Dynamical Synergy of Drug Combinations during Cancer Chemotherapy
Abstract
:1. Introduction
2. Material and Methods
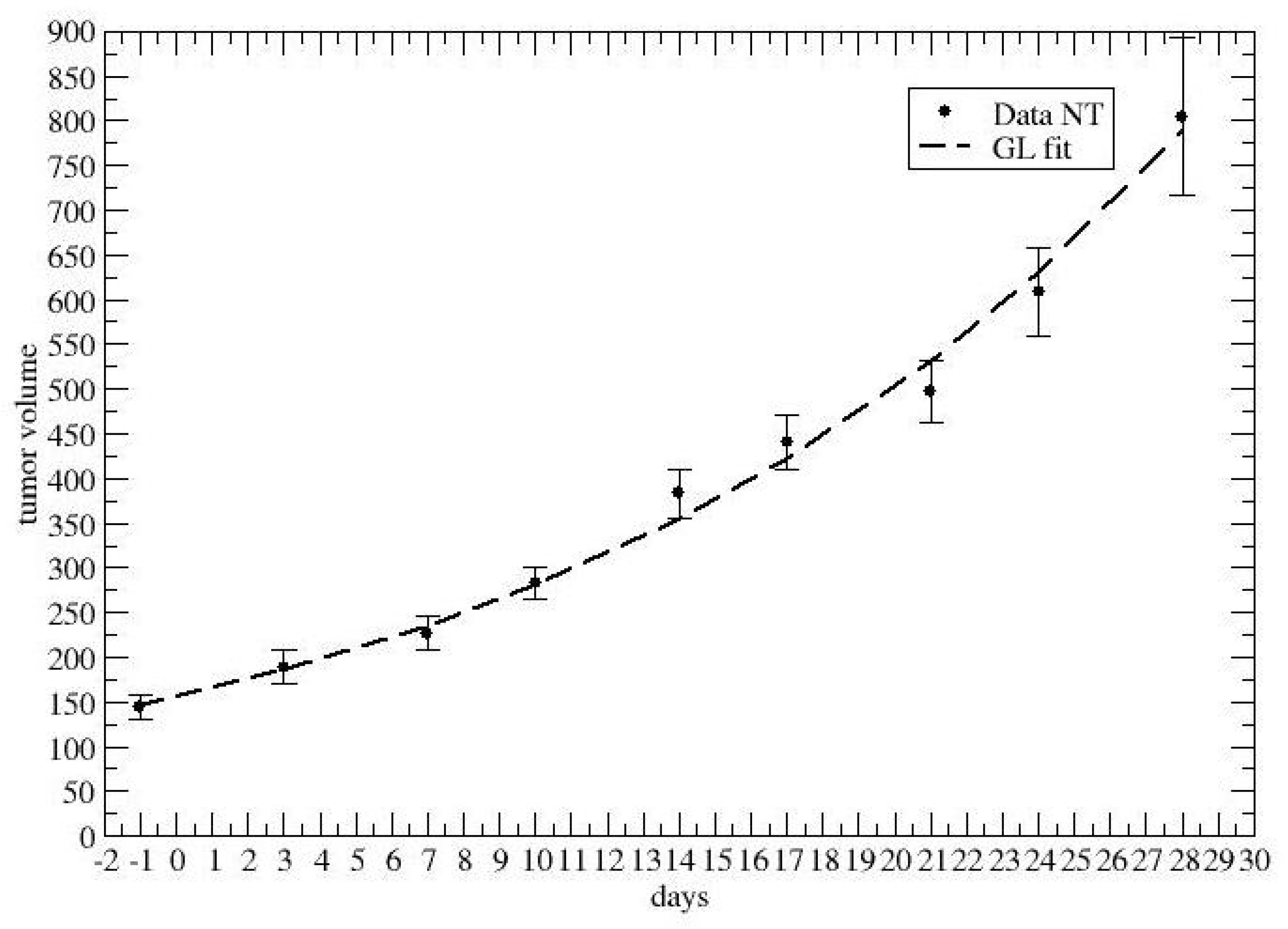
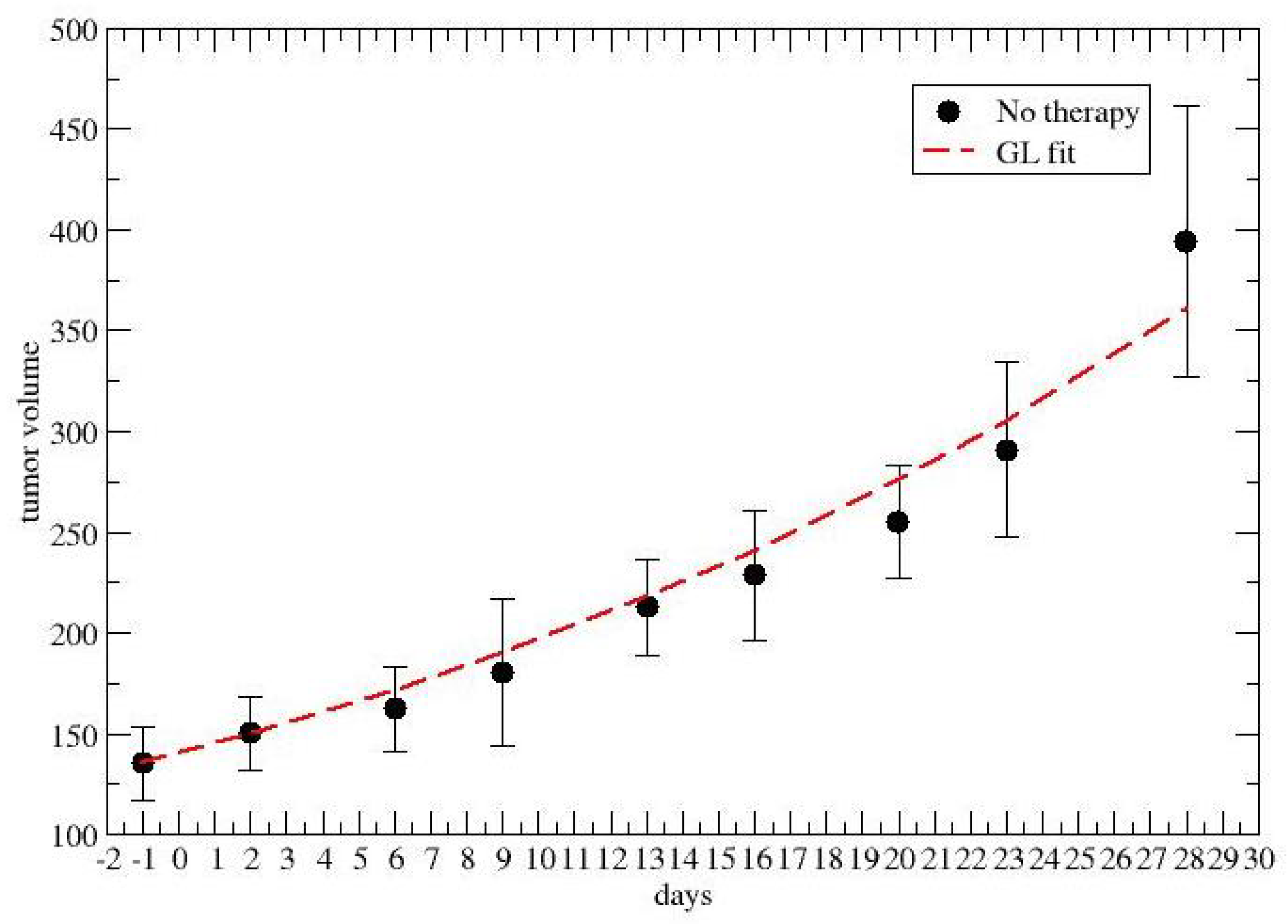
2.1. Time-Dependent Drug Synergism during Chemotherapy: Data Analysis
- Lung cancer dosing protocol:
- CMC plus D5W (placebo treatment no. 1) (0.5% carboxymethyl cellulose +5% dextrose water) 5 mL/kg intravenous weekly (three times), and 10 mL/kg by oral gavage daily for 21 days;
- Cisplatin (treatment no. 2) 2 mg/kg intravenous weekly (three times);
- Docetaxel (treatment no. 3) 6 mg/kg intravenous weekly (three times);
- Trametinib (treatment no. 4) 2 mg/kg oral garage daily for 21 days;
- Trametinib plus docetaxel (treatment no. 5) 2 mg/kg by oral gavage daily for 21 days and 6 mg/kg intravenous weekly (three times)
- Colon cancer dosing protocol
- D5W ((placebo treatment no. 1) (5% dextrose water) 5 mL/kg intravenous weekly (three times);
- 5-FU (treatment no. 2) 20 mg/kg intravenous weekly (three times);
- Cisplatin (treatment no. 3) 2 mg/kg intravenous weekly (three times);
- Oxaliplatin (treatment no. 4) 5 mg/kg intraperitoneal weekly (three times);
- Oxaliplatin plus 5-FU (treatment no. 5) 5 mg/kg intraperitoneal and 20 mg/kg intravenous weekly (three times).
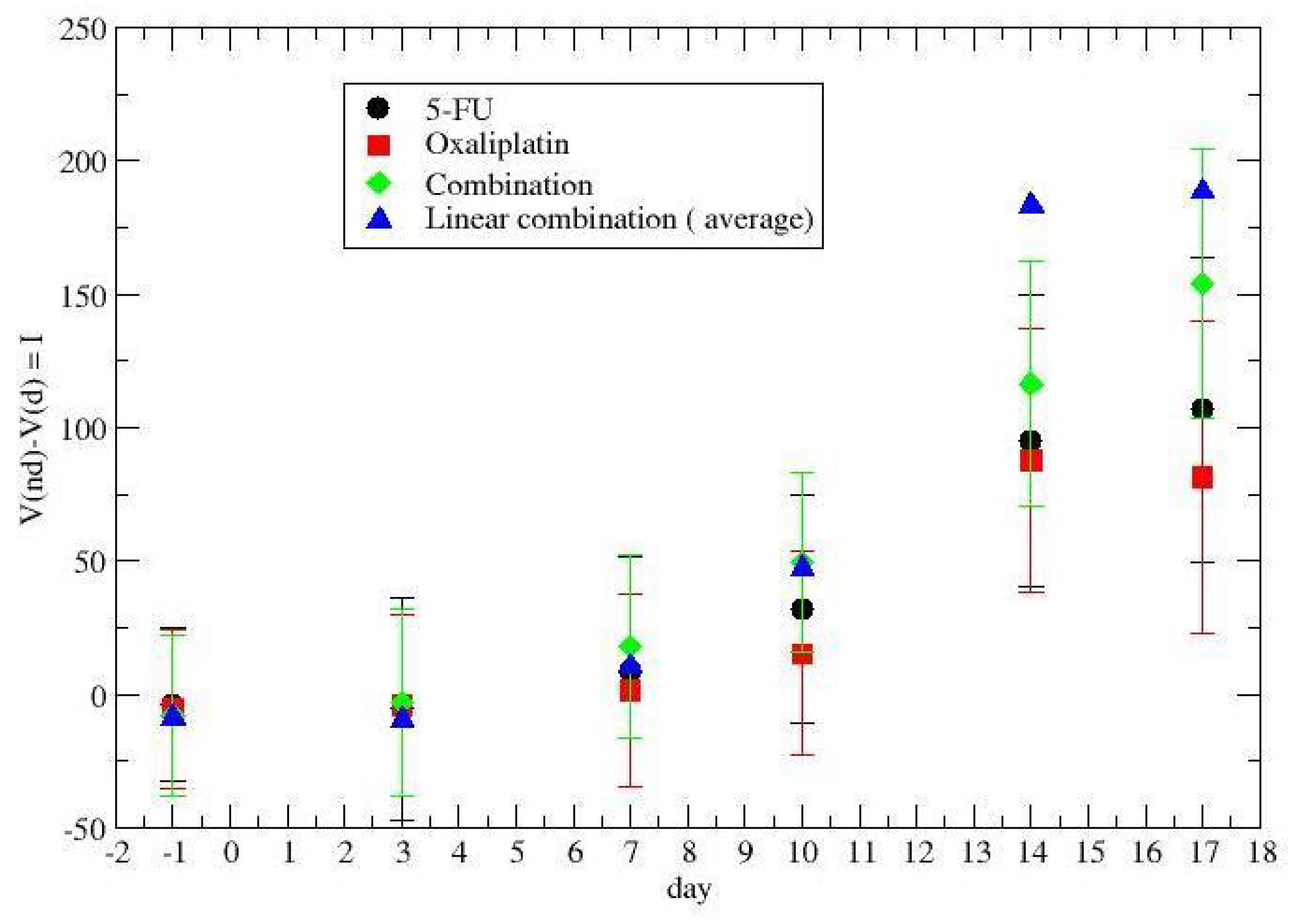
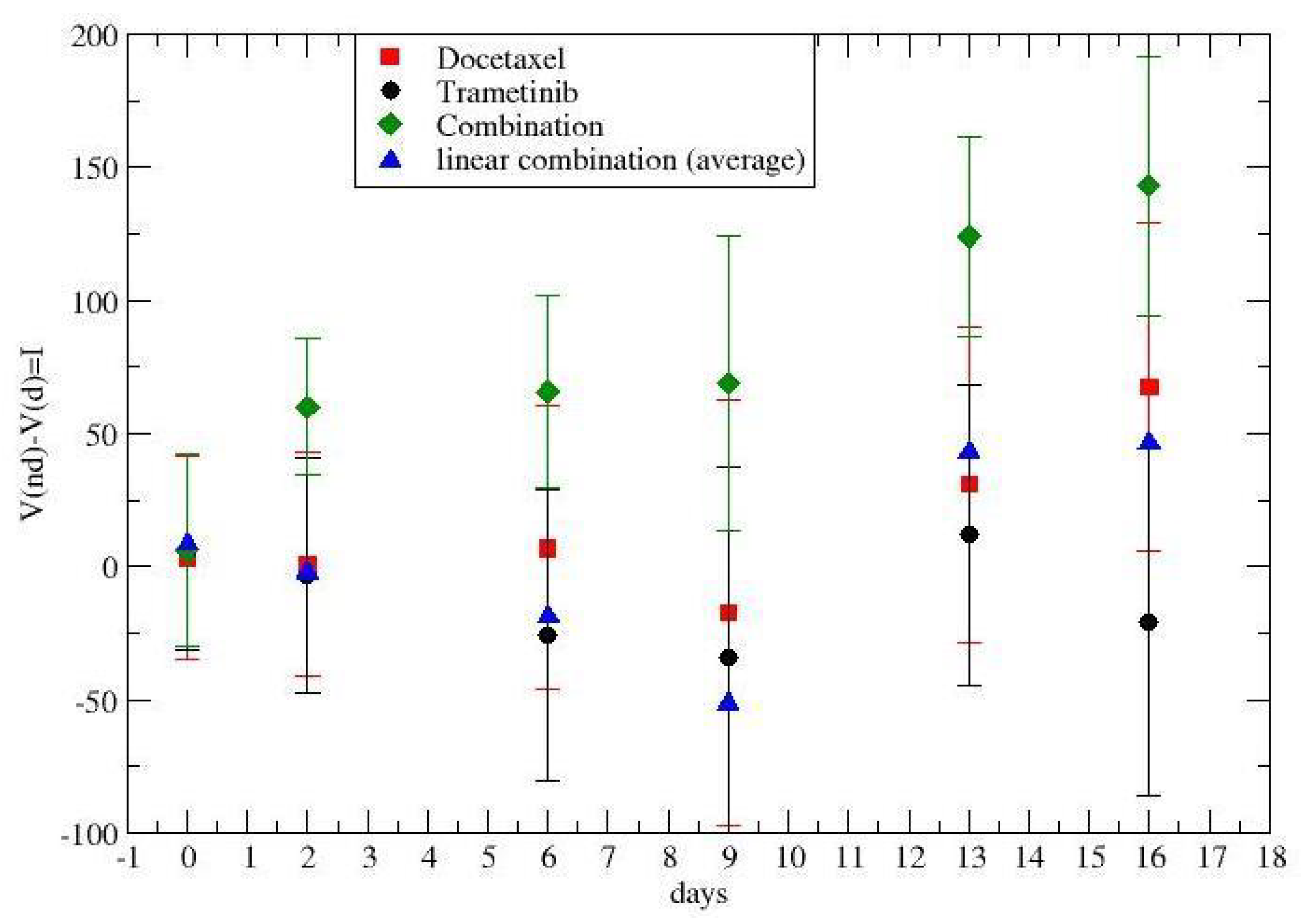
2.2. Time-Dependent Drug Synergism during Chemotherapy: Quantitative Description
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GL | Gompertz law |
| PDX | patient-derived xenograft |
| CC | carrying capacity |
| GTV | gross tumor volume |
| 5-FU | 5-fluorouracil |
| CMC | carboxymethyl cellulose |
| D5W | 5% dextrose water |
Appendix A
References
- Zimmermann, G.R.; Lehar, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Borisy, A.A.; Elliott, P.J.; Hurst, N.W.; Lee, M.S.; Lehár, J.; Price, E.R.; Serbedzija, G.; Zimmermann, G.R.; Foley, M.A.; Stockwell, B.R.; et al. Systematic discovery of multicomponent therapeutics. Proc. Natl. Acad. Sci. USA 2003, 100, 7977–7982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, J.B.; Schoeberl, B.; Nielsen, U.B.; Sorger, P.K. Systems biology and combination therapy in the quest for clinical efficacy. Nat. Chem. Biol. 2006, 2, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Chen, M.; Chen, J.; Padval, M.V.; Kansra, V.V. Biotransformation and in vitro assessment of metabolism-associated drug–drug interaction for CRx-102, a novel combination drug candidate. J. Pharm. Biomed. Anal. 2009, 50, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.R.; Honrath, U.; Sonnenberg, H. Interaction of amiloride and hydrochlorothiazide with atrial natriuretic factor in the medullary collecting duct. Can. J. Physiol. Pharmacol. 1988, 66, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.E. Managing hypertension using combination therapy. Am. Fam. Physician 2008, 77, 1279–1286. [Google Scholar] [PubMed]
- Kumar, V.; Dogra, N. A comprehensive review on deep synergistic drug prediction techniques for cancer. Arch. Comput. Methods Eng. 2021, 29, 1443–1461. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J. Quantitative methods for assessing drug synergism. Genes Cancer 2011, 2, 1003–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geary, N. Understanding synergy. Am. J. Physiol.-Endocrinol. Metab. 2013, 304, E237–E253. [Google Scholar] [CrossRef] [PubMed]
- Doern, C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Liu, X.; Yang, Y.; Yang, H.; Lu, P. Systematic synergy modeling: Understanding drug synergy from a systems biology perspective. BMC Syst. Biol. 2015, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yan, C.C.; Zhang, X.; Zhang, X.; Dai, F.; Yin, J.; Zhang, Y. Drug–target interaction prediction: Databases, web servers and computational models. Briefings Bioinform. 2016, 17, 696–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupke, D.M.; Begley, D.A.; Sundberg, J.P.; Bult, C.J.; Eppig, J.T. The Mouse Tumor Biology Database. Nat. Rev. Cancer 2008, 8, 459–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Gompertz, B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philos. Trans. R. Soc. Lond. 1825, 115, 513–583. [Google Scholar]
- Vaghi, C.; Rodallec, A.; Fanciullino, R.; Ciccolini, J.; Mochel, J.P.; Mastri, M.; Poignard, C.; Ebos, J.M.; Benzekry, S. Population modeling of tumor growth curves and the reduced Gompertz model improve prediction of the age of experimental tumors. PLoS Comput. Biol. 2020, 16, e1007178. [Google Scholar] [CrossRef] [PubMed]
- Wheldon, T.E. Mathematical Models in Cancer Research; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]


| PDX Characteristics | ||||
|---|---|---|---|---|
| Tumor Site | Type | Diagnosis | Stage (AJCC) | Chemotherapy |
| Lung | Primary | Mucinous adenocarcinoma | IV | CMC plus D5W Cisplatin Docetaxel Trametinib Docetaxel plus Trametinib |
| Colorectal | Primary | Adenocarcinoma | IV | D5W Cisplatin 5-FU Oxaliplatin Oxaliplatin plus 5-FU |
| Treatments and Measurements Schedule | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | PDX Lung | PDX Colon | ||||||||||
| Treatments | Meas. | Treatments | Meas. | |||||||||
| #1 | #2 | #3 | #4 | #5 | #1 | #2 | #3 | #4 | #5 | |||
| −1 | X | X | ||||||||||
| 0 | X* | X | X | X | X* | X | X | X | X | X | ||
| 1 | X | X | X | |||||||||
| 2 | X | X | X | X | ||||||||
| 3 | X | X | X | X | ||||||||
| 4 | X | X | X | |||||||||
| 5 | X | X | X | |||||||||
| 6 | X | X | X | X | ||||||||
| 7 | X* | X | X | X | X* | X | X | X | X | X | X | |
| 8 | X | X | X | |||||||||
| 9 | X | X | X | X | ||||||||
| 10 | X | X | X | X | ||||||||
| 11 | X | X | X | |||||||||
| 12 | X | X | X | |||||||||
| 13 | X | X | X | X | ||||||||
| 14 | X* | X | X | X | X* | X | X | X | X | X | X | |
| 15 | X | X | X | |||||||||
| 16 | X | X | X | X | ||||||||
| 17 | X | X | X | X | ||||||||
| 18 | X | X | X | |||||||||
| 19 | X | X | X | |||||||||
| 20 | X | X | X | X | ||||||||
| 21 | X | |||||||||||
| 22 | ||||||||||||
| 23 | ||||||||||||
| 24 | X | |||||||||||
| 25 | ||||||||||||
| 26 | ||||||||||||
| 27 | ||||||||||||
| 28 | X | |||||||||||
| No Therapy | 5-FU | Oxap. | Combination | |
|---|---|---|---|---|
| 0–7 | 0.081 | 0.067 | 0.071 | 0.053 |
| 7–14 | 0.098 | 0.047 | 0.034 | 0.04 |
| 14–21 | 0.043 | 0.051 | 0.053 | 0.037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castorina, P.; Martorana, E.; Forte, S. Dynamical Synergy of Drug Combinations during Cancer Chemotherapy. J. Pers. Med. 2022, 12, 1873. https://doi.org/10.3390/jpm12111873
Castorina P, Martorana E, Forte S. Dynamical Synergy of Drug Combinations during Cancer Chemotherapy. Journal of Personalized Medicine. 2022; 12(11):1873. https://doi.org/10.3390/jpm12111873
Chicago/Turabian StyleCastorina, Paolo, Emanuele Martorana, and Stefano Forte. 2022. "Dynamical Synergy of Drug Combinations during Cancer Chemotherapy" Journal of Personalized Medicine 12, no. 11: 1873. https://doi.org/10.3390/jpm12111873





