Implementation of Routine Endoscopy with Narrow Band Imaging in the Evaluation of Oral and Upper Airways Lesions in Oral Chronic Graft-Versus-Host Disease: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
Study Population and Patients Management
3. Results
4. Discussion
4.1. How to Manage and Stage GVHD?
4.2. Therapies Cause Cancer?
4.3. What about Risk Factors?
4.4. Why NBI?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piccin, A.; Tagnin, M.; Vecchiato, C.; Al-Khaffaf, A.; Beqiri, L.; Kaiser, C.; Agreiter, I.; Negri, G.; Kob, M.; Di Pierro, A.; et al. Graft-versus-host disease (GvHD) of the tongue and of the oral cavity: A large retrospective study. Int. J. Hematol. 2018, 108, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Ruutu, T.; Gratwohl, A.; de Witte, T.; Afanasyev, B.; Apperley, J.; Bacigalupo, A.; Dazzi, F.; Dreger, P.; Duarte, R.; Finke, J.; et al. Prophylaxis and treatment of GVHD: EBMT–ELN working group recommendations for a standardized practice. Bone Marrow Transplant. 2014, 49, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Pavletic, S.Z.; Fowler, D.H. Are we making progress in GVHD prophylaxis and treatment? Hematol. Am. Soc. Hematol. Educ. Program. 2012, 2012, 251–264. [Google Scholar] [CrossRef]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef]
- Fall-Dickson, J.M.; Pavletic, S.Z.; Mays, J.W.; Schubert, M.M. Oral Complications of Chronic Graft-Versus-Host Disease. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz007. [Google Scholar] [CrossRef]
- Travnik, R.; Beckers, M.; Wolff, D.; Holler, E.; Landthaler, M.; Karrer, S. Graft-versus-Host Disease (GvHD)—An update: Part 1: Pathophysiology, clinical features and classification of GvHD. Hautarzt 2011, 62, 139–155. [Google Scholar] [CrossRef]
- Martu, M.A.; Maftei, G.A.; Luchian, I.; Popa, C.; Filioreanu, A.M.; Tatarciuc, D.; Nichitean, G.; Hurjui, L.-L.; Foia, L.-G. Wound healing of periodontal and oral tissues: Part II—Patho-phisiological conditions and metabolic diseases. Rom. J. Oral Rehabil. 2020, 12, 30–40. [Google Scholar]
- Kim, D.; Won, H.H.; Su, S.; Cheng, L.; Xu, W.; Hamad, N.; Uhm, J.; Gupta, V.; Kuruvilla, J.; Messner, H.A.; et al. Risk stratification of organ-specific GVHD can be improved by single-nucleotide polymorphism-based risk models. Bone Marrow Transplant. 2014, 49, 649–656. [Google Scholar] [CrossRef]
- Fall-Dickson, J.M.; Mitchell, S.A.; Marden, S.; Ramsay, E.S.; Guadagnini, J.P.; Wu, T.; St John, L.; Pavletic, S.Z. National Institutes of Health Chronic Graft-versus-Host Disease Study Group. Oral symptom intensity, health-related quality of life, and correlative salivary cytokines in adult survivors of hematopoietic stem cell transplantation with oral chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2010, 16, 948–956. [Google Scholar] [CrossRef]
- Treister, N.S.; Schubert, M.S.; Fall-Dickson, J.M. Oral chronic graft vs host disease. In Chronic Graft versus Host Disease: Interdisciplinary Management; Vogelsang, G.B., Pavletic, S., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 182–198. [Google Scholar]
- Schubert, M.M.; Sullivan, K.M.; Morton, T.H.; Izutsu, K.T.; Peterson, D.E.; Flournoy, N.; Truelove, E.L.; Sale, G.E.; Buckner, C.D.; Storb, R.; et al. Oral manifestations of chronic graft-v-host disease. Arch. Intern. Med. 1984, 144, 1591–1595. [Google Scholar] [CrossRef]
- Castellarin, P.; Stevenson, K.; Biasotto, M.; Yuan, A.; Woo, S.B.; Treister, N.S. Extensive dental caries in patients with oral chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2012, 18, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Curtis, R.E.; Rowlings, P.A.; Deeg, H.J.; Shriner, D.A.; Socíe, G.; Travis, L.B.; Horowitz, M.M.; Witherspoon, R.P.; Hoover, R.N.; Sobocinski, K.A.; et al. Solid cancers after bone marrow transplantation. N. Engl. J. Med. 1997, 336, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.M.; Sy, S.; Louie, P.; Ugarte-Torres, A.; Berka, N.; Sinclair, G.D.; Stewart, D.A.; Russell, J.A.; Storek, J. Genomic instability after allogeneic hematopoietic cell transplantation is frequent in oral mucosa, particularly in patients with a history of chronic graft-versus-host disease, and rare in nasal mucosa. Blood 2010, 116, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Chabrillac, E.; Dupret-Bories, A.; Vairel, B.; Woisard, V.; De Bonnecaze, G.; Vergez, S. Narrow-Band Imaging in oncologic otorhinolaryngology: State of the art. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 138, 451–458. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Lee, J.; Hwang, S.H. Narrow-band imaging for screening of oral premalignant or cancerous lesions: A systematic review and meta-analysis. Clin. Otolaryngol. 2021, 46, 501–507. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Efficacy of non-invasive diagnostic methods in the diagnosis and screening of oral cancer and precancer. Braz. J. Otorhinolaryngol. 2021. [CrossRef]
- Takano, J.H.; Yakushiji, T.; Kamiyama, I.; Nomura, T.; Katakura, A.; Takano, N.; Shibahara, T. Detecting early oral cancer: Narrowband imaging system observation of the oral mucosa microvasculature. Int. J. Oral Maxillofac. Surg. 2010, 39, 208–213. [Google Scholar] [CrossRef]
- Tirelli, G.; Marcuzzo, A.V.; Boscolo Nata, F. Narrow-band imaging pattern classification in oral cavity. Oral Dis. 2018, 24, 1458–1467. [Google Scholar] [CrossRef]
- Piazza, C.; Del Bon, F.; Paderno, A.; Grazioli, P.; Perotti, P.; Barbieri, D.; Majorana, A.; Bardellini, E.; Peretti, G.; Nicolai, P. The diagnostic value of narrow band imaging in different oral and oropharyngeal subsites. Eur. Arch. Otorhinolaryngol. 2016, 273, 3347–3353. [Google Scholar] [CrossRef]
- Tirelli, G.; Piovesana, M.; Gatto, A.; Torelli, L.; Di Lenarda, R.; Boscolo Nata, F. NBI utility in the pre-operative and intra-operative assessment of oral cavity and oropharyngeal carcinoma. Am. J. Otolaryngol. 2017, 38, 65–71. [Google Scholar] [CrossRef]
- Deganello, A.; Paderno, A.; Morello, R.; Fior, M.; Berretti, G.; Del Bon, F.; Alparone, M.; Bardellini, E.; Majorana, A.; Nicolai, P. Diagnostic Accuracy of Narrow Band Imaging in Patients with Oral Lichen Planus: A Prospective Study. Laryngoscope 2021, 131, E1156–E1161. [Google Scholar] [CrossRef] [PubMed]
- Bulfamante, A.M.; D’Agostino Fiorenza, U.; Castellarin, P.; Pipolo, C.; Cacioppo, G.; Felisati, G.; Saibene, A.M. Identification of putative laryngeal and pharyngeal lichen planus lesions: An endoscopic preliminary evaluation in 16 patients. Clin. Otolaryngol. 2021, 46, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Boscolo Nata, F.; Tirelli, G.; Capriotti, V.; Marcuzzo, A.V.; Sacchet, E.; Šuran-Brunelli, A.N.; de Manzini, N. NBI utility in oncologic surgery: An organ by organ review. Surg. Oncol. 2021, 36, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Piazza, C.; Cocco, D.; Del Bon, F.; Mangili, S.; Nicolai, P.; Majorana, A.; Bolzoni Villaret, A.; Peretti, G. Narrow band imaging and high definition television in evaluation of oral and oropharyngeal squamous cell cancer: A prospective study. Oral Oncol. 2010, 46, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Boscolo Nata, F.; Gardenal, N.; Giudici, F.; Tirelli, G. The role of NBI with flexible video-endoscope in the follow-up of head and neck cancer patients: A prospective study. Eur. Arch. Otorhinolaryngol. 2022, 279, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Mays, J.W.; Fassil, H.; Edwards, D.A.; Pavletic, S.Z.; Bassim, C.W. Oral chronic graft-versus-host disease: Current pathogenesis, therapy, and research. Oral Dis. 2013, 19, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Flowers, M.E.; Inamoto, Y.; Carpenter, P.A.; Lee, S.J.; Kiem, H.P.; Petersdorf, E.W.; Pereira, S.E.; Nash, R.A.; Mielcarek, M.; Fero, M.L.; et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 2011, 117, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Filipovich, A.H.; Weisdorf, D.; Pavletic, S.; Socie, G.; Wingard, J.R.; Lee, S.J.; Martin, P.; Chien, J.; Przepiorka, D.; Couriel, D.; et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant. 2005, 11, 945–956. [Google Scholar] [CrossRef]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e381. [Google Scholar] [CrossRef]
- Schoemans, H.M.; Lee, S.J.; Ferrara, J.L.; Wolff, D.; Levine, J.E.; Schultz, K.R.; Shaw, B.E.; Flowers, M.E.; Ruutu, T.; Greinix, H.; et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018, 53, 1401–1415. [Google Scholar] [CrossRef]
- Browning, B.; Thormann, K.; Seshadri, R.; Duerst, R.; Kletzel, M.; Jacobsohn, D.A. Weight loss and reduced body mass index: A critical issue in children with multiorgan chronic graft-versus-host disease. Bone Marrow Transplant. 2006, 37, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.D.; Van Nostran, W. Assessing pain intensity with the visual analog scale: A plea for uniformity. J. Clin. Pharmacol. 2014, 54, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Bassim, C.W.; Fassil, H.; Mays, J.W.; Edwards, D.; Baird, K.; Steinberg, S.M.; Cowen, E.W.; Naik, H.; Datiles, M.; Stratton, P.; et al. Oral disease profiles in chronic graft versus host disease. J. Dent. Res. 2015, 94, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Wolff, D.; Kitko, C.; Koreth, J.; Inamoto, Y.; Jagasia, M.; Pidala, J.; Olivieri, A.; Martin, P.J.; Przepiorka, D.; et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 984–999. [Google Scholar] [CrossRef]
- Treister, N.S.; Stevenson, K.; Kim, H.; Woo, S.B.; Soiffer, R.; Cutler, C. Oral chronic graft-versus-host disease scoring using the NIH consensus criteria. Biol. Blood Marrow Transplant. 2010, 16, 108–114. [Google Scholar] [CrossRef][Green Version]
- Schubert, M.M.; Williams, B.E.; Lloid, M.E.; Donaldson, G.; Chapko, M.K. Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Development of an oral mucositis index. Cancer 1992, 69, 2469–2477. [Google Scholar] [CrossRef]
- Kolb, H.J.; Socié, G.; Duell, T.; Van Lint, M.T.; Tichelli, A.; Apperley, J.F.; Nekolla, E.; Ljungman, P.; Jacobsen, N.; van Weel, M.; et al. Malignant Neoplasms in Long-Term Survivors of Bone Marrow Transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann. Intern. Med. 1999, 131, 738–744. [Google Scholar] [CrossRef]
- Park, M.J.; Roh, J.L.; Choi, S.H.; Nam, S.Y.; Kim, S.Y.; Lee, Y.S. De novo head and neck cancer arising in solid organ transplantation recipients: The Asan Medical Center experience. Auris Nasus Larynx 2018, 45, 838–845. [Google Scholar] [CrossRef]
- Preciado, D.A.; Matas, A.; Adams, G.L. Squamous cell carcinoma of the head and neck in solid organ transplant recipients. Head Neck 2002, 24, 319–325. [Google Scholar] [CrossRef]
- Douglas, C.M.; Jethwa, A.R.; Hasan, W.; Liu, A.; Gilbert, R.; Goldstein, D.; De Almedia, J.; Lipton, J.; Irish, J.C. Long-term survival of head and neck squamous cell carcinoma after bone marrow transplant. Head Neck 2020, 42, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Gyurkocza, B.; Sandmaier, B.M. Conditioning regimens for hematopoietic cell transplantation: One size does not fit all. Blood 2014, 124, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Deeg, H.J.; Maris, M.B.; Scott, B.L.; Warren, E.H. Optimization of allogeneic transplant conditioning: Not the time for dogma. Leukemia 2006, 20, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Sandmaier, B.M.; Mackinnon, S.; Childs, R.W. Reduced intensity conditioning for allogeneic hematopoietic cell transplantation: Current perspectives. Biol. Blood Marrow Transplant. 2007, 13 (Suppl. S1), 87–97. [Google Scholar] [CrossRef][Green Version]
- Alyea, E.P.; Kim, H.T.; Ho, V.; Cutler, C.; Gribben, J.; DeAngelo, D.J.; Lee, S.J.; Windawi, S.; Ritz, J.; Stone, R.M.; et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood 2005, 105, 1810–1814. [Google Scholar] [CrossRef]
- Champlin, R.; Khouri, I.; Shimoni, A.; Gajewski, J.; Kornblau, S.; Molldrem, J.; Ueno, N.; Giralt, S.; Anderlini, P. Harnessing graft-versus-malignancy: Non-myeloablative preparative regimens for allogeneic haematopoietic transplantation, an evolving strategy for adoptive immunotherapy. Br. J. Haematol. 2000, 111, 18–29. [Google Scholar] [CrossRef]
- Socié, G.; Curtis, R.E.; Deeg, H.J.; Sobocinski, K.A.; Filipovich, A.H.; Travis, L.B.; Sullivan, K.M.; Rowlings, P.A.; Kingma, D.W.; Banks, P.M.; et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J. Clin. Oncol. 2000, 18, 348–357. [Google Scholar] [CrossRef]
- Kruse, A.L.; Grätz, K.W. Oral carcinoma after hematopoietic stem cell transplantation—A new classification based on a literature review over 30 years. Head Neck Oncol. 2009, 1, 29. [Google Scholar] [CrossRef]
- Demarosi, F.; Soligo, D.; Lodi, G.; Moneghini, L.; Sardella, A.; Carrassi, A. Squamous cell carcinoma of the oral cavity associated with graft versus host disease: Report of a case and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2005, 100, 63–69. [Google Scholar] [CrossRef]
- Otsubo, H.; Yokoe, H.; Miya, T.; Atsuta, F.; Miura, N.; Tanzawa, H.; Sato, K. Gingival squamous cell carcinoma in a patient with chronic graft-versus-host disease. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1997, 84, 171–174. [Google Scholar] [CrossRef]
- Kruse, A.L.; Bredell, M.; Grätz, K.W. Oral squamous cell carcinoma in non-smoking and non-drinking patients. Head Neck Oncol. 2010, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Filioreanu, A.M.; Stelea, C.; Alexandru Maftei, G.A.; Popescu, E. Prevalence of oral lesions modulated by patients age: The young versus the elderly. Rom. J. Oral Rehabil. 2018, 10, 50–56. [Google Scholar]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Epstein, J.B.; Poh, C.F.; Berean, K.; Lam, W.L.; Zhang, X.; Rosin, M.P. Comparison of HPV infection, p53 mutation and allelic losses in post-transplant and non-posttransplant oral squamous cell carcinomas. J. Oral Pathol. Med. 2002, 31, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.R.; Hoffman, H.T.; Wolf, G.T.; Carey, T.E.; Baker, S.R.; McClatchey, K.D. Squamous carcinoma of the head and neck in organ transplant recipients: Possible role of oncogenic viruses. Laryngoscope 1990, 100 Pt 1, 190–194. [Google Scholar] [CrossRef]






| Patient | Sex | Age | Relevant Comorbidities | Alcohol Consumption and Smoking Habits |
|---|---|---|---|---|
| 1 | F | 67 | Gastric resection for gastric cancer; genital recalcitrant herpes zoster virus infection. | Non-smoker, no alcohol consumption. |
| 2 | M | 39 | Tongue cancer in 2017 and 2020, left tonsillar cancer in 2019, follicular thyroid cancer treated with right hemithyroidectomy. | Non-smoker, no alcohol consumption. |
| 3 | M | 52 | Polymyositis; encephalitis in 2018; squamous cells carcinoma of the lower lip in 2018 treated with surgery; Osteoporosis. | Non-smoker, no alcohol consumption. |
| 4 | M | 49 | Chronic gastritis. | Former smoker, no alcohol consumption. |
| 5 | F | 49 | Hashimoto’s thyroiditis; paroxysmal supraventricular tachycardia; cerebral arteriovenous malformation; major depressive disease. | Non-smoker, no alcohol consumption. |
| 6 | F | 55 | Chronic gastritis; hypertension; abdominal aortic aneurysm. | Active smoker, no alcohol consumption. |
| 7 | F | 67 | Hypertension; right upper lobe lung adenocarcinoma in follow-up; osteoporosis; chemotherapy-treated recurrent colon adenocarcinoma | Non-smoker, no alcohol consumption. |
| 8 | F | 69 | Glucose-6-phosphate dehydrogenase (G6PD) deficiency | Non-smoker no alcohol consumption. |
| 9 | F | 23 | No other comorbidities. | Non-smoker, no alcohol consumption. |
| 10 | F | 72 | Hypertension | Active smoker, no alcohol consumption. |
| 11 | M | 34 | No other comorbidities. | Non-smoker, no alcohol consumption. |
| 12 | M | 50 | No other comorbidities. | Active smoker, no alcohol consumption. |
| 13 | M | 49 | Anxious depressive disorder. | Non-smoker, no alcohol consumption. |
| 14 | M | 39 | Low grade glioma treated with surgery in 2005. | Non-smoker, no alcohol consumption. |
| 15 | M | 62 | Hypertension | Non-smoker, no alcohol consumption. |
| 16 | M | 67 | No other comorbidities. | Non-smoker, no alcohol consumption. |
| 17 | F | 41 | Bilateral sensorineural hearing loss induced by chemotherapy drugs | Non-smoker, no alcohol consumption. |
| 18 | F | 63 | Acute kidney failure in 1970; Hashimoto’s thyroiditis; Major depressive disorder; chronic intestinal polyposis. | Non-smoker, no alcohol consumption. |
| 19 | F | 31 | No other comorbidities. | Non-smoker, no alcohol consumption. |
| 20 | M | 44 | No other comorbidities. | Non-smoker, no alcohol consumption. |
| Diagnosis | CLL | 1 |
| ALL | 4 | |
| CML | 9 | |
| MFI | 1 | |
| HL | 1 | |
| NHL | 2 | |
| PCL | 1 | |
| AML | 1 | |
| Type of Stem Cell Donor | MUD | 16 |
| Haploidentical | 2 | |
| Related | 2 | |
| Conditioning Therapy | ChT RIC | 10 |
| ChT MAC | 7 | |
| ChT + RT MAC | 3 | |
| Acute GVHD | Yes | 7 |
| No | 13 | |
| Chronic Oral GVHD | Yes | 20 |
| No | / | |
| Other cGHVD Sites | ocular | 10 |
| cutaneous | 5 | |
| pulmonary | 2 | |
| articular | 3 | |
| hepatic | 3 | |
| genital | 1 | |
| muscular | 2 | |
| none | 7 | |
| Average Age at Allogeneic-HSTC (years ± DS) | 51.1 ± 14 | |
| Median Time between HSCT and Development of Oral GVHD (months) | 11 |
| Patient | Localization | Oral Examination | Endoscopic Findings | Narrow Band Imaging | Histology |
|---|---|---|---|---|---|
| 1 | Buccal mucosa, gingiva, tongue body, bilateral labial commissure | Diffuse mucositis with erosive areas in the mucosa of the oral cavity. No erosive lesions of the tongue. | Diffuse mucosal erosive lesions without suspicion of malignancy. | No evidence of intrapapillary capillary loops | // |
| 2 | Buccal mucosa bilaterally and left tonsillar pillar with extension at amygdalo-glossus sulcus and oropharynx | Erythroplakia paired with central erosive area | Diffuse mucositis and evidence of erythroplakia with erosive central area suspicious for malignant disease | Evidence of intrapapillary capillary loops | Squamocellular carcinoma of the oropharynx. |
| 3 | Right buccal mucosa and lateral right surface of the tongue. | Leukoplakia of the right surface of the tongue with an indurated area in the lower portion. | Reticular lichenoid-like lesions on the right buccal mucosa without suspicious of malignancy | Evidence of intrapapillary capillary loops. | Epitelial verrucous hyperplasia |
| 4 | Bilateral buccal mucosa. | Reticular lesions with scleroatrophic changes of the mucosa. | Reticular lichenoid-like changes of the buccal mucosa bilaterally without suspicion of malignancy. | No evidence of intrapapillary capillary loops. | // |
| 5 | Bilateral buccal mucosa | Reticular lesions of the mucosa and evidence at buccal mucosa of the right side of small ulceration. | Lichenoid-like changes with an erosive area at the right buccal mucosa non-suspicious for malignancy | No evidence of intrapapillary capillary loops. | // |
| 6 | Oral vestibular mucosa, buccal mucosa, hard and soft palate. | Exophytic lesions with lichenoid-like changes on buccal mucosa and oral vestibular mucosa. | Lichenoid-like lesions on hard, soft palate and on oral pelvis without suspicious of malignancy | No evidence of intrapapillary capillary loops. | // |
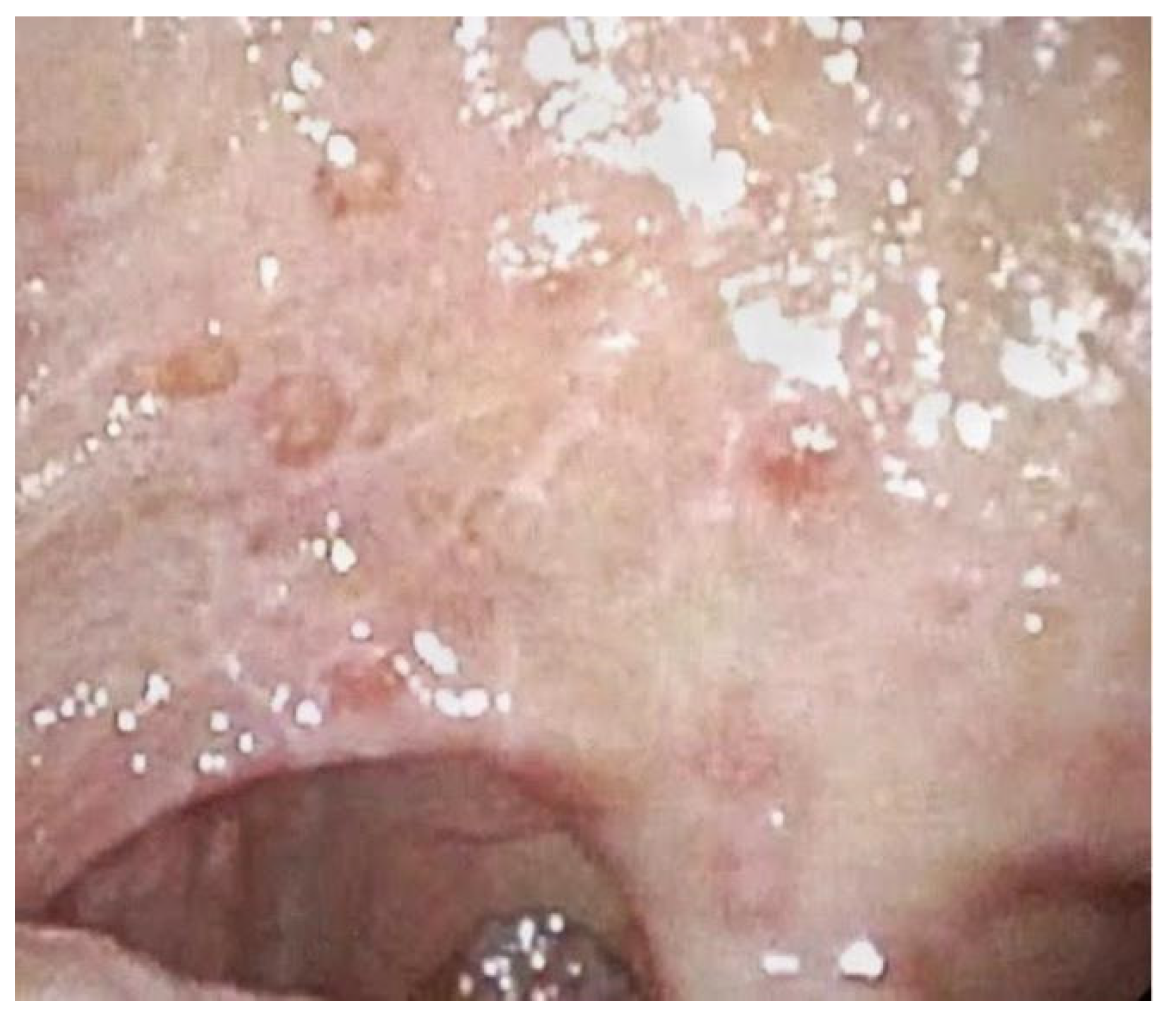
| 7 | Bilateral buccal mucosa and tongue. | Ulcerative lesion on the left buccal mucosa with augmented peripheral vascularization. Erythematous appearance of the tongue with inflammation and edema of the lingual papillae. | Evidence of ulcerative lesion of the left buccal mucosa with erythroplasia-like changes and abnormal vascularization on its margins. | Evidence of dark spots on the border of the ulcerative lesion of the left buccal mucosa. | Delayed because of recurrence of intestinal adenocarcinoma. |
| 8 | Buccal mucosa, tongue, adherent gingiva, and hard palate. | Inflammation of the adherent gingiva with diffuse mucositis. | Erythematous lesions on tongue surface and buccal mucosa without suspicious of malignancy | No evidence of intrapapillary capillary loops. | // |
| 9 | Buccal mucosa and hard/soft palate | Blisters on hard and soft palate. | Lichenoid changes of the buccal mucosa bilaterally without suspicious of malignancy | No evidence of intrapapillary capillary loops. | // |
| 10 | Tongue, buccal mucosa, hard and soft palate. | Erosive lesions on the right buccal mucosa and on the left tongue lateral surface. | Diffuse erosive lesions on buccal mucosa bilaterally, lateral tongue surface, and lichenoid changes on the hard and soft palate. No suspicious of malignancy | No evidence of intrapapillary capillary loops. | // |
| 11 | Oral vestibular mucosa and buccal mucosa | Edema of the vestibular and buccal mucosa with florid sclero-atrophic lesions on cheek mucosa bilaterally | Diffuse lichenoid-like lesions of the oral cavity, no involvement of the tongue. No suspicious of malignancy | No evidence of intrapapillary capillary loops. | // |
| 12 | Oropharynx, buccal mucosa, and hard and soft palate | No evidence of lesions, diffuse hyperemia of the oral cavity | Diffuse mucositis of the oral cavity and oropharynx without suspicious of malignancy | No evidence of intrapapillary capillary loops | // |
| 13 | Oropharynx and buccal mucosa | Mucositis of the oral cavity and oropharynx with reticular lesions on buccal mucosa bilaterally. | Lichenoid-like chances of the mucosa without suspicion of malignancy. | No evidence of intrapapillary capillary loops | // |
| 14 | Buccal mucosa and hard palate | Diffuse mucositis of the oral cavity | Mucositis with hyperplasia of the buccal mucosa and hard palate bilaterally without suspicious of malignancy. | No evidence of intrapapillary capillary loops | // |
| 15 | Tongue and buccal mucosa | Glossitis and evidence of reticular lesions localized on buccal mucosa bilaterally | Lichenoid-like chances of the mucosa without suspicion of malignancy. | No evidence of intrapapillary capillary loops | // |
| 16 | Buccal mucosa and tongue base. | Ulcerative lesions localized at buccal mucosa bilaterally. Fungal infection at the tongue base. | No suspicious lesions. | No evidence of intrapapillary capillary loops | // |
| 17 | Buccal mucosa and oropharynx | Xerostomia and mucositis. | Enhanced vascularization pattern on buccal mucosa bilaterally without suspicious of malignancy | No evidence of intrapapillary capillary loops. | // |
| 18 | Buccal mucosa and hard palate | Erosive lesions on buccal mucosa bilaterally and hard palate | No suspicion of malignancy. | No evidence of intrapapillary capillary loops | // |
| 19 | Buccal mucosa and vestibular mucosa. | Diffuse blisters on the mucosa of the oral cavity and diffuse mucositis. | Demucosized area at left buccal mucosa without suspicious of malignancy. | No evidence of intrapapillary capillary loops | // |
| 20 | Buccal mucosa | Mucositis and lichenoid changing of the buccal mucosa bilaterally. | Bilateral erosive areas at cheek mucosa (major extension at left) without suspicious of malignancy. | No evidence of intrapapillary capillary loops | // |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitro, L.; Pipolo, C.; Castellarin, P.; Sardella, A.; Bulfamante, A.M.; De Marco, B.; Magliano, G.; Grillo, G.; Felisati, G.; Saibene, A.M. Implementation of Routine Endoscopy with Narrow Band Imaging in the Evaluation of Oral and Upper Airways Lesions in Oral Chronic Graft-Versus-Host Disease: A Preliminary Study. J. Pers. Med. 2022, 12, 1628. https://doi.org/10.3390/jpm12101628
Nitro L, Pipolo C, Castellarin P, Sardella A, Bulfamante AM, De Marco B, Magliano G, Grillo G, Felisati G, Saibene AM. Implementation of Routine Endoscopy with Narrow Band Imaging in the Evaluation of Oral and Upper Airways Lesions in Oral Chronic Graft-Versus-Host Disease: A Preliminary Study. Journal of Personalized Medicine. 2022; 12(10):1628. https://doi.org/10.3390/jpm12101628
Chicago/Turabian StyleNitro, Letizia, Carlotta Pipolo, Paolo Castellarin, Andrea Sardella, Antonio Mario Bulfamante, Beatrice De Marco, Gabriele Magliano, Giovanni Grillo, Giovanni Felisati, and Alberto Maria Saibene. 2022. "Implementation of Routine Endoscopy with Narrow Band Imaging in the Evaluation of Oral and Upper Airways Lesions in Oral Chronic Graft-Versus-Host Disease: A Preliminary Study" Journal of Personalized Medicine 12, no. 10: 1628. https://doi.org/10.3390/jpm12101628
APA StyleNitro, L., Pipolo, C., Castellarin, P., Sardella, A., Bulfamante, A. M., De Marco, B., Magliano, G., Grillo, G., Felisati, G., & Saibene, A. M. (2022). Implementation of Routine Endoscopy with Narrow Band Imaging in the Evaluation of Oral and Upper Airways Lesions in Oral Chronic Graft-Versus-Host Disease: A Preliminary Study. Journal of Personalized Medicine, 12(10), 1628. https://doi.org/10.3390/jpm12101628






