Selection of Representative Questionnaire Items from the Asthma Control Test
Abstract
:1. Introduction
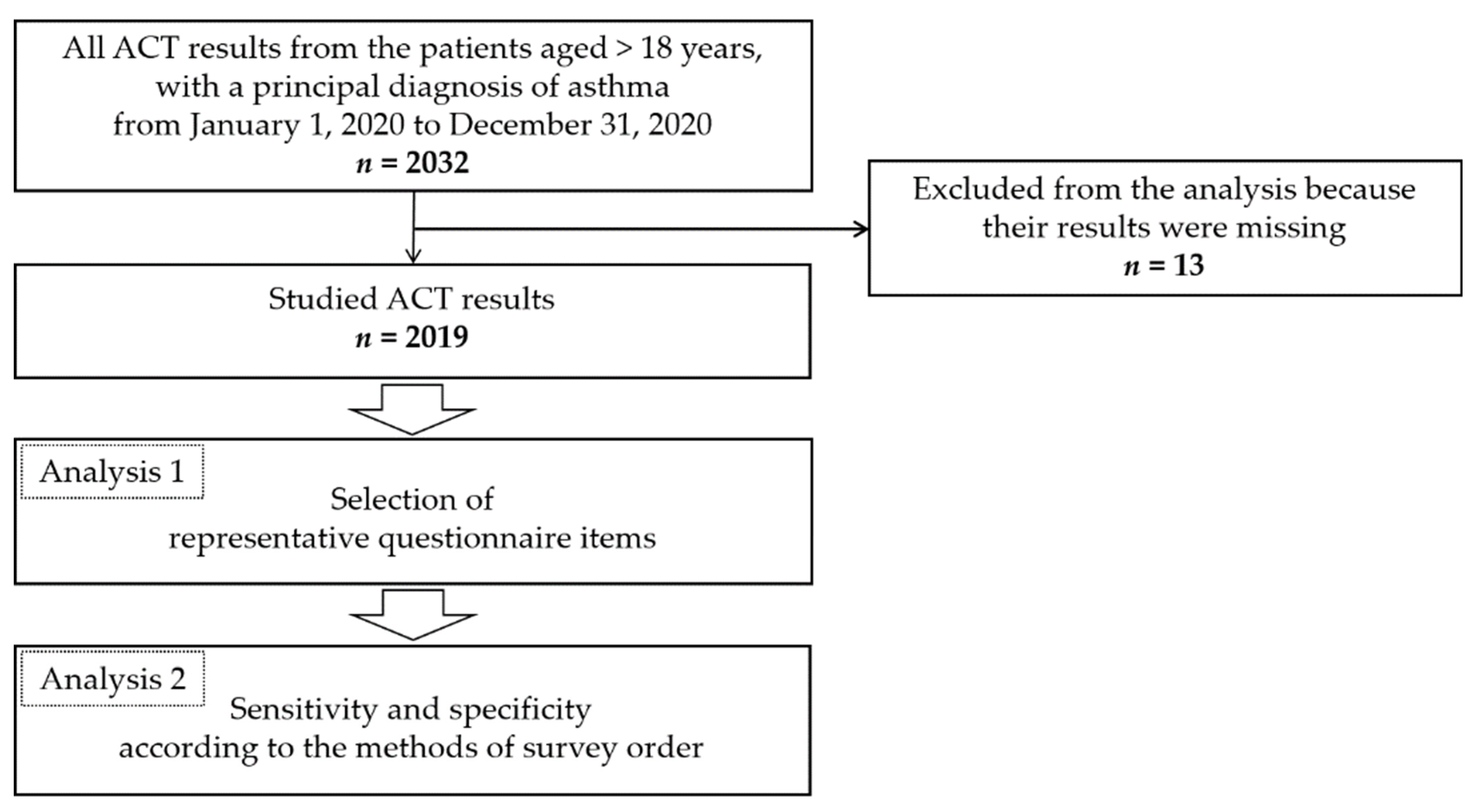
2. Materials and Methods
2.1. Participants of the Study
2.2. Methods
2.2.1. ACT
2.2.2. Selection of Representative Questionnaire Items (Analysis 1)
2.2.3. Sensitivity and Specificity According to the Methods of Survey Order Using the Selected Representative Questionnaire Items (Analysis 2)
2.2.4. Statistical Analysis
3. Results
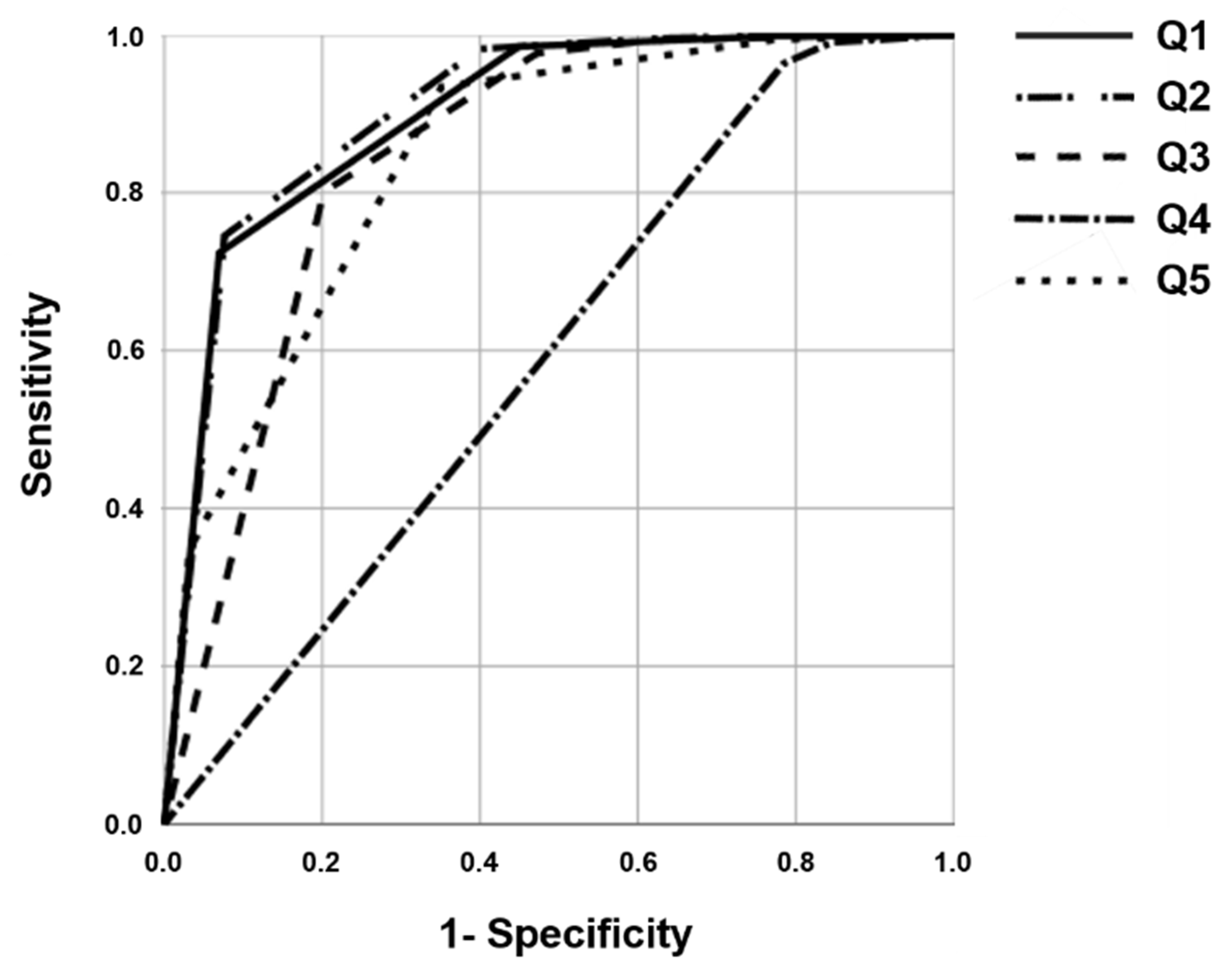
3.1. Selection of Representative Questionnaire Items
3.2. Sensitivity and Specificity According to the Methods of Survey Order Using the Selected Representative Questionnaire Items
- (1)
- Only examine Q2 to check for abnormal findings,
- (2)
- Examine Q2 and Q1 to check for at least one abnormal finding,
- (3)
- Examine Q2 and Q1 to check for both abnormal findings,
- (4)
- Examine Q2, Q1, and Q3 to check for at least one abnormal finding,
- (5)
- Examine Q2, Q1, and Q3 to check for at least two abnormal findings,
- (6)
- Examine Q2, Q1, and Q3 to check for all three abnormal findings,
- (7)
- Examine Q2 and one questionnaire item out of Q1 and Q3 to calculate the sum of the two (7-1: Questionnaire items 2 and 1, 7-2: Questionnaire items 2 and 3).
- (8)
- Examine Q2, Q1, and Q3 to calculate the sum of all three.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCracken, J.L.; Veeranki, S.P.; Ameredes, B.T.; Calhoun, W.J. Diagnosis and management of asthma in adults: A review. JAMA 2017, 318, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Ventola, C.L. Mobile devices and apps for health care professionls: Uses and benefits. P T 2014, 39, 356–364. [Google Scholar] [PubMed]
- Chongmelaxme, B.; Lee, S.; Dhippayom, T.; Saokaew, S.; Chaiyakunapruk, N.; Dilokthornsakul, P. The effects of telemedicine on asthma control and patients’ quality of life in adults: A systematic review and meta-analysis. J. Allergy Clin. Immunol. Pract. 2019, 7, 199–216.e11. [Google Scholar] [CrossRef] [PubMed]
- Marcano Belisario, J.S.; Huckvale, K.; Greenfield, G.; Car, J.; Gunn, L.H. Smartphone and tablet self management apps for asthma. Cochrane Database Syst. Rev. 2013, 11, CD010013. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhai, Y.K.; Zhu, W.J.; Sun, D.X. Effectiveness of telemedicine for controlling asthma symptoms: A systematic review and meta-analysis. Telemed J. E Health 2015, 21, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Lee, S.Y.; Jo, E.J.; Lee, S.E.; Kang, M.G.; Song, W.J.; Kim, S.H.; Cho, S.H.; Min, K.U.; Ahn, K.H.; et al. Feasibility of a smartphone application based action plan and monitoring in asthma. Asia Pac. Allergy 2016, 6, 174–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anhøj, J.; Nielsen, L. Quantitative and qualitative usage data of an Internet-based asthma monitoring tool. J. Med. Internet Res. 2004, 6, e23. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, V.; Cvoriscec, B.; Ostojic, S.B.; Reznikoff, D.; Stipic-Markovic, A.; Tudjman, Z. Improving asthma control through telemedicine: A study of short-message service. Telemed J. E Health 2005, 11, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.; O’Connor, G.; Friedmann, R.H. Development and implementation of the home asthma telemonitoring (HAT) system to facilitate asthma self-care, IOS Press, Amsterdam, Netherlands. Stud. Health Technol. Inform. 2001, 84, 810–814. [Google Scholar]
- GINA, Global Initiative for Asthma—Global Strategy for Asthma Management and Prevention. 2021. Available online: https://ginasthma.org/ (accessed on 15 January 2022).
- Juniper, E.F.; O’Byrne, P.M.; Guyatt, G.H.; Ferrie, P.J.; King, D.R. Development and validation of a questionnaire to measure asthma control. Eur. Respir. J. 1999, 14, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.A.; Sorkness, C.A.; Kosinski, M.; Schatz, M.; Li, J.T.; Marcus, P.; Murray, J.J.; Pendergraft, T.B. Development of the asthma control test: A survey for assessing asthma control. J. Allergy Clin. Immunol. 2004, 113, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Sorkness, C.A.; Li, J.T.; Marcus, P.; Murray, J.J.; Nathan, R.A.; Kosinski, M.; Pendergraft, T.B.; Jhingran, P. Asthma Control Test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J. Allergy Clin. Immunol. 2006, 117, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.H.; Zeiger, R.; Sorkness, C.; Mahr, T.; Ostrom, N.; Burgess, S.; Rosenzweig, J.C.; Manjunath, R. Development and cross-sectional validation of the Childhood Asthma Control Test. J. Allergy Clin. Immunol. 2007, 119, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Gemicioğlu, B.; Mungan, D.; Bavbek, S.; Yıldız, F.; Polatlı, M.; Naycı, S.; Erkekol, F.Ö.; Türker, H.; Günen, H.; Çamsarı, G.; et al. Validity and reliability of the assessment tool for asthma (ATA) questionnaire: The ATA study. Turk. Thorac. J. 2020, 21, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Juniper, E.F.; Svensson, K.; Mörk, A.C.; Ståhl, E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir. Med. 2005, 99, 553–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiou, A.; Pearson, M. Measuring outcomes with tools of proven feasibility and utility: The example of a patient-focused asthma measure. J. Eval. Clin. Pract. 2002, 8, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Pinnock, H.; Burton, C.; Campbell, S.; Gruffydd-Jones, K.; Hannon, K.; Hoskins, G.; Lester, H.; Price, D. Clinical implications of the Royal College of Physicians three questions in routine asthma care: A real-life validation study. Prim. Care Respir. J. 2012, 21, 288–294. [Google Scholar] [CrossRef] [PubMed]
| Group 1 a (n = 473) | Group 2 b (n = 1546) | Total (n = 2019) | ||
|---|---|---|---|---|
| Sex | Men | 206 | 670 | 876 |
| Women | 267 | 876 | 1143 | |
| Age Group | 10–19 | 5 | 4 | 9 |
| 20–29 | 26 | 46 | 72 | |
| 30–39 | 27 | 84 | 111 | |
| 40–49 | 35 | 147 | 182 | |
| 50–59 | 46 | 266 | 312 | |
| 60–69 | 125 | 416 | 541 | |
| 70–79 | 119 | 370 | 489 | |
| 80–89 | 86 | 197 | 283 | |
| 90–99 | 4 | 16 | 20 | |
| Q1 | Q2 | Q3 | Q4 | Q5 | |
|---|---|---|---|---|---|
| Pearson Correlation Coefficient | 0.818 | 0.822 | 0.778 | 0.393 | 0.739 |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Correlation of the Q1 Score with the Total Score Excluding Q1 | Correlation of the Q2 Score with the Total Score Excluding Q2 | Correlation of the Q3 Score with the Total Score Excluding Q3 | Correlation of the Q4 Score with the Total Score Excluding Q4 | Correlation of the Q5 Score with the Total Score Excluding Q5 | |
|---|---|---|---|---|---|
| Pearson Correlation Coefficient | 0.684 | 0.652 | 0.591 | 0.226 | 0.592 |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Q1 | Q2 | Q3 | Q4 | Q5 | |
|---|---|---|---|---|---|
| Area under the curve | 0.909 | 0.910 | 0.860 | 0.599 | 0.855 |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Method 1 | Q2 score ≤ 4 | Q2 score = 5 | |
| Group 1 | 558 | 46 | |
| Group 2 | 360 | 1055 | |
| Sensitivity (%) | 92.0 | Specificity (%) | 74.0 |
| Method 2 | One of two scores (Q1, Q2) ≤ 4 | Both scores = 5 | |
| Group 1 | 596 | 8 | |
| Group 2 | 535 | 880 | |
| Sensitivity (%) | 98.6 | Specificity (%) | 62.0 |
| Method 3 | Both scores (Q1, Q2) ≤ 4 | One of two scores = 5 | |
| Group 1 | 524 | 80 | |
| Group 2 | 144 | 1024 | |
| Sensitivity (%) | 86.6 | Specificity (%) | 84.7 |
| Method 4 | One of three scores (Q1, Q2, Q3) ≤ 4 | All three scores = 5 | |
| Group 1 | 603 | 1 | |
| Group 2 | 660 | 755 | |
| Sensitivity (%) | 99.0 | Specificity (%) | 53.0 |
| Method 5 | Two of three scores (Q1, Q2, Q3) ≤ 4 | ||
| Group 1 | 574 | 30 | |
| Group 2 | 260 | 1155 | |
| Sensitivity (%) | 96.0 | Specificity (%) | 82.0 |
| Method 6 | All three scores (Q1, Q2, Q3) ≤ 4 | ||
| Group 1 | 425 | 179 | |
| Group 2 | 73 | 1342 | |
| Sensitivity (%) | 70.0 | Specificity (%) | 95.0 |
| Method 7-1 | Sum of two questionnaire item scores (Q1 + Q2) ≤ 8 | Higher than 9 | |
| Group 1 | 577 | 27 | |
| Group 2 | 239 | 1176 | |
| Sensitivity (%) | 96.0 | Specificity (%) | 83.0 |
| Method 7-2 | Sum of two questionnaire item scores (Q2 + Q3) ≤ 8 | Higher than 9 | |
| Group 1 | 568 | 36 | |
| Group 2 | 154 | 1261 | |
| Sensitivity (%) | 94.0 | Specificity (%) | 89.0 |
| Method 8 | Sum of three questionnaire item scores (Q1 + Q2 +Q3) ≤ 12 | Higher than 13 | |
| Group 1 | 585 | 19 | |
| Group 2 | 122 | 1293 | |
| Sensitivity (%) | 97.0 | Specificity (%) | 91.0 |
| Correlation of the Sum of Q1 and Q2 Scores and the Total Score | Correlation of the Sum of Q1 and Q2 Scores and the Total Score Minus the Scores of Q1 and Q2 | Correlation of the Sum of Q2 and Q3 Scores and the Total Score | Correlation of the Sum of Q2 and Q3 Scores and the Total Score Minus the Scores of Q2 and Q3 | |
|---|---|---|---|---|
| Pearson’s Correlation Coefficient | 0.914 | 0.656 | 0.919 | 0.707 |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, M.-S.; Yu, I.; Park, S.; Lee, J.-H.; Lee, S.J.; Lee, W.-Y.; Yong, S.J.; Jo, M.; Kim, S.-H. Selection of Representative Questionnaire Items from the Asthma Control Test. J. Pers. Med. 2022, 12, 1913. https://doi.org/10.3390/jpm12111913
Chang M-S, Yu I, Park S, Lee J-H, Lee SJ, Lee W-Y, Yong SJ, Jo M, Kim S-H. Selection of Representative Questionnaire Items from the Asthma Control Test. Journal of Personalized Medicine. 2022; 12(11):1913. https://doi.org/10.3390/jpm12111913
Chicago/Turabian StyleChang, Min-Seok, Iseul Yu, Sunmin Park, Ji-Ho Lee, Seok Jeong Lee, Won-Yeon Lee, Suk Joong Yong, Meounggun Jo, and Sang-Ha Kim. 2022. "Selection of Representative Questionnaire Items from the Asthma Control Test" Journal of Personalized Medicine 12, no. 11: 1913. https://doi.org/10.3390/jpm12111913
APA StyleChang, M.-S., Yu, I., Park, S., Lee, J.-H., Lee, S. J., Lee, W.-Y., Yong, S. J., Jo, M., & Kim, S.-H. (2022). Selection of Representative Questionnaire Items from the Asthma Control Test. Journal of Personalized Medicine, 12(11), 1913. https://doi.org/10.3390/jpm12111913








