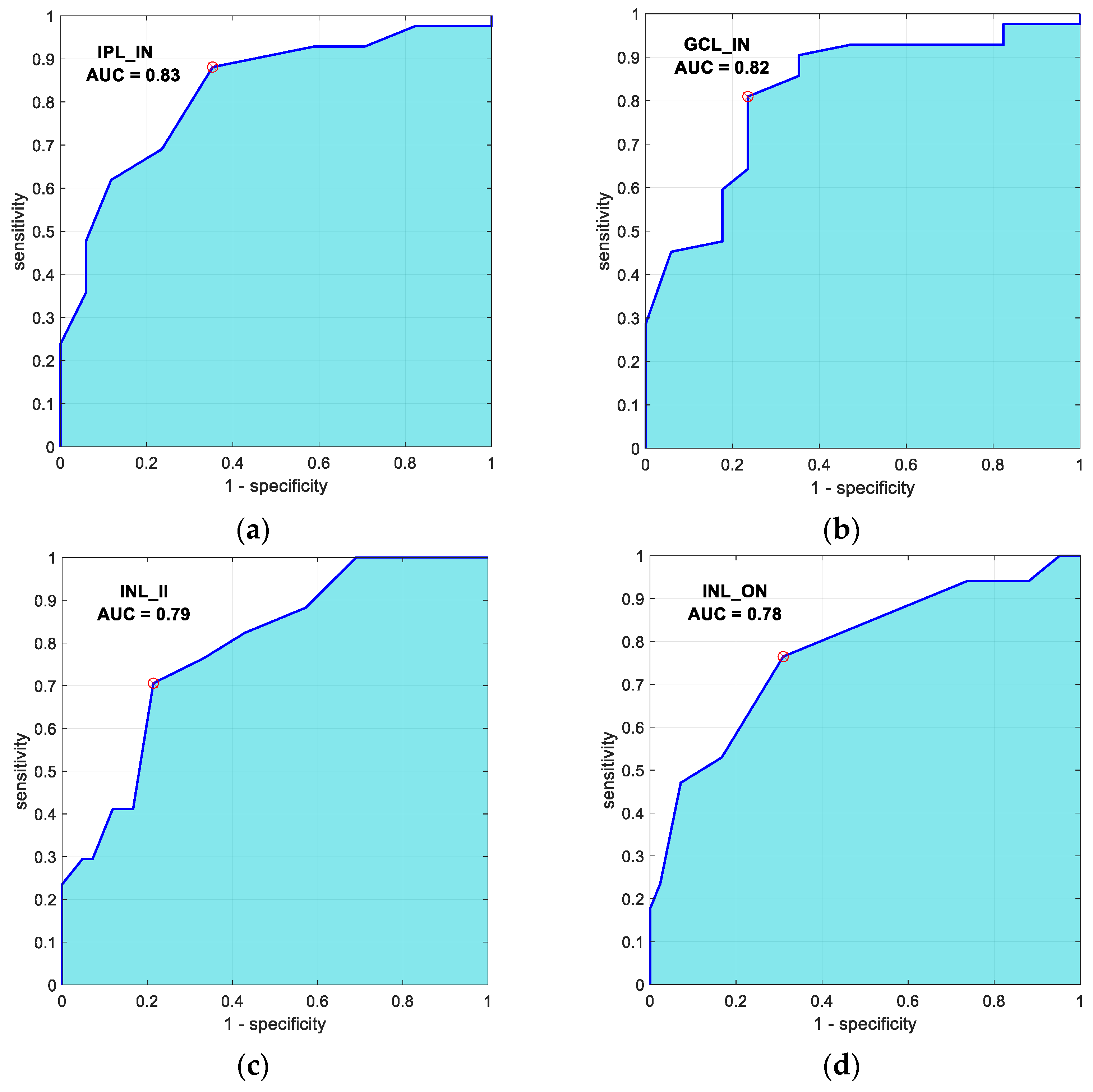
3.3. Automatic Classification
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bauer, M.; Andreassen, O.A.; Geddes, J.R.; Kessing, L.V.; Lewitzka, U.; Schulze, T.G.; Vieta, E. Areas of uncertainties and unmet needs in bipolar disorders: Clinical and research perspectives. Lancet Psychiatry 2018, 5, 930–939. [Google Scholar] [CrossRef]
- Lublóy, Á.; Keresztúri, J.L.; Németh, A.; Mihalicza, P. Exploring factors of diagnostic delay for patients with bipolar disorder: A population-based cohort study. BMC Psychiatry 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieta, E.; Salagre, E.; Grande, I.; Carvalho, A.F.; Fernandes, B.S.; Berk, M.; Birmaher, B.; Tohen, M.; Suppes, T. Early Intervention in Bipolar Disorder. Am. J. Psychiatry 2018, 175, 411–426. [Google Scholar] [CrossRef]
- Teixeira, A.L.; Colpo, G.D.; Fries, G.R.; Bauer, I.E.; Selvaraj, S. Biomarkers for bipolar disorder: Current status and challenges ahead. Expert Rev. Neurother. 2018, 19, 67–81. [Google Scholar] [CrossRef]
- Garcia-Martin, E.; Ara, J.R.; Martin, J.; Almarcegui, C.; Dolz, I.; Vilades, E.; Gil-Arribas, L.; Fernandez, F.J.; Polo, V.; Larrosa, J.M.; et al. Retinal and Optic Nerve Degeneration in Patients with Multiple Sclerosis Followed up for 5 Years. Ophthalmology 2017, 124, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Schönfeldt-Lecuona, C.; Kregel, T.; Schmidt, A.; Kassubek, J.; Dreyhaupt, J.; Freudenmann, R.W.; Connemann, B.J.; Gahr, M.; Pinkhardt, E.H. Retinal single-layer analysis with optical coherence tomography (OCT) in schizophrenia spectrum disorder. Schizophr. Res. 2019, 219, 5–12. [Google Scholar] [CrossRef]
- García-Martín, E.; Pueyo, V.; Fernández, J.; Almárcegui, C.; Dolz, I.; Martín, J.; Ara, J.; Honrubia, F.M. Atrofia de la capa de fibras nerviosas de la retina en pacientes con esclerosis múltiple. Estudio prospectivo con dos años de seguimiento. Arch. Soc. Esp. Oftalmol. 2010, 85, 179–186. [Google Scholar] [CrossRef]
- Chan, V.T.; Sun, Z.; Tang, S.; Chen, L.J.; Wong, A.; Tham, C.Y.C.; Wong, T.Y.; Chen, C.; Ikram, M.K.; Whitson, H.E.; et al. Spectral-Domain OCT Measurements in Alzheimer’s Disease: A Systematic Review and Meta-analysis. Ophthalmology 2019, 126, 497–510. [Google Scholar] [CrossRef]
- Mokbul, M.I. Optical Coherence Tomography: Basic Concepts and Applications in Neuroscience Research. J. Med. Eng. 2017, 2017, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Lapiscina, E.H.; Arnow, S.; Wilson, J.A.; Saidha, S.; Preiningerova, J.L.; Oberwahrenbrock, T.; Brandt, A.U.; Pablo, L.E.; Guerrieri, S.; González-Suárez, I.; et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: A cohort study. Lancet Neurol. 2016, 15, 574–584. [Google Scholar] [CrossRef] [Green Version]
- Satue, M.; Obis, J.; Rodrigo, M.J.; Otin, S.; Fuertes, M.I.; Vilades, E.; Gracia, H.; Ara, J.R.; Alarcia, R.; Polo, V.; et al. Optical Coherence Tomography as a Biomarker for Diagnosis, Progression, and Prognosis of Neurodegenerative Diseases. J. Ophthalmol. 2016, 2016, 8503859. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, S.M.; Demmin, D.L.; Schallek, J.B.; Fradkin, S.I. Measures of Retinal Structure and Function as Biomarkers in Neurology and Psychiatry. Biomark. Neuropsychiatry 2020, 2, 100018. [Google Scholar] [CrossRef]
- Wollenhaupt-Aguiar, B.; Kapczinski, F.; Pfaffenseller, B. Biological Pathways Associated with Neuroprogression in Bipolar Disorder. Brain Sci. 2021, 11, 228. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, E.; Gavin, A.; Garcia-Campayo, J.; Vilades, E.; Orduna, E.; Polo, V.; Larrosa, J.M.; Pablo, L.E.; Satue, M. Vision function and retinal changes in patients with bipolar disorder. Retina 2019, 39, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Ching, C.R.K.; Hibar, D.P.; Gurholt, T.P.; Nunes, A.; Thomopoulos, S.I.; Abé, C.; Agartz, I.; Brouwer, R.M.; Cannon, D.M.; de Zwarte, S.M.C.; et al. What we learn about bipolar disorder from large-scale neuroimaging: Findings and future directions from the ENIGMA Bipolar Disorder Working Group. Hum. Brain Mapp. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hajek, T.; Cullis, J.; Novak, T.; Kopecek, M.; Höschl, C.; Blagdon, R.; O’Donovan, C.; Bauer, M.; Young, L.T.; MacQueen, G.; et al. Hippocampal volumes in bipolar disorders: Opposing effects of illness burden and lithium treatment. Bipolar Disord. 2012, 14, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Hajek, T.; Cullis, J.; Novák, T.; Kopecek, M.; Blagdon, R.; Propper, L.; Stopkova, P.; Duffy, A.; Hoschl, C.; Uher, R.; et al. Brain Structural Signature of Familial Predisposition for Bipolar Disorder: Replicable Evidence For Involvement of the Right Inferior Frontal Gyrus. Biol. Psychiatry 2013, 73, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Kalmar, J.H.; Wang, F.; Spencer, L.; Edmiston, E.; Lacadie, C.M.; Martin, A.; Constable, R.; Duncan, J.S.; Staib, L.H.; Papademetris, X.; et al. Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J. Int. Neuropsychol. Soc. 2009, 15, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.L.; Swartz, H. A Critical Appraisal of Neuroimaging Studies of Bipolar Disorder: Toward a New Conceptualization of Underlying Neural Circuitry and a Road Map for Future Research. Am. J. Psychiatry 2014, 171, 829–843. [Google Scholar] [CrossRef]
- Harrison, P.J.; Colbourne, L.; Harrison, C.H. The neuropathology of bipolar disorder: Systematic review and meta-analysis. Mol. Psychiatry 2018, 25, 1787–1808. [Google Scholar] [CrossRef] [Green Version]
- Lizano, P.; Bannai, D.; Lutz, O.; Kim, L.A.; Miller, J.; Keshavan, M. A Meta-analysis of Retinal Cytoarchitectural Abnormalities in Schizophrenia and Bipolar Disorder. Schizophr. Bull. 2019, 46, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Polo, V.; Satue, M.; Gavin, A.; Vilades, E.; Orduna, E.; Cipres, M.; Garcia-Campayo, J.; Navarro-Gil, M.; Larrosa, J.M.; Pablo, L.E.; et al. Ability of swept source OCT to detect retinal changes in patients with bipolar disorder. Eye 2018, 33, 549–556. [Google Scholar] [CrossRef]
- Kalenderoglu, A.; Sevgi-Karadag, A.; Celik, M.; Eğilmez, O.B.; Han-Almis, B.; Ozen, M.E. Can the retinal ganglion cell layer (GCL) volume be a new marker to detect neurodegeneration in bipolar disorder? Compr. Psychiatry 2016, 67, 66–72. [Google Scholar] [CrossRef]
- Alici, S.; Onur, Ö.Ş.; Çavuşoğlu, E.; Onur, I.U.; Erkiran, M. Optical coherence tomography findings in bipolar disorder: A preliminary receiver operating characteristic analysis on ganglion cell layer volume for diagnosis. Arch. Clin. Psychiatry 2019, 46, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.A.; Saleh, A.A.; Gohar, S.; Khalil, D.H.; Said, M. Optical coherence tomography findings in patients with bipolar disorder. J. Affect. Disord. 2017, 218, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Mehraban, A.; Samimi, S.M.; Entezari, M.; Seifi, M.H.; Nazari, M.; Yaseri, M. Peripapillary retinal nerve fiber layer thickness in bipolar disorder. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 254, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.W.; Tajunisah, I.; Sharmilla, K.; Peyman, M.; Subrayan, V. Retinal Nerve Fiber Layer Structure Abnormalities in Schizophrenia and Its Relationship to Disease State: Evidence From Optical Coherence Tomography. Investig. Opthalmol. Vis. Sci. 2013, 54, 7785–7792. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, T.M.P.; Andrade, S.M.; De Andrade, M.J.O.; Nogueira, R.M.T.B.L.; Santos, N.A. Colour discrimination thresholds in type 1 Bipolar Disorder: A pilot study. Sci. Rep. 2017, 7, 16405. [Google Scholar] [CrossRef] [PubMed]
- Schallmo, M.-P.; Sponheim, S.; Olman, C. Reduced contextual effects on visual contrast perception in schizophrenia and bipolar affective disorder. Psychol. Med. 2015, 45, 3527–3537. [Google Scholar] [CrossRef] [Green Version]
- Yıldız, M.; Alim, S.; Batmaz, S.; Demir, S.; Songur, E.; Ortak, H.; Demirci, K. Duration of the depressive episode is correlated with ganglion cell inner plexifrom layer and nasal retinal fiber layer thicknesses: Optical coherence tomography findings in major depression. Psychiatry Res. Neuroimaging 2016, 251, 60–66. [Google Scholar] [CrossRef]
- Librenza-Garcia, D.; Kotzian, B.J.; Yang, J.; Mwangi, B.; Cao, B.; Lima, L.N.P.; Bermudez, M.B.; Boeira, M.V.; Kapczinski, F.; Passos, I.C. The impact of machine learning techniques in the study of bipolar disorder: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Tashiro, T.; Uehara, K. Deep Neural Generative Model of Functional MRI Images for Psychiatric Disorder Diagnosis. IEEE Trans. Biomed. Eng. 2019, 66, 2768–2779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthik, S.; Sudha, M. Predicting bipolar disorder and schizophrenia based on non-overlapping genetic phenotypes using deep neural network. Evol. Intell. 2020, 14, 619–634. [Google Scholar] [CrossRef]
- Achalia, R.; Sinha, A.; Jacob, A.; Achalia, G.; Kaginalkar, V.; Venkatasubramanian, G.; Rao, N.P. A proof of concept machine learning analysis using multimodal neuroimaging and neurocognitive measures as predictive biomarker in bipolar disorder. Asian J. Psychiatry 2020, 50, 101984. [Google Scholar] [CrossRef] [PubMed]
- Claude, L.-A.; Houenou, J.; Duchesnay, E.; Favre, P. Will machine learning applied to neuroimaging in bipolar disorder help the clinician? A critical review and methodological suggestions. Bipolar Disord. 2020, 22, 334–355. [Google Scholar] [CrossRef] [PubMed]
- Arlot, S.; Celisse, A. A survey of cross-validation procedures for model selection. Stat. Surv. 2010, 4, 40–79. [Google Scholar] [CrossRef]
- Wu, X.; Kumar, V.; Quinlan, J.R.; Ghosh, J.; Yang, Q.; Motoda, H.; McLachlan, G.J.; Ng, S.K.; Liu, B.; Yu, P.S.; et al. Top 10 algorithms in data mining. Knowl. Inf. Syst. 2007, 14, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Cover, T.; Hart, P. Nearest neighbor pattern classification. IEEE Trans. Inf. Theory 1967, 13, 21–27. [Google Scholar] [CrossRef]
- Abu Alfeilat, H.A.; Hassanat, A.; Lasassmeh, O.; Tarawneh, A.S.; Alhasanat, M.B.; Salman, H.S.E.; Prasath, S. Effects of Distance Measure Choice on K-Nearest Neighbor Classifier Performance: A Review. Big Data 2019, 7, 221–248. [Google Scholar] [CrossRef] [Green Version]
- Vapnik, V.N. An overview of statistical learning theory. IEEE Trans. Neural Netw. 1999, 10, 988–999. [Google Scholar] [CrossRef] [Green Version]
- Passos, I.C.; Ballester, P.L.; Barros, R.C.; Librenza-Garcia, D.; Mwangi, B.; Birmaher, B.; Brietzke, E.; Hajek, T.; Jaramillo, C.L.; Mansur, R.B.; et al. Machine learning and big data analytics in bipolar disorder: A position paper from the International Society for Bipolar Disorders Big Data Task Force. Bipolar Disord. 2019, 21, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Kapczinski, F.; Andreazza, A.; Dean, O.; Giorlando, F.; Maes, M.; Yücel, M.; Gama, C.; Dodd, S.; Dean, B.; et al. Pathways underlying neuroprogression in bipolar disorder: Focus on inflammation, oxidative stress and neurotrophic factors. Neurosci. Biobehav. Rev. 2010, 35, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Kapczinski, N.S.; Mwangi, B.; Cassidy, R.; Librenza-Garcia, D.; Bermudez, M.B.; Kauer-Sant’Anna, M.; Kapczinski, F.; Passos, I.C. Neuroprogression and illness trajectories in bipolar disorder. Expert Rev. Neurother. 2016, 17, 277–285. [Google Scholar] [CrossRef]
- Svetozarskiy, S.; Kopishinskaya, S. Retinal Optical Coherence Tomography in Neurodegenerative Diseases (Review). Sovrem. Tehnol. Med. 2015, 7, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Morla, E.M.; López-Villarreal, A.; Jiménez-López, E.; Aparicio, A.I.; Martínez-Vizcaíno, V.; Roberto, R.-J.; Vieta, E.; Santos, J.-L. Impact of number of episodes on neurocognitive trajectory in bipolar disorder patients: A 5-year follow-up study. Psychol. Med. 2018, 49, 1299–1307. [Google Scholar] [CrossRef]
- López-Jaramillo, C.; Vásquez, J.P.L.; Gallo, A.; Ospina-Duque, J.; Bell, V.; Torrent, C.; Martinez-Aran, A.; Vieta, E. Effects of recurrence on the cognitive performance of patients with bipolar I disorder: Implications for relapse prevention and treatment adherence. Bipolar Disord. 2010, 12, 557–567. [Google Scholar] [CrossRef]
- Ascaso, F.J.; Rodriguez-Jimenez, R.; Cabezón, L.; López-Antón, R.; Santabárbara, J.; De la Cámara, C.; Modrego, P.J.; Quintanilla, M.A.; Bagney, A.; Gutierrez, L.; et al. Retinal nerve fiber layer and macular thickness in patients with schizophrenia: Influence of recent illness episodes. Psychiatry Res. 2015, 229, 230–236. [Google Scholar] [CrossRef]
- Carlson, S.; Dixon, C.E. Lithium Improves Dopamine Neurotransmission and Increases Dopaminergic Protein Abundance in the Striatum after Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2827–2836. [Google Scholar] [CrossRef]
- Masoudnia, S.; Ebrahimpour, R. Mixture of experts: A literature survey. Artif. Intell. Rev. 2012, 42, 275–293. [Google Scholar] [CrossRef]
- Yang, T.-K.; Huang, X.-G.; Yao, J.-Y. Effects of Cigarette Smoking on Retinal and Choroidal Thickness: A Systematic Review and Meta-Analysis. J. Ophthalmol. 2019, 2019, 8079127. [Google Scholar] [CrossRef]
- Berk, M.; Dandash, O.; Daglas, R.; Cotton, S.; Allott, K.; Fornito, A.; Suo, C.; Klauser, P.; Liberg, B.; Henry, L.; et al. Neuroprotection after a first episode of mania: A randomized controlled maintenance trial comparing the effects of lithium and quetiapine on grey and white matter volume. Transl. Psychiatry 2017, 7, e1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Tong, N.; Gong, Y.; Qiu, Q.; Yin, L.; Lv, X.; Wu, X. Valproate protects the retina from endoplasmic reticulum stress-induced apoptosis after ischemia–reperfusion injury. Neurosci. Lett. 2011, 504, 88–92. [Google Scholar] [CrossRef] [PubMed]
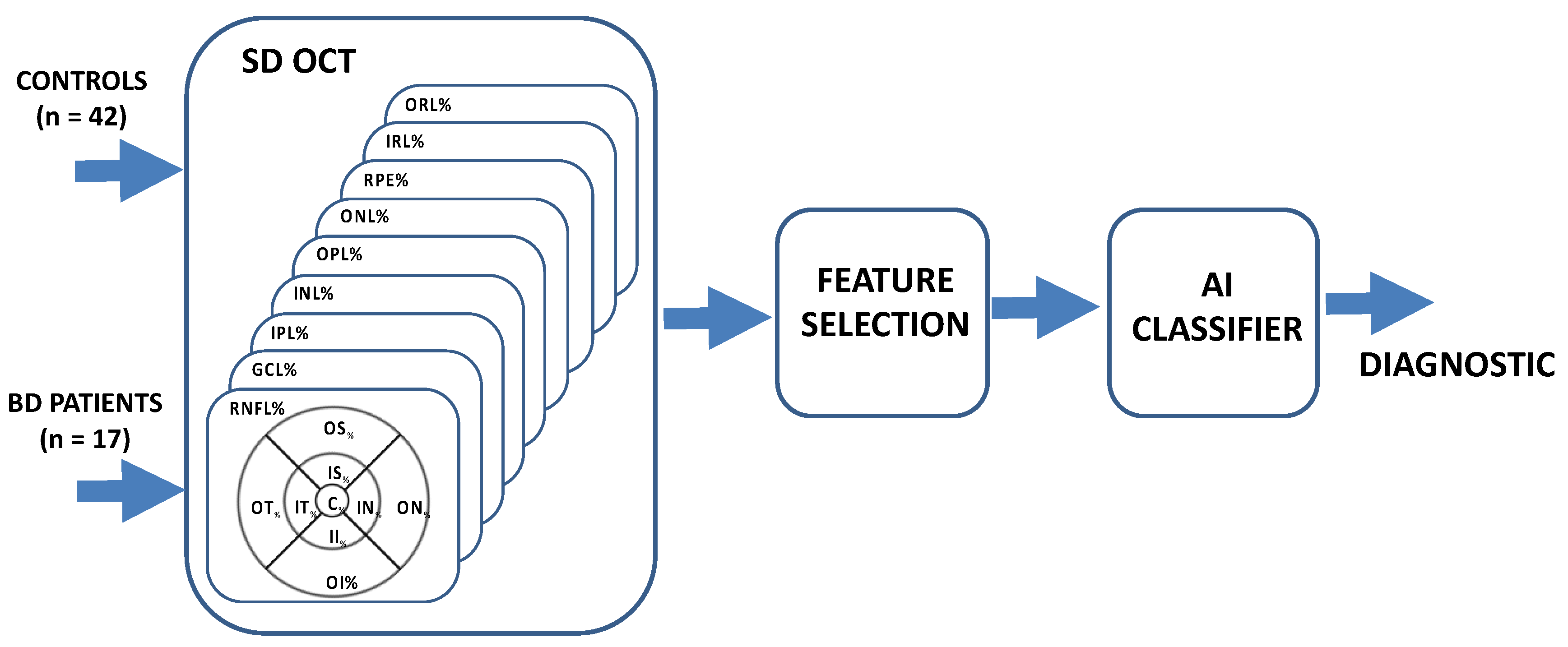
| Controls n = 42 | BD n = 17 | p Value | |
|---|---|---|---|
| Mean age (years ± SD) | 49.74 ± 17.01 | 51.47 ± 11.94 | p (t-test) = 0.703 |
| Male:Female ratio | 13:29 | 7:10 | χ2(1) = 0.56, p = 0.45 |
| Number of R:L eyes analyzed | 18:24 | 9:8 | χ2(1) = 0.49, p = 0.48 |
| Duration of disease (years ± SD) | -- | 20.64 ± 6.48 | -- |
| Age when disease diagnosed (years ± SD) | -- | 30.00 ± 13.84 | -- |
| Number of hospitalizations | -- | 2 [3.0] | -- |
| Number of manic episodes | -- | 8.33 ± 4.22 | -- |
| Layer | RNFL | GCL | IPL | INL | OPL | ONL | RPE | IRL | ORL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | BD | C | BD | C | BD | C | BD | C | BD | C | BD | C | BD | C | BD | C | BD | |
| Central | 12.0 [3.0] | 11.65 ± 2.09 | 16.62 ± 3.78 | 14.0 [5.5] | 21.26 ± 3.51 | 20.12 ± 4.86 | 20.76 ± 6.26 | 18.0 [10.0] | 25.71 ± 5.60 | 23.0 [8.5] | 95.0 [16.25] | 92.0 [15.0] | 16.0 [2.0] | 15.0 [2.0] | 189.45 ± 25.03 | 185.06 ± 20.48 | 90.0 [5.0] | 88.18 ± 4.59 |
| p (U-test) = 0.14 AUC = 0.62 | p (U-test) = 0.17 AUC = 0.61 | p (t-test) = 0.32 AUC = 0.61 | p (U-test) = 0.86 AUC = 0.51 | p (U-test) = 0.47 AUC = 0.56 | p (U-test) = 0.46 AUC = 0.56 | p (U-test) = 0.056 AUC = 0.66 | p (t-test) = 0.52 AUC = 0.58 | p (U-test) = 0.28 AUC = 0.59 | ||||||||||
| Inner Nasal (IN) | 22.21 ± 2.88 | 20.47 ± 3.78 | 53.0 [6.25] | 44.71 ± 6.94 | 43.0 [4.25] | 38.59 ± 4.12 | 40.50 [5.0] | 43.47 ± 3.73 | 32.0 [7.0] | 35.47 ± 8.46 | 76.0 [16.25] | 70.94 ± 16.15 | 15.05 ± 1.62 | 15.0 [3.5] | 267.0 [25.0] | 254.06 ± 15.31 | 82.50 ± 3.09 | 81.82 ± 3.09 |
| p (t-test) = 0.06 AUC = 0.68 | p (U-test) = 0.00 AUC = 0.82 | p (U-test) = 0.00 AUC = 0.83 | p (U-test) = 0.041 AUC = 0.67 # | p (U-test) = 0.56 AUC = 0.55 # | p (U-test) = 0.79 AUC = 0.52 | p (U-test) = 0.71 AUC = 0.53 # | p (U-test) = 0.009 AUC = 0.72 | p (t-test) = 0.45 AUC = 0.56 | ||||||||||
| Outer Nasal (ON) | 51.90 ± 7.99 | 44 [18] | 38.00 [6.0] | 36.82 ± 5.08 | 30.0 [3.25] | 29.35 ± 3.46 | 34.0 [4.0] | 37.12 ± 3.39 | 28.0 [4.25] | 29.0 [4.0] | 55.81 ± 7.98 | 55.18 ± 9.63 | 13.0 [2.0] | 13.00 ± 1.77 | 239.0 [18.75] | 239.29 ± 11.27 | 78.95 ± 2.84 | 77.71 ± 2.78 |
| p (U-test) = 0.18 AUC = 0.61 | p (U-test) = 0.62 AUC = 0.54 | p (U-test) = 0.91 AUC = 0.51 | p (U-test) = 0.001 AUC = 0.78 # | p (U-test) = 0.055 AUC = 0.66 # | p (t-test) = 0.79 AUC = 0.52 | p (U-test) = 0.66 AUC = 0.54 | p (U-test) = 0.87 AUC = 0.51 # | p (t-test) = 0.13 AUC = 0.62 | ||||||||||
| Inner Superior (IS) | 25.07 ± 2.62 | 24.0 [3.5] | 52.0 [5.25] | 51.00 [7.0] | 42.0 [4.0] | 40.06 ± 3.36 | 40.0 [4.5] | 43.29 ± 2.69 | 31.0 [6.0] | 30.0 [11.0] | 73.50 [11.0] | 69.94 ± 11.80 | 16.0 [3.0] | 16.0 [3.0] | 264.0 [23.75] | 263.0 [17.5] | 82.0 [3.5] | 81.0 [3.5] |
| p (U-test) = 0.12 AUC = 0.63 | p (U-test) = 0.021 AUC = 0.69 | p (U-test) = 0.051 AUC = 0.66 | p (U-test) = 0.009 AUC = 0.72 # | p (U-test) = 0.83 AUC = 0.52 | p (U-test) = 0.65 AUC = 0.54 | p (U-test) = 0.97 AUC = 0.50 | p (U-test) = 0.46 AUC = 0.56 | p (U-test) = 0.14 AUC = 0.62 | ||||||||||
| Outer superior (OS) | 39.57 ± 5.56 | 38.0 [.8.0] | 36.0 [5.0] | 33.88 ± 3.66 | 29.0 [3.25] | 27.76 ± 2.61 | 31.83 ± 2.58 | 33.82 ± 2.83 | 25.0 [3.0] | 26.0 [3.0] | 61.45 ± 8.55 | 59.12 ± 6.70 | 14.0 [2.25] | 13.06 ± 1.30 | 225.5 [15.25] | 229.0 [19.0] | 80.00 ± 2.78 | 78.00 ± 2.47 |
| p (U-test) = 0.50 AUC = 0.56 | p (U-test) = 0.16 AUC = 0.62 | p (U-test) = 0.13 AUC = 0.63 | p (t-test) = 0.012 AUC = −0.69 # | p (U-test) = 0.56 AUC = 0.55 # | p (t-test) = 0.32 AUC = 0.58 | p (U-test) = 0.18 AUC = 0.61 | p (U-test) = 0.70 AUC = 0.53 | p (t-test) = 0.013 AUC = 0.70 | ||||||||||
| Inner Temporal (IT) | 17.0 [3.0] | 17.06 ± 1.68 | 47.50 [6.5] | 44.0 [11.0] | 41.50 [4.25] | 40.0 [7.5] | 37.0 [5.0] | 40.47 ± 4.53 | 29.5 [5.0] | 31.0 [3.5] | 73.50 [9.0] | 71.35 ± 10.71 | 14.0 [3.0] | 15.0 [3.0] | 250.5 [21.0] | 246.0 [18.5] | 82.0 [4.25] | 80.94 ± 3.34 |
| p (U-test) = 0.36 AUC = 0.57 | p (U-test) = 0.020 AUC = 0.69 | p (U-test) = 0.18 AUC = 0.61 | p (U-test) = 0.017 AUC= 0.70 # | p (U-test) = 0.28 AUC = 0.59 # | p (U-test) = 0.32 AUC = 0.58 | p (U-test) = 0.82 AUC = 0.52 | p (U-test) = 0.20 AUC = 0.61 | p (U-test) = 0.24 AUC = 0.60 | ||||||||||
| Outer Temporal (OT) | 19.0 [2.0] | 20.0 [3.5] | 37.5 [7.0] | 35.0 [6.5] | 32.50 [3.0] | 32.12 ± 3.59 | 33.50 [4.25] | 34.94 ± 2.08 | 26.5 [2.25.] | 28.65 ± 2.71 | 57.57 ± 7.36 | 54.94 ± 7.06 | 13.0 [1.0] | 12.24 ± 1.09 | 208.0 [15.25] | 207.0 [19.5] | 78.40 ± 2.67 | 77.06 ± 2.05 |
| p (U-test) = 0.43 AUC= 0.56 # | p (U-test) = 0.27 AUC = 0.59 | p (U-test) = 0.57 AUC = 0.55 | p (U-test) = 0.042 AUC = 0. 67 # | p (U-test) = 0.020 AUC = 0. 69 # | p (t-test) = 0.21 AUC = 0.60 | p (U-test) = 0.27 AUC = 0.59 | p (U-test) = 0.72 AUC = 0.53 # | p (t-test) = 0.067 AUC = 0.65 | ||||||||||
| Inner Inferior (II) | 26.62 ± 4.36 | 24.76 ± 4.24 | 53.0 [6.0] | 50.0 [8.0] | 41.0 [4.0] | 39.0 [6.5] | 41.0 [4.0] | 45.47 ± 4.16 | 34.57 ± 8.54 | 37.47 ± 8.54 | 63.95 ± 11.69 | 61.12 ± 15.81 | 14.43 ± 1.65 | 14.29 ± 1.45 | 262.5 [19.5] | 259.0 [18.5] | 80.02 ± 2.78 | 79.41 ± 2.74 |
| p (t-test) = 0.14 AUC = 0.64 | p (U-test) = 0.014 AUC = 0.70 | p (U-test) = 0.035 AUC = 0.67 | p (U-test) = 0.001 AUC = 0 79 # | p (t-test) = 0.24 AUC = 0.59 # | p (t-test) = 0.51 AUC = 0.53 | p (t-test) = 0.77 AUC = 0.52 | p (U-test) = 0.20 AUC = 0.61 | p (t-test) = 0.44 AUC = 0.56 | ||||||||||
| Outer Inferior (OI) | 41.07 ± 7.83 | 38.82 ± 9.19 | 31.50 [5.25] | 32.0 [4.0] | 26.0 [4.0] | 26.35 ± 2.32 | 31.0 [3.0] | 32.94 ± 2.11 | 27.0 [5.0] | 27.59 ± 2.55 | 50.83 ± 6.98 | 49.35 ± 7.75 | 12.50 [1.25] | 12.0 [2.0] | 208.5 [18.5] | 208.0 [15.5] | 76.95 ± 2.95 | 76.41 ± 2.87 |
| p (t-test) = 0.35 AUC = 0.62 | p (U-test) = 0.84 AUC = 0.52 # | p (U-test) = 0.73 AUC = 0.53 | p (U-test) = 0.004 AUC = 0.74 # | p (U-test) = 0.29 AUC = 0.58 # | p (t-test) = 0.48 AUC = 0.53 | p (U-test) = 0.38 AUC = 0.57 | p (U-test) = 0.79 AUC = 0.52 | p (t-test) = 0.52 AUC = 0.54 | ||||||||||
| GCL_IN | IPL_IN | INL_ON | INL_II | |
|---|---|---|---|---|
| GCL_IN | 1 | 0.938 p < 0.01 | 0.184 p = 0.16 | 0.36 p = 0.005 |
| IPL_IN | -- | 1 | 0.25 p = 0.056 | 0.46 p < 0.01 |
| INL_ON | -- | -- | 1 | 0.63 p < 0.01 |
| INL_II | -- | -- | -- | 1 |
| Classifier | Input Features | Accuracy | AUC | |||
|---|---|---|---|---|---|---|
| GCL_IN | IPL_IN | INL_ON | INL_II | |||
| Gaussian Naive Bayes | X | X | X | X | 0.89 | 0.91 |
| KNN (k = 3, Euclidean) | X | X | X | 0.92 | 0.95 | |
| KNN (k = 3, Cubic) | X | X | X | 0.92 | 0.95 | |
| KNN (k = 3, Cosine) | X | X | X | 0.89 | 0.95 | |
| KNN (k = 3, Weighted) | X | X | X | 0.89 | 0.92 | |
| SVM (Linear, C = 2) | X | X | X | 0.95 | 0.97 | |
| SVM (Quadratic, p = 2, C = 2) | X | X | 0.89 | 0.90 | ||
| SVM (, C = 2) | X | X | X | 0.87 | 0.92 | |
| SVM (, C = 2) | X | X | 0.92 | 0.92 | ||
| Predicted Control | Predicted BD | |
|---|---|---|
| Actual control | 40 | 2 |
| Actual BD | 1 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Morla, E.M.; Fuentes, J.L.; Miguel-Jiménez, J.M.; Boquete, L.; Ortiz, M.; Orduna, E.; Satue, M.; Garcia-Martin, E. Automatic Diagnosis of Bipolar Disorder Using Optical Coherence Tomography Data and Artificial Intelligence. J. Pers. Med. 2021, 11, 803. https://doi.org/10.3390/jpm11080803
Sánchez-Morla EM, Fuentes JL, Miguel-Jiménez JM, Boquete L, Ortiz M, Orduna E, Satue M, Garcia-Martin E. Automatic Diagnosis of Bipolar Disorder Using Optical Coherence Tomography Data and Artificial Intelligence. Journal of Personalized Medicine. 2021; 11(8):803. https://doi.org/10.3390/jpm11080803
Chicago/Turabian StyleSánchez-Morla, Eva M., Juan L. Fuentes, Juan M. Miguel-Jiménez, Luciano Boquete, Miguel Ortiz, Elvira Orduna, María Satue, and Elena Garcia-Martin. 2021. "Automatic Diagnosis of Bipolar Disorder Using Optical Coherence Tomography Data and Artificial Intelligence" Journal of Personalized Medicine 11, no. 8: 803. https://doi.org/10.3390/jpm11080803
APA StyleSánchez-Morla, E. M., Fuentes, J. L., Miguel-Jiménez, J. M., Boquete, L., Ortiz, M., Orduna, E., Satue, M., & Garcia-Martin, E. (2021). Automatic Diagnosis of Bipolar Disorder Using Optical Coherence Tomography Data and Artificial Intelligence. Journal of Personalized Medicine, 11(8), 803. https://doi.org/10.3390/jpm11080803







