Higher Waist Hip Ratio Genetic Risk Score Is Associated with Reduced Weight Loss in Patients with Severe Obesity Completing a Meal Replacement Programme
Abstract
:1. Background
2. Methods
2.1. Study Design
2.2. Setting
2.3. Study Population
2.4. Inclusion and Exclusion Criteria
2.5. Milk Diet Intervention
2.6. Anthropometric Measurements
2.7. Blood Samples
2.8. Genotyping
2.9. Statistical Analysis Plan
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stegenga, H.; Haines, A.; Jones, K.; Wilding, J. Identification, assessment, and management of overweight and obesity: Summary of updated NICE guidance. BMJ 2014, 349, g6608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khera, R.; Murad, M.H.; Chandar, A.K.; Dulai, P.S.; Wang, Z.; Prokop, L.J.; Loomba, R.; Camilleri, M.; Singh, S. Association of Pharmacological Treatments for Obesity with Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2424–2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindstrom, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemio, K.; Hamalainen, H.; Harkonen, P.; Keinanen-Kiukaanniemi, S.; Laakso, M.; et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. [Google Scholar] [CrossRef]
- Clark, A.M.; Hartling, L.; Vandermeer, B.; McAlister, F.A. Meta-analysis: Secondary prevention programs for patients with coronary artery disease. Ann. Intern. Med. 2005, 143, 659–672. [Google Scholar] [CrossRef]
- Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; Espeland, M.A.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef]
- Gregg, E.W.; Jakicic, J.M.; Blackburn, G.; Bloomquist, P.; Bray, G.A.; Clark, J.M.; Coday, M.; Curtis, J.M.; Egan, C.; Evans, M.; et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016, 4, 913–921. [Google Scholar]
- Brazil, J.F.; Gibson, I.; Dunne, D.; Hynes, L.B.; Harris, A.; Bakir, M.; Keegan, D.; McGuire, B.; Hynes, M.; Collins, C.; et al. Improved Quality of Life, Fitness, Mental Health and Cardiovascular Risk Factors with a Publicly Funded Bariatric Lifestyle Intervention for Adults with Severe Obesity: A Prospective Cohort Study. Nutrients 2021, 13, 4172. [Google Scholar] [CrossRef]
- O’Rahilly, S.; Barroso, I.; Wareham, N.J. Genetic factors in type 2 diabetes: The end of the beginning? Science 2005, 307, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Ehtisham, S.; Crabtree, N.; Clark, P.; Shaw, N.; Barrett, T. Ethnic differences in insulin resistance and body composition in United Kingdom adolescents. J. Clin. Endocrinol. Metab. 2005, 90, 3963–3969. [Google Scholar] [CrossRef] [Green Version]
- Medici, F.; Hawa, M.; Ianari, A.; Pyke, D.A.; Leslie, R.D. Concordance rate for type II diabetes mellitus in monozygotic twins: Actuarial analysis. Diabetologia 1999, 42, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, S.; Ahlbom, A.; Lichtenstein, P.; Andersson, T. Shared genetic influence of BMI physical activity and type 2 diabetes: A twin study. Diabetologia 2013, 56, 1031–1035. [Google Scholar] [CrossRef] [Green Version]
- Azzolini, F.; Berentsen, G.D.; Skaug, H.J.; Hjelmborg, J.V.B.; Kaprio, J.A. The heritability of BMI varies across the range of BMI-a heritability curve analysis in a twin cohort. Int. J. Obes. 2022, 46, 1786–1791. [Google Scholar] [CrossRef]
- Thamer, C.; Stumvoll, M.; Niess, A.; Tschritter, O.; Haap, M.; Becker, R.; Shirkavand, F.; Bachmann, O.; Rett, K.; Volk, A.; et al. Reduced skeletal muscle oxygen uptake and reduced beta-cell function: Two early abnormalities in normal glucose-tolerant offspring of patients with type 2 diabetes. Diabetes Care 2003, 26, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Garcia, R.; Shulman, G.I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 2004, 350, 664–671. [Google Scholar] [CrossRef] [Green Version]
- Kriketos, A.D.; Greenfield, J.R.; Peake, P.W.; Furler, S.M.; Denyer, G.S.; Charlesworth, J.A.; Campbell, L.V. Inflammation, insulin resistance, and adiposity: A study of first-degree relatives of type 2 diabetic subjects. Diabetes Care 2004, 27, 2033–2040. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Pearson, E.; Avery, P.J.; McCarthy, M.I.; Levy, J.C.; Hitman, G.A.; Sampson, M.; Walker, M.; Hattersley, A.T. Reduced beta cell function in offspring of mothers with young-onset type 2 diabetes. Diabetologia 2006, 49, 1876–1880. [Google Scholar] [CrossRef] [Green Version]
- Loos, R.J.F.; Yeo, G.S.H. The bigger picture of FTO: The first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 2014, 10, 51–61. [Google Scholar] [CrossRef]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [Green Version]
- Speliotes, E.K.; Willer, C.J.; Berndt, S.I.; Monda, K.L.; Thorleifsson, G.; Jackson, A.U.; Lango Allen, H.; Lindgren, C.M.; Luan, J.; Magi, R.; et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010, 42, 937–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grarup, N.; Sandholt, C.H.; Hansen, T.; Pedersen, O. Genetic susceptibility to type 2 diabetes and obesity: From genome-wide association studies to rare variants and beyond. Diabetologia 2014, 57, 1528–1541. [Google Scholar] [CrossRef] [PubMed]
- Jocken, J.W.; Blaak, E.E.; Schiffelers, S.; Arner, P.; van Baak, M.A.; Saris, W.H. Association of a beta-2 adrenoceptor (ADRB2) gene variant with a blunted in vivo lipolysis and fat oxidation. Int. J. Obes. 2007, 31, 813–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teran-Garcia, M.; Santoro, N.; Rankinen, T.; Bergeron, J.; Rice, T.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Bergman, R.N.; Despres, J.P.; et al. Hepatic lipase gene variant -514C>T is associated with lipoprotein and insulin sensitivity response to regular exercise: The HERITAGE Family Study. Diabetes 2005, 54, 2251–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindi, V.I.; Uusitupa, M.I.; Lindstrom, J.; Louheranta, A.; Eriksson, J.G.; Valle, T.T.; Hamalainen, H.; Ilanne-Parikka, P.; Keinanen-Kiukaanniemi, S.; Laakso, M.; et al. Association of the Pro12Ala polymorphism in the PPAR-gamma2 gene with 3-year incidence of type 2 diabetes and body weight change in the Finnish Diabetes Prevention Study. Diabetes 2002, 51, 2581–2586. [Google Scholar] [CrossRef] [Green Version]
- Florez, J.C.; Jablonski, K.A.; Bayley, N.; Pollin, T.I.; de Bakker, P.I.; Shuldiner, A.R.; Knowler, W.C.; Nathan, D.M.; Altshuler, D. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N. Engl. J. Med. 2006, 355, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Christensen, P. Comparison of a low-energy diet and a very low-energy diet in sedentary obese individuals: A pragmatic randomized controlled trial. Clin. Obes. 2011, 1, 31–40. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Delany, J.P.; Otto, A.D.; Kuller, L.; Vockley, J.; South-Paul, J.E.; Thomas, S.B.; Brown, J.; McTigue, K.; Hames, K.C.; et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. JAMA 2010, 304, 1795–1802. [Google Scholar] [CrossRef] [Green Version]
- Johansson, K.; Neovius, M.; Lagerros, Y.T.; Harlid, R.; Rossner, S.; Granath, F.; Hemmingsson, E. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: A randomised controlled trial. BMJ 2009, 339, b4609. [Google Scholar] [CrossRef] [Green Version]
- Ryan, D.H.; Johnson, W.D.; Myers, V.H.; Prather, T.L.; McGlone, M.M.; Rood, J.; Brantley, P.J.; Bray, G.A.; Gupta, A.K.; Broussard, A.P.; et al. Nonsurgical weight loss for extreme obesity in primary care settings: Results of the Louisiana Obese Subjects Study. Arch. Intern. Med. 2010, 170, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Rafey, M.F.; Murphy, C.F.; Abdalgwad, R.; Kilkelly, K.; Griffin, H.; Beatty, N.; O’Shea, P.M.; Collins, C.; McGrath, R.; Hynes, M.; et al. Effects of a Milk-Based Meal Replacement Program on Weight and Metabolic Characteristics in Adults with Severe Obesity. Diabetes Metab. Syndr. Obes. 2020, 13, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Sun, T.-H.; Shao, Y.-H.J.; Mao, C.-L.; Hung, M.-N.; Lo, Y.-Y.; Ko, T.-M.; Hsiao, T.-H. A Novel Quality-Control Procedure to Improve the Accuracy of Rare Variant Calling in SNP Arrays. Front. Genet. 2021, 12, 736390. [Google Scholar] [CrossRef]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Day, F.R.; Gustafsson, S.; Buchkovich, M.L.; Na, J.; Bataille, V.; Cousminer, D.L.; Dastani, Z.; Drong, A.W.; Esko, T.; et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat. Commun. 2016, 7, 10495. [Google Scholar] [CrossRef] [Green Version]
- Yaghootkar, H.; Lotta, L.A.; Tyrrell, J.; Smit, R.A.J.; Jones, S.E.; Donnelly, L.; Beaumont, R.; Campbell, A.; Tuke, M.A.; Hayward, C.; et al. Genetic Evidence for a Link Between Favorable Adiposity and Lower Risk of Type 2 Diabetes, Hypertension, and Heart Disease. Diabetes 2016, 65, 2448–2460. [Google Scholar] [CrossRef] [Green Version]
- Justice, A.E.; Karaderi, T.; Highland, H.M.; Young, K.L.; Graff, M.; Lu, Y.; Turcot, V.; Auer, P.L.; Fine, R.S.; Guo, X.; et al. Protein-Coding Variants Implicate Novel Genes Related to Lipid Homeostasis Contributing to Body Fat Distribution. Nat. Genet. 2019, 51, 452–469. [Google Scholar] [CrossRef] [Green Version]
- Dastani, Z.; Hivert, M.-F.; Timpson, N.; Perry, J.R.B.; Yuan, X.; Scott, R.A.; Henneman, P.; Heid, I.M.; Kizer, J.R.; Lyytikäinen, L.-P.; et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: A multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012, 8, e1002607. [Google Scholar] [CrossRef] [Green Version]
- Scott, R.A.; Scott, L.J.; Mägi, R.; Marullo, L.; Gaulton, K.J.; Kaakinen, M.; Pervjakova, N.; Pers, T.H.; Johnson, A.D.; Eicher, J.D.; et al. An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes 2017, 66, 2888–2902. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience 2015, 4, s13742-015. [Google Scholar] [CrossRef] [PubMed]
- Magi, R.; Manning, S.; Yousseif, A.; Pucci, A.; Santini, F.; Karra, E.; Querci, G.; Pelosini, C.; McCarthy, M.I.; Lindgren, C.M.; et al. Contribution of 32 GWAS-identified common variants to severe obesity in European adults referred for bariatric surgery. PLoS ONE 2013, 8, e70735. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, I.B.; Fries, G.R.; Bucker, J.; Scotton, E.; von Diemen, L.; Kauer-Sant’Anna, M. The FKBP5 polymorphism rs1360780 is associated with lower weight loss after bariatric surgery: 26 months of follow-up. Surg. Obes. Relat. Dis. 2016, 12, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Resende, C.M.M.; Durso, D.F.; Borges, K.B.G.; Pereira, R.M.; Rodrigues, G.K.D.; Rodrigues, K.F.; Silva, J.L.P.; Rodrigues, E.C.; Franco, G.R.; Alvarez-Leite, J.I. The polymorphism rs17782313 near MC4R gene is related with anthropometric changes in women submitted to bariatric surgery over 60 months. Clin. Nutr. 2017, 37, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.K.; Resende, C.M.; Durso, D.F.; Rodrigues, L.A.; Silva, J.L.; Reis, R.C.; Pereira, S.S.; Ferreira, D.C.; Franco, G.R.; Alvarez-Leite, J. A single FTO gene variant rs9939609 is associated with body weight evolution in a multiethnic extremely obese population that underwent bariatric surgery. Nutrition 2015, 31, 1344–1350. [Google Scholar] [CrossRef]
- Cooiman, M.I.; Kleinendorst, L.; Aarts, E.O.; Janssen, I.M.C.; van Amstel, H.K.P.; Blakemore, A.I.; Hazebroek, E.J.; Meijers-Heijboer, H.J.; van der Zwaag, B.; Berends, F.J.; et al. Genetic Obesity and Bariatric Surgery Outcome in 1014 Patients with Morbid Obesity. Obes. Surg. 2020, 30, 470–477. [Google Scholar] [CrossRef]
- Dashti, H.S.; Levy, D.E.; Hivert, M.F.; Alimenti, K.; McCurley, J.L.; Saxena, R.; Thorndike, A.N. Genetic risk for obesity and the effectiveness of the ChooseWell 365 workplace intervention to prevent weight gain and improve dietary choices. Am. J. Clin. Nutr. 2022, 115, 180–188. [Google Scholar] [CrossRef]
- McCaffery, J.M.; Jablonski, K.A.; Pan, Q.; Astrup, A.; Revsbech Christiansen, M.; Corella, D.; Corso, L.M.L.; Florez, J.C.; Franks, P.W.; Gardner, C.; et al. Genetic Predictors of Change in Waist Circumference and Waist-to-Hip Ratio With Lifestyle Intervention: The Trans-NIH Consortium for Genetics of Weight Loss Response to Lifestyle Intervention. Diabetes 2022, 71, 669–676. [Google Scholar] [CrossRef]
- De Toro-Martín, J.; Guénard, F.; Tchernof, A.; Pérusse, L.; Marceau, S.; Vohl, M.C. Polygenic risk score for predicting weight loss after bariatric surgery. JCI Insight 2018, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Ciudin, A.; Fidilio, E.; Gutiérrez-Carrasquilla, L.; Caixàs, A.; Vilarrasa, N.; Pellitero, S.; Simó-Servat, A.; Vilallonga, R.; Ruiz, A.; de la Fuente, M.; et al. A Clinical-Genetic Score for Predicting Weight Loss after Bariatric Surgery: The OBEGEN Study. J. Pers. Med. 2021, 11, 1040. [Google Scholar] [CrossRef]



| Type 2 Diabetes | No Diabetes | p-Value | |||
|---|---|---|---|---|---|
| n | 33 | 60 | |||
| Age (years) | 52.7 | ±8.9 | 50.6 | ±12 | 0.39 |
| Sex (female) | 15 | (45.5%) | 35 | (58.3%) | 0.23 |
| Ethnicity (White Irish) | 30 | (90.9%) | 54 | (90%) | 0.62 |
| Height (m) | 1.69 | ±0.1 | 1.68 | ±0.1 | 0.67 |
| Weight (kg) * | 131 | (117.0, 159.4) | 145.3 | (130, 167.1) | 0.056 |
| Ideal Body Weight (kg) * | 70.6 | (65.6, 76.6) | 68.9 | (64.4, 76.6) | 0.67 |
| Excess Body Weight (kg) * | 56.8 | (44.6, 91.3) | 76.5 | (64.1, 94.3) | 0.031 |
| Excess Body Weight (%) | 95.9 | ±40.6 | 111.6 | ±30.3 | 0.05 |
| Body Mass Index (kgm−2) | 49 | ±10.1 | 52.9 | ±7.6 | 0.037 |
| Total Cholesterol (mmol/l) | 4.3 | ±1.2 | 4.9 | ±0.8 | 0.0042 |
| LDL-Cholesterol (mmol/l) | 2.3 | ±0.9 | 2.8 | ±0.8 | 0.0033 |
| HDL-Cholesterol (mmol/l) * | 1 | (0.9, 1.2) | 1.2 | (1.1, 1.5) | 0.0045 |
| Triglycerides (mmol/l) * | 1.9 | (1.5, 2.5) | 1.6 | (1.3, 1.9) | 0.047 |
| Triglyceride: HDL Ratio * | 4.6 | (3.3, 5.6) | 3.1 | (2.1, 3.8) | 0.0019 |
| HbA1c (mmol/mol) | 67 | (55, 73) | 39 | (36, 41) | <0.001 |
| GRS—Body Mass Index | 2.06 | ±0.16 | 2.07 | ±0.14 | 0.62 |
| GRS—Body Fat Percent | 0.35 | ±0.07 | 0.35 | ±0.07 | 0.95 |
| GRS—Favourable Adiposity | 0.17 | ±0.03 | 0.17 | ±0.03 | 0.64 |
| GRS—Waist–Hip Ratio | 1.14 | ±0.10 | 1.14 | ±0.13 | 0.83 |
| GRS—Type 2 Diabetes | 10.25 | ±0.40 | 10.01 | ±0.49 | 0.022 |
| GRS—Adiponectin | 0.28 | ±0.08 | 0.30 | ±0.06 | 0.074 |
| On metformin | 30 | (90.9%) | 7 | (11.7%) | <0.001 |
| On insulin | 9 | (27.3%) | 0 | (0%) | <0.001 |
| On sulphonylurea | 13 | (39.4%) | 0 | (0%) | <0.001 |
| On DPPIV-inhibitor | 6 | (18.2%) | 0 | (0%) | 0.001 |
| On SGLT2-inhibitor | 6 | (18.2%) | 0 | (0%) | 0.001 |
| On GLP1-receptor agonist | 13 | (39.4%) | 3 | (5%) | <0.001 |
| On PPARγ-receptor agonist | 1 | (3%) | 0 | (0%) | 0.18 |
| Variable | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | [95% C.I.] | p | β | [95% C.I.] | p | β | [95% C.I.] | p | |
| BMI GRS Score: | |||||||||
| Baseline Weight (kg) | 30.0 | [−10.9, 70.8] | 0.148 | 27.3 | [−6.2, 60.8] | 0.108 | 36.5 | [2.3, 70.7] | 0.037 |
| Baseline BMI (kg m−2) | 10.6 | [−1.6, 22.8] | 0.087 | 10.5 | [−1.3, 22.2] | 0.081 | 13.0 | [0.8, 25.3] | 0.038 |
| Follow-up Weight (kg) | −5.6 | [−18.8, 7.6] | 0.401 | −6.2 | [−19.5, 7.0] | 0.353 | −5.5 | [−20.1, 9.0] | 0.451 |
| Follow-up BMI (kg m−2) | −2.4 | [−7.0, 2.3] | 0.312 | −2.3 | [−7.0, 2.3] | 0.319 | −2.0 | [−7.1, 3.1] | 0.441 |
| Change in Total Body Weight (%) | 4.5 | [−4.5, 13.5] | 0.328 | 4.3 | [−4.7, 13.3] | 0.345 | 3.1 | [−6.7, 12.8] | 0.532 |
| Change in Excess Body Weight (%) | 16.2 | [−3.5, 36.0] | 0.106 | 15.8 | [−3.8, 35.4] | 0.112 | 15.6 | [−5.4, 36.6] | 0.143 |
| Body Fat Percentage GRS Score: | |||||||||
| Baseline Weight (kg) | 60.8 | [−20.2, 141.9] | 0.139 | 42.9 | [−24.6, 110.5] | 0.210 | 37.9 | [−29.5, 105.3] | 0.266 |
| Baseline BMI (kg m−2) | 14.6 | [−9.9, 39.2] | 0.239 | 15.7 | [−8.1, 39.5] | 0.193 | 14.5 | [−9.7, 38.6] | 0.237 |
| Follow-up Weight (kg) | −11.8 | [−37.8, 14.2] | 0.368 | −11.3 | [−37.5, 14.8] | 0.392 | −9.6 | [−37.2, 17.9] | 0.488 |
| Follow-up BMI (kg m−2) | −4.4 | [−13.5, 4.7] | 0.336 | −3.7 | [−12.9, 5.5] | 0.424 | −3.0 | [−12.6, 6.7] | 0.545 |
| Change in Total Body Weight (%) | 7.2 | [−10.6, 24.9] | 0.426 | 5.6 | [−12.3, 23.5] | 0.537 | 3.8 | [−15.1, 22.6] | 0.693 |
| Change in Excess Body Weight (%) | 26.7 | [−12.1, 65.6] | 0.175 | 24.3 | [−14.5, 63.1] | 0.217 | 20.4 | [−20.3, 61.1] | 0.321 |
| Favourable Adiposity GRS Score: | |||||||||
| Baseline Weight (kg) | −9.9 | [−205.8, 186.1] | 0.921 | 8.8 | [−157.8, 175.5] | 0.916 | −21.0 | [−191.1, 149.0] | 0.806 |
| Baseline BMI (kg m−2) | 8.9 | [−49.8, 67.5] | 0.765 | 9.6 | [−47.2, 66.4] | 0.738 | 1.3 | [−57.9, 60.6] | 0.965 |
| Follow-up Weight (kg) | 2.7 | [−60.4, 65.8] | 0.931 | 0.8 | [−62.2, 63.8] | 0.979 | 6.1 | [−61.9, 74.2] | 0.858 |
| Follow-up BMI (kg m−2) | 0.0 | [−22.1, 22.0] | 0.998 | −0.8 | [−22.8, 21.2] | 0.944 | −0.3 | [−24.2, 23.6] | 0.980 |
| Change in Total Body Weight (%) | −3.9 | [−47.6, 39.7] | 0.858 | −2.5 | [−46.0, 41.0] | 0.909 | −4.3 | [−51.3, 42.7] | 0.857 |
| Change in Excess Body Weight (%) | 5.8 | [−89.7, 101.3] | 0.904 | 9.2 | [−85.3, 103.7] | 0.847 | 2.0 | [−99.3, 103.2] | 0.969 |
| Waist–Hip Ratio GRS Score: | |||||||||
| Baseline Weight (kg) | −3.2 | [−56.3, 49.9] | 0.905 | 7.3 | [−37.3, 52.0] | 0.745 | 10.8 | [−32.4, 54.1] | 0.619 |
| Baseline BMI (kg m−2) | −3.0 | [−19.2, 13.1] | 0.709 | −1.6 | [−17.2, 13.9] | 0.835 | −1.2 | [−16.6, 14.2] | 0.877 |
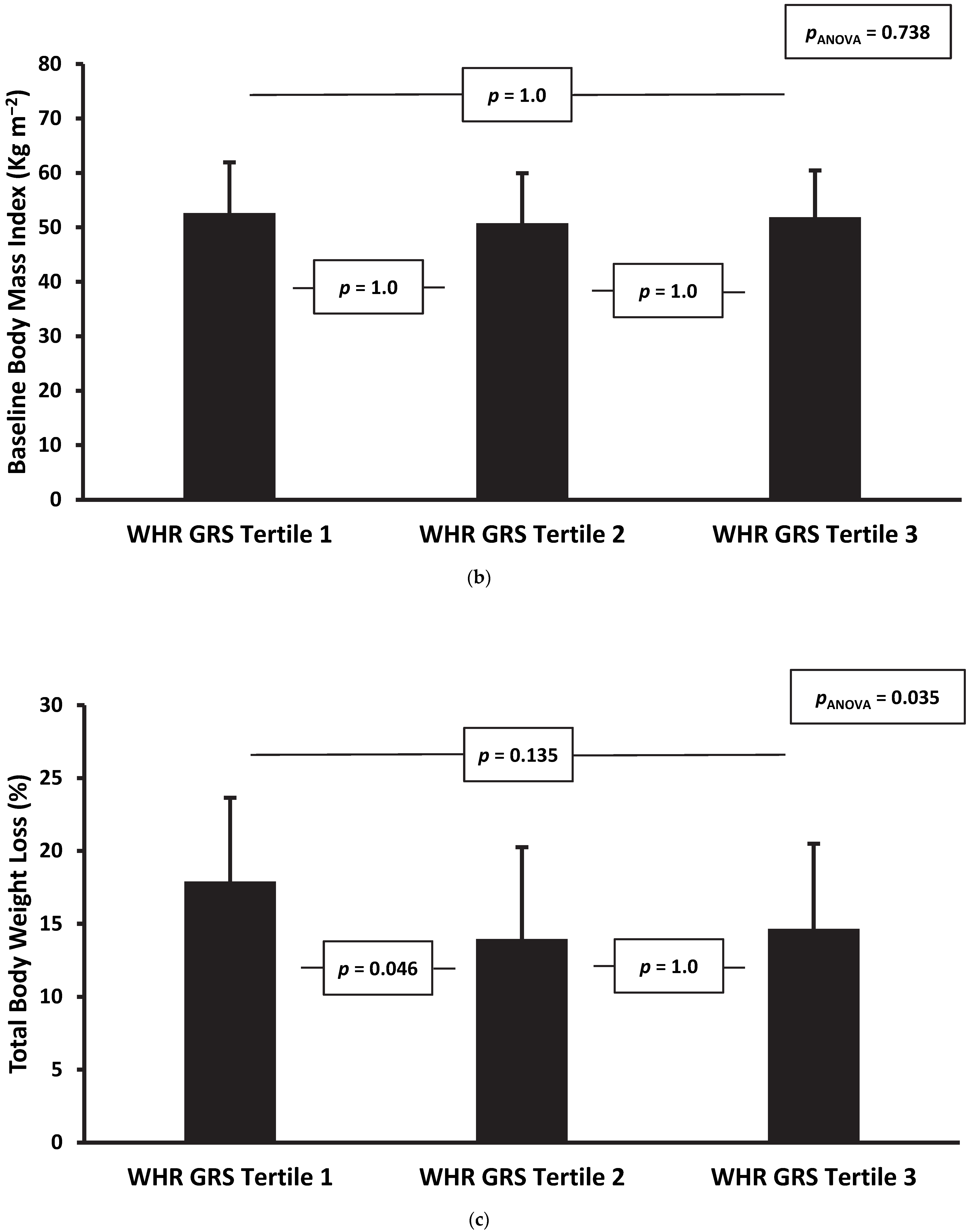
| Follow-up Weight (kg) | 15.1 | [−0.5, 30.7] | 0.057 | 14.5 | [−1.3, 30.2] | 0.071 | 16.5 | [0.0, 33.0] | 0.050 |
| Follow-up BMI (kg m−2) | 5.5 | [0.0, 10.9] | 0.051 | 5.3 | [−0.3, 10.8] | 0.062 | 5.8 | [0.0, 11.6] | 0.050 |
| Change in Total Body Weight (%) | −10.8 | [−21.6, 0.0] | 0.049 | −10.4 | [−21.3, 0.5] | 0.060 | −11.6 | [−23.0, −0.3] | 0.045 |
| Change in Excess Body Weight (%) | −23.9 | [−48.2, 0.4] | 0.054 | −22.1 | [−46.3, 2.0] | 0.072 | −24.1 | [−49.2, 1.0] | 0.060 |
| Adiponectin GRS Score: | |||||||||
| Baseline Weight (kg) | 137.3 | [49.7, 225.0] | 0.002 | 100.7 | [24.4, 177.0] | 0.010 | 46.7 | [−39.4, 132.7] | 0.283 |
| Baseline BMI (kg m−2) | 36.0 | [9.1, 62.9] | 0.009 | 36.1 | [9.6, 62.5] | 0.008 | 22.3 | [−8.1, 52.6] | 0.149 |
| Follow-up Weight (kg) | −14.0 | [−44.7, 16.6] | 0.366 | −13.4 | [−44.1, 17.3] | 0.386 | −10.0 | [−44.6, 24.7] | 0.569 |
| Follow-up BMI (kg m−2) | −5.7 | [−16.3, 4.8] | 0.285 | −4.3 | [−15.0, 6.4] | 0.429 | −3.9 | [−16.1, 8.4] | 0.532 |
| Change in Total Body Weight (%) | 6.7 | [−13.5, 26.9] | 0.512 | 3.7 | [−16.8, 24.2] | 0.722 | 2.3 | [−21.5, 26.1] | 0.850 |
| Change in Excess Body Weight (%) | 43.8 | [0.5, 87.2] | 0.047 | 38.1 | [−5.7, 81.9] | 0.088 | 27.9 | [−22.9, 78.7] | 0.278 |
| Type 2 Diabetes GRS Score: | |||||||||
| Baseline Weight (kg) | −4.5 | [−17.2, 8.1] | 0.479 | −2.5 | [−13.4, 8.4] | 0.649 | −1.3 | [−12.6, 10.0] | 0.818 |
| Baseline BMI (kg m−2) | −0.2 | [−4.1, 3.6] | 0.901 | −0.9 | [−4.7, 2.9] | 0.633 | −0.6 | [−4.6, 3.4] | 0.765 |
| Follow-up Weight (kg) | 3.4 | [−0.5, 7.3] | 0.086 | 3.3 | [−0.8, 7.3] | 0.111 | 3.5 | [−1.0, 7.9] | 0.123 |
| Follow-up BMI (kg m−2) | 1.2 | [−0.2, 2.6] | 0.090 | 1.1 | [−0.3, 2.5] | 0.133 | 1.2 | [−0.4, 2.7] | 0.138 |
| Change in Total Body Weight (%) | −2.2 | [−4.9, 0.5] | 0.109 | −2.0 | [−4.8, 0.8] | 0.160 | −2.3 | [−5.4, 0.8] | 0.140 |
| Change in Excess Body Weight (%) | −4.9 | [−10.9, 1.1] | 0.109 | −4.9 | [−10.9, 1.2] | 0.114 | −5.1 | [−11.7, 1.6] | 0.134 |
| Change in Excess Body Weight (%) | −10.4 | [−47.3, 26.4] | 0.575 | −10.7 | [−47.1, 25.7] | 0.641 | −11.4 | [−51.2, 28.3] | 0.567 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handley, D.; Rafey, M.F.; Almansoori, S.; Brazil, J.F.; McCarthy, A.; Amin, H.A.; O’Donnell, M.; Blakemore, A.I.; Finucane, F.M. Higher Waist Hip Ratio Genetic Risk Score Is Associated with Reduced Weight Loss in Patients with Severe Obesity Completing a Meal Replacement Programme. J. Pers. Med. 2022, 12, 1881. https://doi.org/10.3390/jpm12111881
Handley D, Rafey MF, Almansoori S, Brazil JF, McCarthy A, Amin HA, O’Donnell M, Blakemore AI, Finucane FM. Higher Waist Hip Ratio Genetic Risk Score Is Associated with Reduced Weight Loss in Patients with Severe Obesity Completing a Meal Replacement Programme. Journal of Personalized Medicine. 2022; 12(11):1881. https://doi.org/10.3390/jpm12111881
Chicago/Turabian StyleHandley, Dale, Mohammed Faraz Rafey, Sumaya Almansoori, John F. Brazil, Aisling McCarthy, Hasnat A. Amin, Martin O’Donnell, Alexandra I. Blakemore, and Francis M. Finucane. 2022. "Higher Waist Hip Ratio Genetic Risk Score Is Associated with Reduced Weight Loss in Patients with Severe Obesity Completing a Meal Replacement Programme" Journal of Personalized Medicine 12, no. 11: 1881. https://doi.org/10.3390/jpm12111881
APA StyleHandley, D., Rafey, M. F., Almansoori, S., Brazil, J. F., McCarthy, A., Amin, H. A., O’Donnell, M., Blakemore, A. I., & Finucane, F. M. (2022). Higher Waist Hip Ratio Genetic Risk Score Is Associated with Reduced Weight Loss in Patients with Severe Obesity Completing a Meal Replacement Programme. Journal of Personalized Medicine, 12(11), 1881. https://doi.org/10.3390/jpm12111881






