Venous Wall of Patients with Chronic Venous Disease Exhibits a Glycolytic Phenotype
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Sample Processing
2.3. Analysis of Gene Expression Using RT-qPCR
2.4. Study of Protein Expression through Immunohistochemistry
2.5. Histological Observation and Statistical Analysis
3. Results
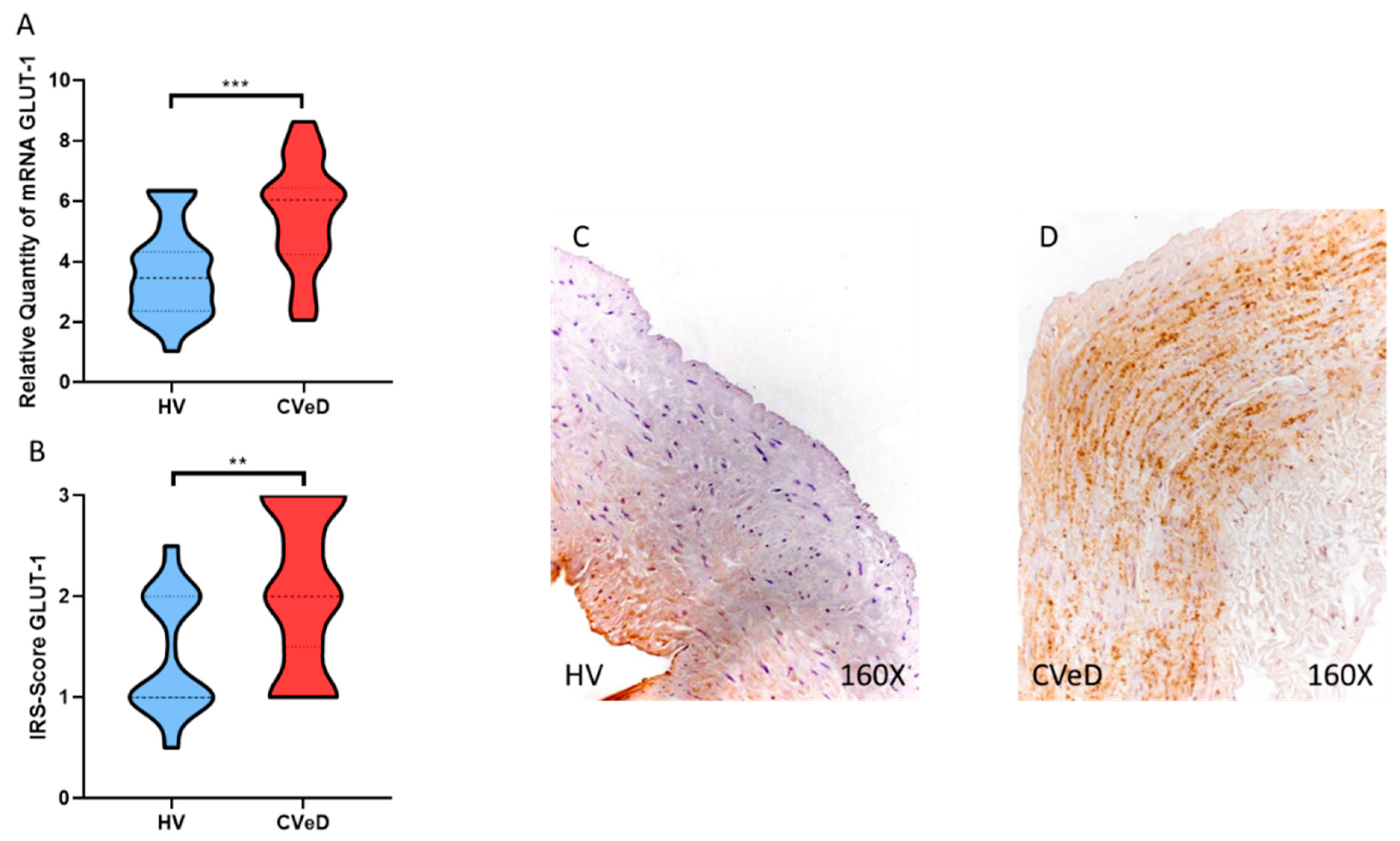
3.1. CVeD Patients Show Increased Expression of GLUT-1 in the Venous Wall
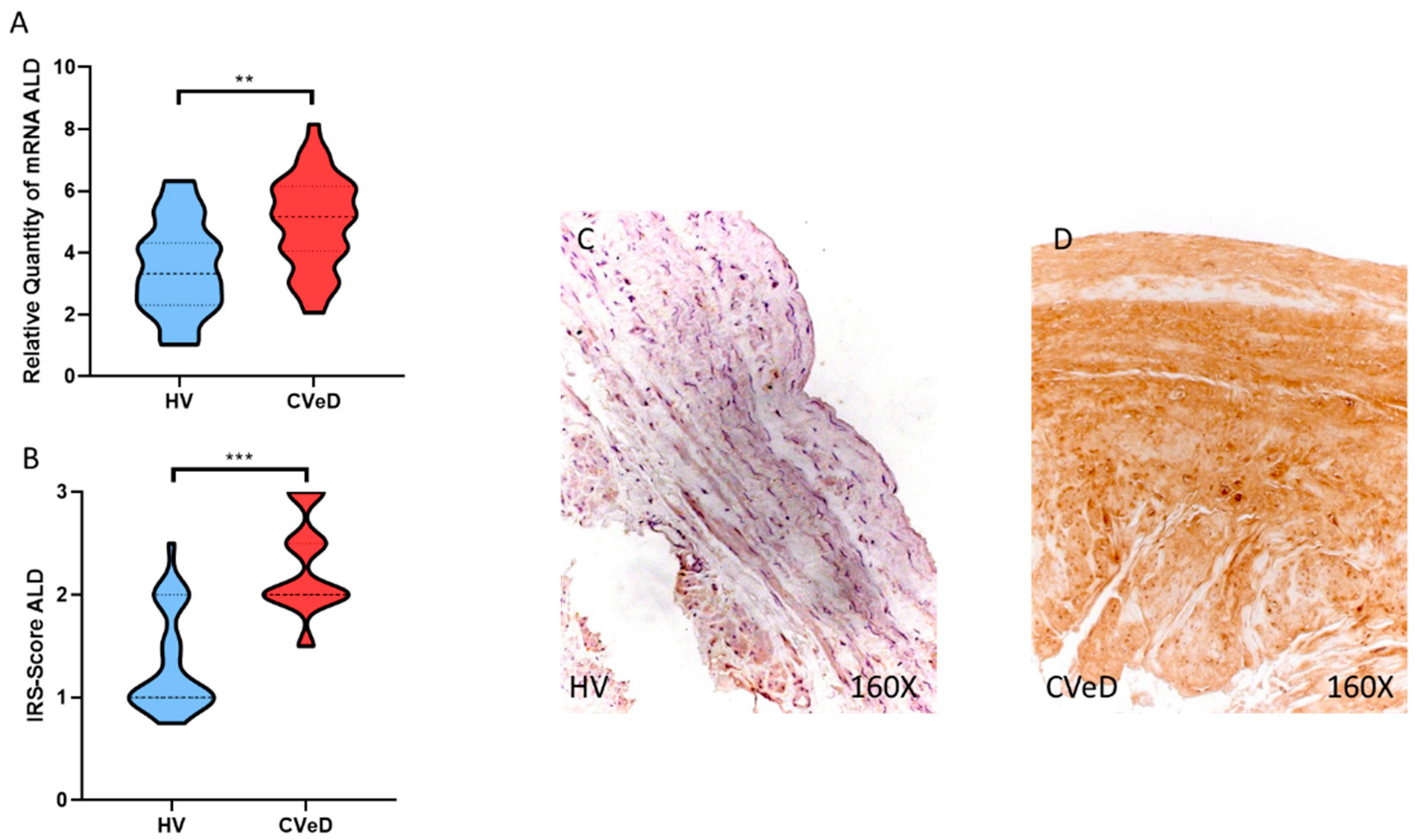
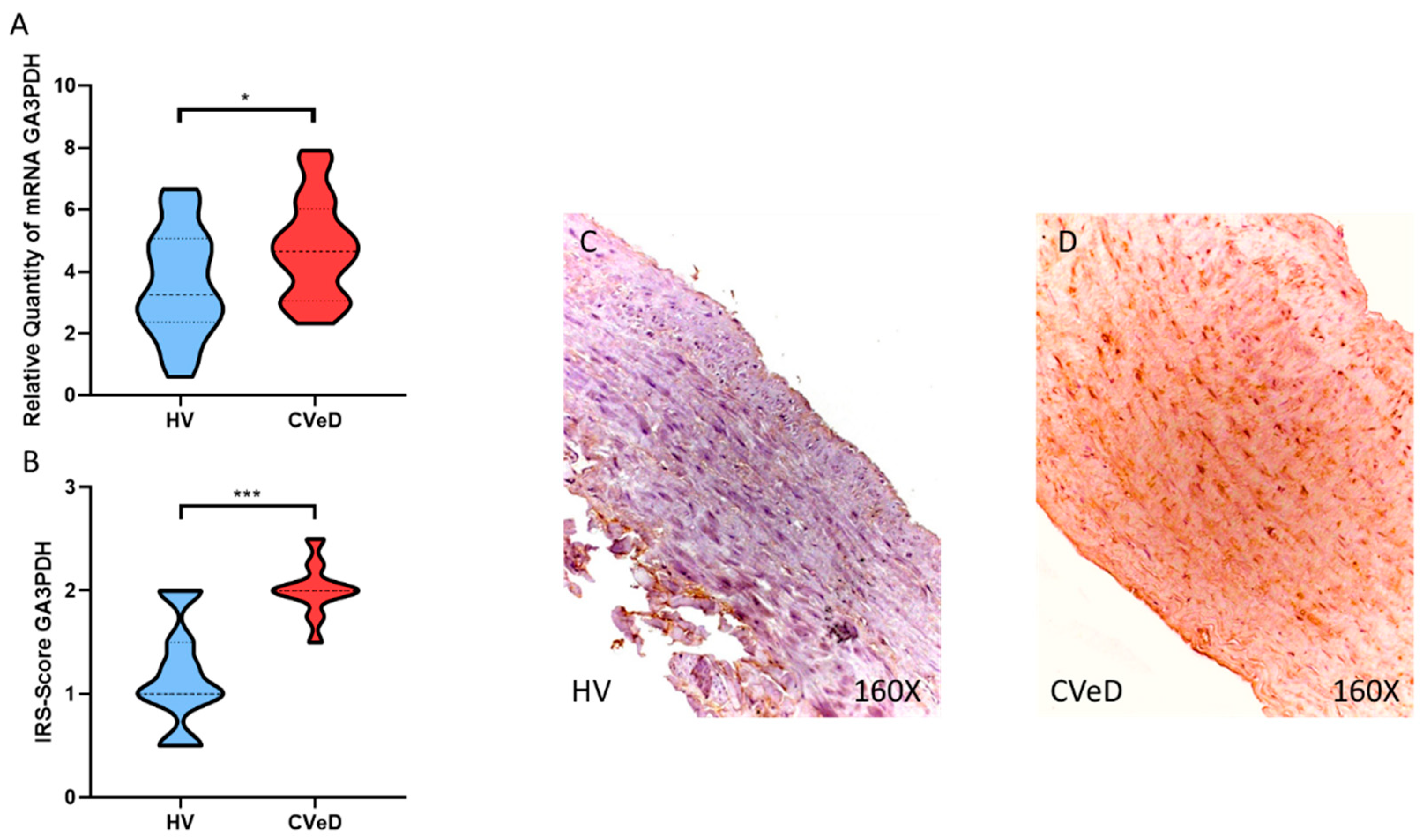
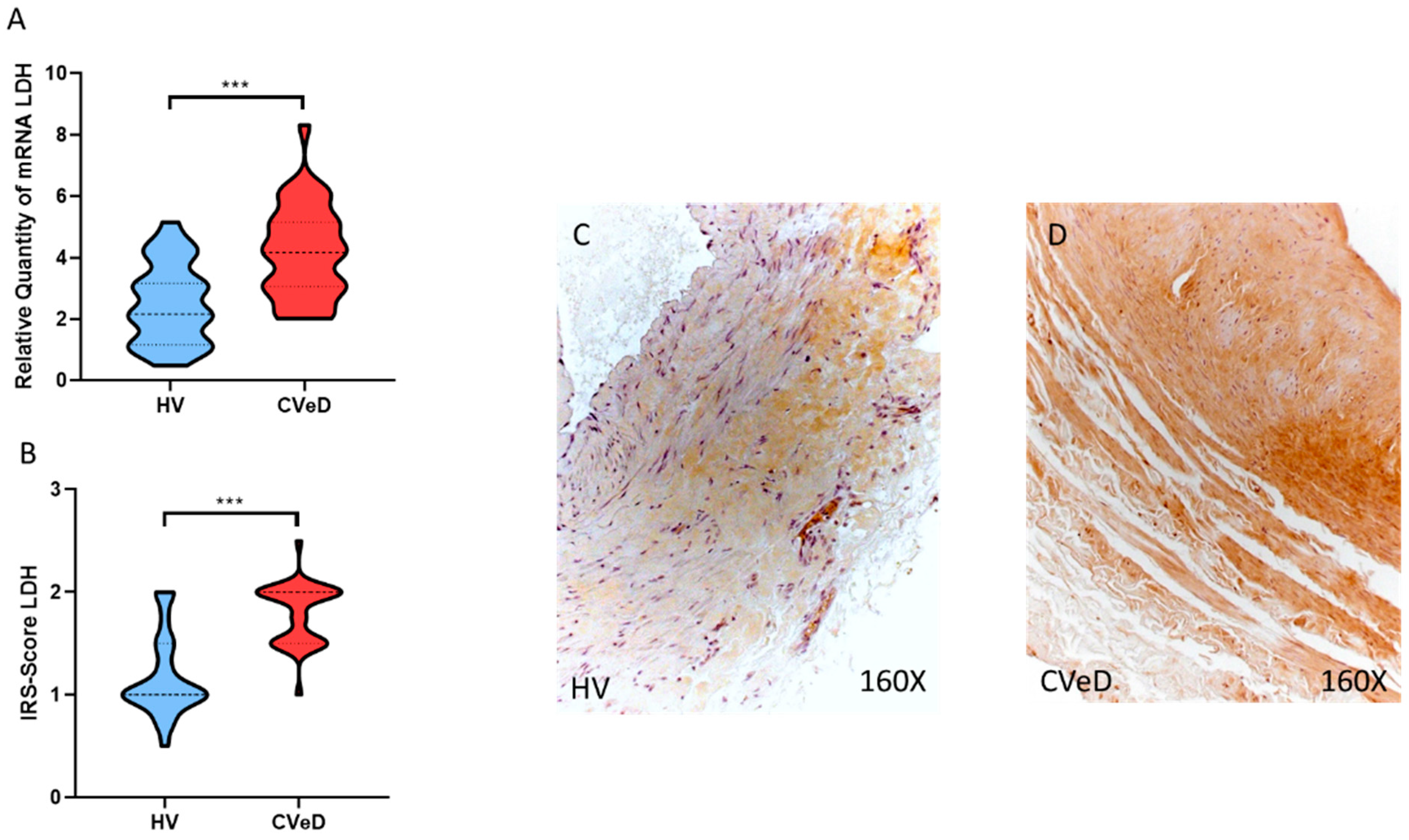
3.2. CVeD Patients Show Increased Expression of Glycolytic Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Imbernón-Moya, A.; Ortiz-de Frutos, F.J.; Sanjuan-Alvarez, M.; Portero-Sanchez, I. Chronic Venous Disease of Legs. Med. Clin. 2017, 148, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Rabe, E.; Guex, J.J.; Puskas, A.; Scuderi, A.; Fernandez Quesada, F.; Coordinators, V. Epidemiology of Chronic Venous Disorders in Geographically Diverse Populations: Results from the Vein Consult Program—PubMed. Int. Angiol. A J. Int. Union Angiol. 2012, 31, 105–115. [Google Scholar]
- Rabe, E.; Régnier, C.; Goron, F.; Salmat, G.; Pannier, F. The Prevalence, Disease Characteristics and Treatment of Chronic Venous Disease: An International Web-Based Survey. J. Comp. Eff. Res. 2020, 9, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Onida, S.; Davies, A.H. Predicted Burden of Venous Disease. Phlebology 2016, 31, 74–79. [Google Scholar] [CrossRef]
- Kim, Y.; Png, C.Y.M.; Sumpio, B.J.; DeCarlo, C.S.; Dua, A. Defining the Human and Health Care Costs of Chronic Venous Insufficiency. Semin. Vasc. Surg. 2021, 34, 59–64. [Google Scholar] [CrossRef]
- Lohr, J.M.; Bush, R.L. Venous Disease in Women: Epidemiology, Manifestations, and Treatment. J. Vasc. Surg. 2013, 57, 37S–45S. [Google Scholar] [CrossRef] [Green Version]
- Brand, F.N.; Dannenberg, A.L.; Abbott, R.D.; Kannel, W.B. The Epidemiology of Varicose Veins: The Framingham Study. Am. J. Prev. Med. 1988, 4, 96–101. [Google Scholar] [CrossRef]
- Vlajinac, H.D.; Radak, D.J.; Marinković, J.M.; Maksimović, M.Ž. Risk Factors for Chronic Venous Disease. Phlebology 2012, 27, 416–422. [Google Scholar] [CrossRef]
- Criqui, M.H.; Denenberg, J.O.; Bergan, J.; Langer, R.D.; Fronek, A. Risk Factors for Chronic Venous Disease: The San Diego Population Study. J. Vasc. Surg. 2007, 46, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Zolotukhin, I.A.; Seliverstov, E.I.; Shevtsov, Y.N.; Avakiants, I.P.; Nikishkov, A.S.; Tatarintsev, A.M.; Kirienko, A.I. Prevalence and Risk Factors for Chronic Venous Disease in the General Russian Population. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Raffetto, J.D.; Mannello, F. Pathophysiology of Chronic Venous Disease. Int. Angiol. 2014, 33, 212–221. [Google Scholar]
- Mansilha, A.; Sousa, J. Pathophysiological Mechanisms of Chronic Venous Disease and Implications for Venoactive Drug Therapy. Int. J. Mol. Sci. 2018, 19, 1669. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Ruiz-Grande, F.; Álvarez-Mon, M.A.; Monserrat, J.; Guijarro, L.G.; Coca, S.; Álvarez-Mon, M.; Bujan, J.; et al. Contribution of the Elastic Component and Venous Wall Arterialization in Patients with Venous Reflux. J. Pers. Med. 2022, 12, 12. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Pekarek, L.; Alvarez-Mon, M.A.; Asúnsolo, Á.; Sanchez-Trujillo, L.; Coca, S.; Buján, J.; Álvarez-Mon, M.; García-Honduvilla, N.; et al. Defective Expression of the Peroxisome Regulators PPARα Receptors and Lysogenesis with Increased Cellular Senescence in the Venous Wall of Chronic Venous Disorder. Histol. Histopathol. 2021, 36, 18322. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Ruiz-Grande, F.; Barrena, S.; Montoya, H.; Pekarek, L.; Zoullas, S.; Alvarez-Mon, M.A.; Sainz, F.; et al. Chronic Venous Disease Patients Show Increased IRS-4 Expression in the Great Saphenous Vein Wall. J. Int. Med. Res. 2021, 49. [Google Scholar] [CrossRef]
- Hossein Ghaderian, S.M.; Lindsey, N.J.; Graham, A.M.; Homer-Vanniasinkam, S.; Najar, R.A. Pathogenic Mechanisms in Varicose Vein Disease: The Role of Hypoxia and Inflammation. Pathology 2010, 42, 446–453. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Álvarez-Mon, M.A.; Chaowen, C.; Ruiz-Grande, F.; Pekarek, L.; Monserrat, J.; Asúnsolo, A.; García-Honduvilla, N.; et al. Understanding Chronic Venous Disease: A Critical Overview of Its Pathophysiology and Medical Management. J. Clin. Med. 2021, 10, 10. [Google Scholar] [CrossRef]
- Spiridon, M.; Corduneanu, D. Chronic Venous Insufficiency: A Frequently Underdiagnosed and Undertreated Pathology. Maedica 2017, 12, 59–61. [Google Scholar] [PubMed]
- Vuylsteke, M.E.; Thomis, S.; Guillaume, G.; Modliszewski, M.L.; Weides, N.; Staelens, I. Epidemiological Study on Chronic Venous Disease in Belgium and Luxembourg: Prevalence, Risk Factors, and Symptomatology. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Davies, A.H. The Seriousness of Chronic Venous Disease: A Review of Real-World Evidence—PubMed. Adv. Ther. 2019, 36 (Suppl. S1), 5–12. [Google Scholar] [CrossRef]
- Sritharan, K.; Lane, T.R.A.; Davies, A.H. The Burden of Depression in Patients with Symptomatic Varicose Veins. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 480–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, M.A.; Adesina-Georgiadis, K.N.; Spagou, K.; Vorkas, P.A.; Li, J.V.; Shalhoub, J.; Holmes, E.; Davies, A.H. A Comprehensive Characterisation of the Metabolic Profile of Varicose Veins; Implications in Elaborating Plausible Cellular Pathways for Disease Pathogenesis. Sci. Rep. 2017, 7, 2989. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, X.; Du, J.; Cui, Q.; Huang, Y.; Jin, H. Metabolic Reprogramming of Vascular Endothelial Cells: Basic Research and Clinical Applications. Front. Cell Dev. Biol. 2021, 9, 243. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Cheng, A.; Xu, G.; He, F. Metabolism of Vascular Smooth Muscle Cells in Vascular Diseases. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H613–H631. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.E.; Hanford, K.M.L.; Kroon, J. Vascular Metabolism as Driver of Atherosclerosis: Linking Endothelial Metabolism to Inflammation. Immunometabolism 2021, 3, e210020. [Google Scholar] [CrossRef]
- Li, X.; Kumar, A.; Carmeliet, P. Metabolic Pathways Fueling the Endothelial Cell Drive. Annu. Rev. Physiol. 2019, 81, 483–503. [Google Scholar] [CrossRef]
- Ortega, M.A.; Sáez, M.A.; Fraile-Martínez, O.; Álvarez-Mon, M.A.; García-Montero, C.; Guijarro, L.G.; Asúnsolo, Á.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; et al. Overexpression of Glycolysis Markers in Placental Tissue of Pregnant Women with Chronic Venous Disease: A Histological Study. Int. J. Med. Sci. 2022, 19, 186. [Google Scholar] [CrossRef]
- Kuo, C.J.; Liang, S.S.; Hsi, E.; Chiou, S.H.; Lin, S.D. Quantitative Proteomics Analysis of Varicose Veins: Identification of a Set of Differentially Expressed Proteins Related to ATP Generation and Utilization. Kaohsiung J. Med. Sci. 2013, 29, 594–605. [Google Scholar] [CrossRef] [Green Version]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; de Maeseneer, M.; et al. The 2020 Update of the CEAP Classification System and Reporting Standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef]
- Ortega, M.A.; Asúnsolo, Á.; Pekarek, L.; Alvarez-Mon, M.A.; Delforge, A.; Sáez, M.A.; Coca, S.; Sainz, F.; Mon, M.Á.; Buján, J.; et al. Histopathological Study of JNK in Venous Wall of Patients with Chronic Venous Insufficiency Related to Osteogenesis Process. Int. J. Med. Sci. 2021, 18, 1921–1934. [Google Scholar] [CrossRef]
- García-Honduvilla, N.; Asúnsolo, Á.; Ortega, M.A.; Sainz, F.; Leal, J.; Lopez-Hervas, P.; Pascual, G.; Buján, J. Increase and Redistribution of Sex Hormone Receptors in Premenopausal Women Are Associated with Varicose Vein Remodelling. Oxidative Med. Cell. Longev. 2018, 2018, 3974026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Vallone, P.M.; Butler, J.M. AutoDimer: A Screening Tool for Primer-Dimer and Hairpin Structures. Biotechniques 2004, 37, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, M.A.; Romero, B.; Asúnsolo, Á.; Martínez-Vivero, C.; Sainz, F.; Bravo, C.; de León-Luis, J.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Pregnancy-Associated Venous Insufficiency Course with Placental and Systemic Oxidative Stress. J. Cell. Mol. Med. 2020, 24, 4157–4170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanmartín-Salinas, P.; Guijarro, L.G. Overexpression of IRS-4 Correlates with Procaspase 3 Levels in Tumoural Tissue of Patients with Colorectal Cancer. J. Oncol. 2018, 2018, 3812581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, S.W.S.; Shi, Y. The Glycolytic Process in Endothelial Cells and Its Implications. Acta Pharm. Sin. 2022, 43, 251–259. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of Glycolysis by the Hypoxia-Inducible Factor (HIF): Implications for Cellular Physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1: Mediator of Physiological and Pathophysiological Responses to Hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.S.; Kiriakidis, S.; Paleolog, E.M.; Davies, A.H. Increased Activation of the Hypoxia-Inducible Factor Pathway in Varicose Veins. J. Vasc. Surg. 2012, 55, 1427–1439. [Google Scholar] [CrossRef] [Green Version]
- Ortega, M.A.; Asúnsolo, Á.; Leal, J.; Romero, B.; Alvarez-Rocha, M.J.; Sainz, F.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Implication of the PI3K/Akt/MTOR Pathway in the Process of Incompetent Valves in Patients with Chronic Venous Insufficiency and the Relationship with Aging. Oxidative Med. Cell. Longev. 2018, 2018, 1495170. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Yan, G.; Qiao, Y.; Zhu, B.; Liu, B.; Tang, C. Hypoxia Induces Lactate Secretion and Glycolytic Efflux by Downregulating Mitochondrial Pyruvate Carrier Levels in Human Umbilical Vein Endothelial Cells. Mol. Med. Rep. 2018, 18, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Ren, L.; Hamblin, M.H.; Fan, Y. Endothelial Cell Glucose Metabolism and Angiogenesis. Biomedicines 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Romero, B.; Asúnsolo, Á.; Sainz, F.; Martinez-Vivero, C.; Álvarez-Mon, M.; Buján, J.; Garc-a-Honduvilla, N. Behavior of Smooth Muscle Cells under Hypoxic Conditions: Possible Implications on the Varicose Vein Endothelium. BioMed Res. Int. 2018, 2018, 7156150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pragallapati, S.; Manyam, R. Glucose Transporter 1 in Health and Disease. J. Oral Maxillofac. Pathol. JOMFP 2019, 23, 443. [Google Scholar] [CrossRef]
- Adhikari, N.; Basi, D.L.; Carlson, M.; Mariash, A.; Hong, Z.; Lehman, U.; Mullegama, S.; Weir, E.K.; Hall, J.L. Increase in GLUT1 in Smooth Muscle Alters Vascular Contractility and Increases Inflammation in Response to Vascular Injury. Arter. Thromb. Vasc. Biol. 2011, 31, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buján, J.; Gimeno, M.J.; Jimínez, J.A.; Kielty, C.M.; Mecham, R.P.; Bellón, J.M. Expression of Elastic Components in Healthy and Varicose Veins. World J. Surg. 2003, 27, 901–905. [Google Scholar] [CrossRef]
- Pascual, G.; Mendieta, C.; García-Honduvilla, N.; Corrales, C.; Bellón, J.M.; Buján, J. TGF-Β1 Upregulation in the Aging Varicose Vein. J. Vasc. Res. 2007, 44, 192–201. [Google Scholar] [CrossRef]
- Qian, X.; Li, X.; Shi, Z.; Xia, Y.; Cai, Q.; Xu, D.; Tan, L.; Du, L.; Zheng, Y.; Zhao, D.; et al. PTEN Suppresses Glycolysis by Dephosphorylating and Inhibiting Autophosphorylated PGK1. Mol. Cell 2019, 76, 516–527. [Google Scholar] [CrossRef]
- Hu, H.; Juvekar, A.; Lyssiotis, C.A.; Lien, E.C.; Albeck, J.G.; Oh, D.; Varma, G.; Hung, Y.P.; Ullas, S.; Lauring, J.; et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell 2016, 164, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Ortega, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Martínez-Vivero, C.; Pekarek, L.; Coca, S.; Guijarro, L.G.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N.; et al. Chronic Venous Disease Patients Showed Altered Expression of IGF-1/PAPP-A/STC-2 Axis in the Vein Wall. BioMed Res. Int. 2020, 2020, 6782659. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Guzhova, I.V.; Margulis, B.A. Glyceraldehyde-3-Phosphate Dehydrogenase Is a Multifaceted Therapeutic Target. Pharmaceutics 2020, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Sukhanov, S.; Higashi, Y.; Shai, S.Y.; Itabe, H.; Ono, K.; Parthasarathy, S.; Delafontaine, P. Novel Effect of Oxidized Low-Density Lipoprotein: Cellular ATP Depletion via Downregulation of Glyceraldehyde-3-Phosphate Dehydrogenase. Circ. Res. 2006, 99, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Chiche, J.; Pommier, S.; Beneteau, M.; Mondragón, L.; Meynet, O.; Zunino, B.; Mouchotte, A.; Verhoeyen, E.; Guyot, M.; Pagès, G.; et al. GAPDH Enhances the Aggressiveness and the Vascularization of Non-Hodgkin’s B Lymphomas via NF-ΚB-Dependent Induction of HIF-1α. Leukemia 2015, 29, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-Y. Lactate: A Multifunctional Signaling Molecule. Yeungnam Univ. J. Med. 2021, 38, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gao, L.; Nickel, T.; Yang, J.; Zhou, J.; Gilbertsen, A.; Geng, Z.; Johnson, C.; Young, B.; Henke, C.; et al. Lactate Promotes Synthetic Phenotype in Vascular Smooth Muscle Cells. Circ. Res. 2017, 121, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.S.; Viji, R.I.; Kiran, M.S.; Sudhakaran, P.R. Endothelial Cell Response to Lactate: Implication of PAR Modification of VEGF. J. Cell. Physiol. 2007, 211, 477–485. [Google Scholar] [CrossRef]
- Bruczko-Goralewska, M.; Romanowicz, L.; Baczyk, J.; Wolańska, M.; Sobolewski, K.; Kowalewski, R. Peptide Growth Factors and Their Receptors in the Vein Wall. J. Investig. Med. 2019, 67, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- García-Róspide, V.; Peñafiel-Marfil, R.; Moreno-Padilla, F.; González-Ríos, J.; Ramos-Bruno, J.J.; Ros-Díe, E. Enzymatic and Isoenzymatic Study of Varicose Veins. Phlebol. J. Venous Dis. 2016, 6, 187–198. [Google Scholar] [CrossRef]

| Gene | Sequence Fwd (5′ → 3′) | Sequence Rev (5′ → 3′) | Tm |
|---|---|---|---|
| TBP | TGCACAGGAGCCAAGAGTGAA | CACATCACAGCTCCCCACCA | 60 °C |
| GLUT-1 | GGCCGGTAAGTAGGAGAGGT | ATTGAATTCCGCCTGGGGAC | 61 °C |
| PGK1 | TGGACTGTGGTCCTGAAAGC | GTTCTCCATTCCACCTTCCTCT | 58 °C |
| ALD | CCAGAAGGGTCCAGCTTCAA | CAACACCGCCCTTGGATTTG | 58 °C |
| GA3PDH | GGA AGG TGA AGG TCG GAG TCA | GTC ATT GAT GGC AAC AAT ATC CAC T | 60 °C |
| LDH | CAGGTGGTTGAGAGGGTCTT | AGGGTTGCCCAAGAATAGCC | 59 °C |
| Antigen | Species | Dilution | Provider | Protocol Specifications |
|---|---|---|---|---|
| PGK1 | Rabbit | 1:500 | Abcam (ab154613) | 10 mM Sodium citrate pH = 6 before incubation with the blocking solution. |
| GLUT1 | Mouse | 1: 200 | Abcam (ab40084) | 10 mM Sodium citrate pH = 6 before incubation with the blocking solution. |
| ALD | Rabbit | 1:250 | Abcam (ab252953) | 0.1% Triton with PBS, 10 min, before incubation with the blocking solution |
| GA3PDH | Rabbit | 1:500 | Abcam (ab 134187) | 0.1% Triton with PBS, 10 min, before incubation with the blocking solution |
| LDH | Rabbit | 1:750 | Abcam (ab 199553) | 0.1% Triton with PBS, 10 min, before incubation with the blocking solution |
| Antigen | Species | Dilution | Provider | Protocol Specifications |
|---|---|---|---|---|
| IgG (Mouse) | Goat | 1:300 | Sigma (F2012/045K6072) | |
| IgG (Rabbit) | Mouse | 1: 1000 | Sigma (RG-96/B5283) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraile-Martinez, O.; García-Montero, C.; Alvarez-Mon, M.Á.; Gomez-Lahoz, A.M.; Monserrat, J.; Llavero-Valero, M.; Ruiz-Grande, F.; Coca, S.; Alvarez-Mon, M.; Buján, J.; et al. Venous Wall of Patients with Chronic Venous Disease Exhibits a Glycolytic Phenotype. J. Pers. Med. 2022, 12, 1642. https://doi.org/10.3390/jpm12101642
Fraile-Martinez O, García-Montero C, Alvarez-Mon MÁ, Gomez-Lahoz AM, Monserrat J, Llavero-Valero M, Ruiz-Grande F, Coca S, Alvarez-Mon M, Buján J, et al. Venous Wall of Patients with Chronic Venous Disease Exhibits a Glycolytic Phenotype. Journal of Personalized Medicine. 2022; 12(10):1642. https://doi.org/10.3390/jpm12101642
Chicago/Turabian StyleFraile-Martinez, Oscar, Cielo García-Montero, Miguel Ángel Alvarez-Mon, Ana M. Gomez-Lahoz, Jorge Monserrat, Maria Llavero-Valero, Fernando Ruiz-Grande, Santiago Coca, Melchor Alvarez-Mon, Julia Buján, and et al. 2022. "Venous Wall of Patients with Chronic Venous Disease Exhibits a Glycolytic Phenotype" Journal of Personalized Medicine 12, no. 10: 1642. https://doi.org/10.3390/jpm12101642







