Optimal Prandial Timing of Insulin Bolus in Youths with Type 1 Diabetes: A Systematic Review
Abstract
:1. Introduction
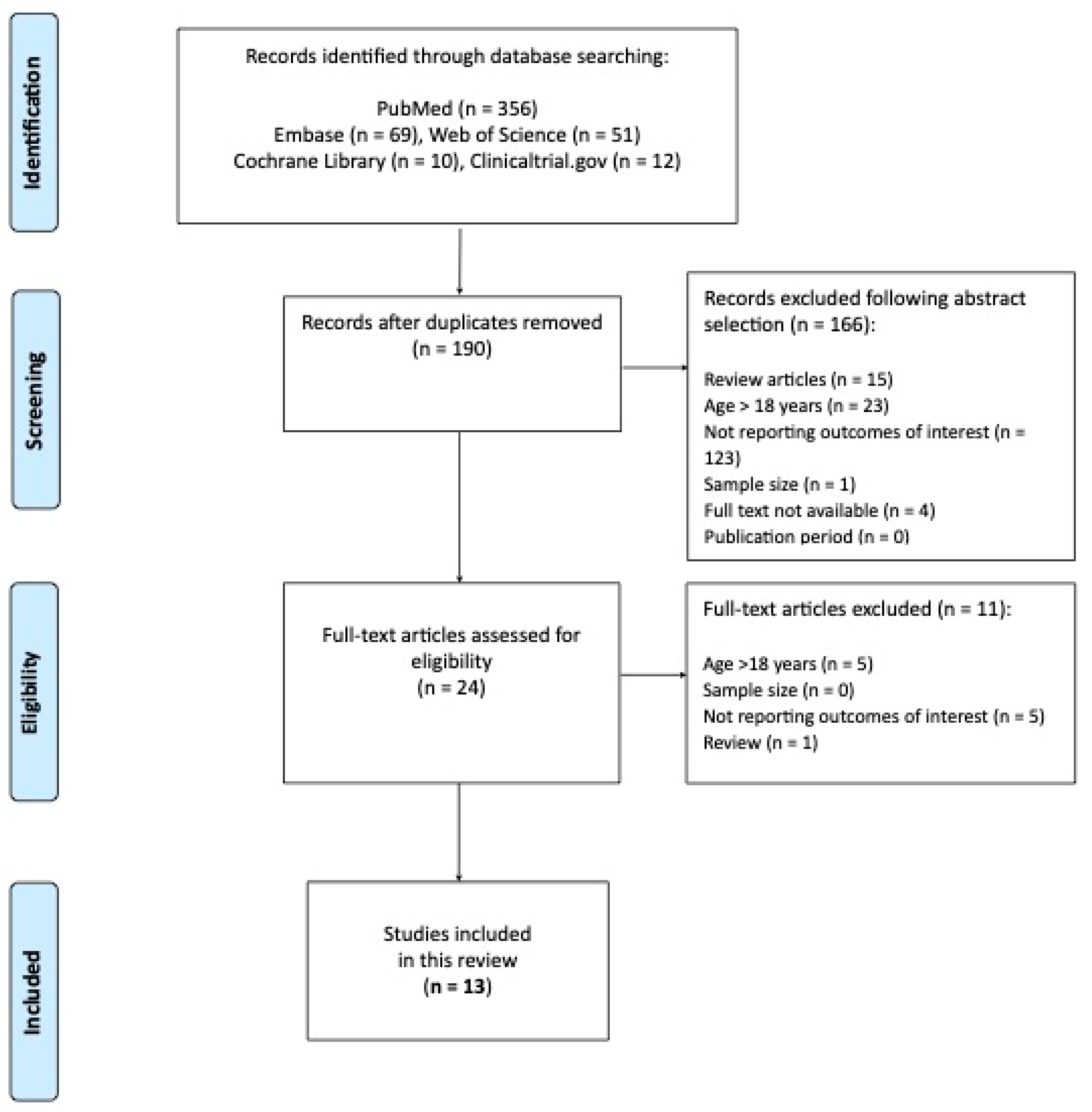
2. Material and Methods
2.1. Search Strategy
2.2. Criteria for Study Selection
| Population | Children and adolescents (1–18 years) with T1D. |
| Intervention | Insulin bolus given immediately before meal (START: ‒2 to 0 min) or post-meal (POST: 10–20 min after the start of the meal) Rapid analogue insulin bolus or mealtime (START) or post-meal (POST) fast-acting insulin analogue bolus. |
| Comparison | Pre-meal bolus (‒20 to ‒10 min), the gold standard in adults. |
| Outcomes | (i) post-prandial glucose levels, blood glucose area under the curve (AUC), maximum blood glucose level; (ii) HbA1c, number of hypoglycemic episodes, diabetic ketoacidosis (DKA) episodes, total daily insulin dose, time in range, time below range; (iii) BMI; (iv) treatment satisfaction. |
| Study design | Randomized clinical trials (RCTs), observational studies (cohort, case-control, cross-sectional studies), exploratory studies, mix of qualitative and quantitative studies. |
2.3. Data Extraction and Management
2.4. Assessment of the Certainty of the Evidence
| High | The authors have a lot of confidence that the true effect is similar to the estimated effect. |
| Moderate | The authors believe that the true effect is probably close to the estimated effect. |
| Low | The true effect might be markedly different from the estimated effect. |
| Very low | The true effect is probably markedly different from the estimated effect. |
3. Evidence from Clinical Studies
3.1. Glycemic Outcomes
3.2. Total Daily Insulin Dose and BMI
3.3. Treatment Satisfaction
4. Discussion
- Similar to adults, in the pediatric population, individuals using pre-meal insulin injection showed better glycemic outcomes (post-prandial BG, HbA1c, and hypoglycemia) compared with those on post-meal injections.
- Studies on fast-acting analogues confirmed the feasibility of post-meal dosing, which could contribute to lower BG levels for two hours after the meal according to their pharmacokinetic properties [10].
- The available data on treatment satisfaction are insufficient to make any conclusion about a negative effect on quality of life associated with pre-meal compared to post-meal bolusing.
- Only a few studies reported CGM data, which are a very important tool to move towards a personalized approach for the timing of insulin boluses based on individual characteristics, age groups, and meal composition. CGM data also provides valuable information on the individual’s glucose trends (stable, increasing, or decreasing levels) to adapt insulin timing or dose and improve time in range (TIR) [28].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiMeglio, L.A.; Acerini, C.L.; Codner, E.; Craig, M.E.; Hofer, S.E.; Pillay, K.; Maahs, D.M. ISPAD Clinical Practice Consensus Guidelines 2018, Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr. Diabetes 2018, 19, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novo Nordisk A/S. NovoRapid Summary of Product Characteristics. 2011. Available online: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000258/WC500030372.pdf (accessed on 22 October 2022).
- Eli Lilly. Humalog Summary of Product Characteristics. 2011. Available online: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000088/WC500050332.pdf (accessed on 22 October 2022).
- Sanofi-Aventis. Apidra Summary of Product Characteristics. 2011. Available online: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000557/WC500025250.pdf (accessed on 22 October 2022).
- Home, P.D. The pharmacokinetics and pharmacodynamics of rapid-acting insulin analogues and their clinical consequences. Diabetes Obes. Metab. 2012, 14, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Duran-Valdez, E.; Burge, M.R.; Broderick, P.; Shey, L.; Valentine, V.; Schrader, R.M.; Schade, D.S. Insulin timing: A patient-centered approach to improve control in type 1 diabetes. Endocr. Pract. 2017, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Pettus, J.; Price, D.A.; Edelman, S.V. How patients with type 1 diabetes translate continuous glucose monitoring data into diabetes management decisions. Endocr. Pract. 2015, 21, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Slattery, D.; Amiel, S.A.; Choudhary, P. Optimal prandial timing of bolus insulin in diabetes management: A review. Diabet. Med. 2018, 35, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Pettus, J. Time to get serious about insulin timing. Endocr. Pract. 2017, 23, 503–505. [Google Scholar] [CrossRef]
- Fath, M.; Danne, T.; Biester, T.; Erichsen, L.; Kordonouri, O.; Haahr, H. Faster-acting insulin aspart provides faster onset and greater early exposure vs. insulin aspart in children and adolescents with type 1 diabetes mellitus. Pediatr. Diabetes 2017, 18, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Boughton, C.K.; Hartnell, S.; Allen, J.M.; Hovorka, R. The importance of prandial insulin bolus timing with hybrid closed-loop systems. Diabet. Med. 2019, 36, 1716–1717. [Google Scholar] [CrossRef]
- Sy, S.L.; Munshi, M.M.; Toschi, E. Can Smart Pens Help Improve Diabetes Management? J. Diabetes Sci. Technol. 2022, 16, 628–634. [Google Scholar] [CrossRef]
- Johansen, M.D.; Gjerløv, I.; Christiansen, J.S.; Hejlesen, O.K. Interindividual and intraindividual variations in postprandial glycemia peak time complicate precise recommendations for self-monitoring of glucose in persons with type 1 diabetes mellitus. J. Diabetes Sci. Technol. 2012, 6, 356–361. [Google Scholar] [CrossRef]
- Simmons, J.H.; Chen, V.; Miller, K.M.; McGill, J.B.; Bergenstal, R.M.; Goland, R.S.; Harlan, D.M.; Largay, J.F.; Massaro, E.M.; Beck, R.W.; et al. Differences in the management of type 1 diabetes among adults under excellent control compared with those under poor control in the T1D Exchange Clinic Registry. Diabetes Care 2013, 36, 3573–3577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schünemann, H.J.; Cuello, C.; Akl, E.A.; Mustafa, R.A.; Meerpohl, J.J.; Thayer, K.; Morgan, R.L.; Gartlehner, G.; Kunz, R.; Katikireddi, S.V.; et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J. Clin. Epidemiol. 2019, 111, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobry, E.; McFann, K.; Messer, L.; Gage, V.; VanderWel, B.; Horton, L.; Chase, H.P. Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with type 1 diabetes. Diabetes Technol. Ther. 2010, 12, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Danne, T.; Aman, J.; Schober, E.; Deiss, D.; Jacobsen, J.L.; Friberg, H.H.; Jensen, L.H.; ANA 1200 Study Group. A comparison of postprandial and preprandial administration of insulin aspart in children and adolescents with type 1 diabetes. Diabetes Care 2003, 26, 2359–2364. [Google Scholar] [CrossRef] [Green Version]
- Rohilla, L.; Dayal, D.; Gujjar, N.; Walia, P.; Kumar, R.; Yadav, J. Mealtime bolus insulin dose timing in children with type 1 diabetes: Real-life data from a tertiary care centre in northern India. Acta Endocrinol. 2021, 17, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Lane, W.; Lambert, E.; George, J.; Rathor, N.; Thalange, N. Exploring the Burden of Mealtime Insulin Dosing in Adults and Children With Type 1 Diabetes. Clin. Diabetes 2021, 39, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Van Name, M.A.; Thorsted, B.L.; Piltoft, J.S.; Tamborlane, W.V. Postprandial dosing of bolus insulin in patients with type 1 diabetes: A cross-sectional study using data from the T1D Exchange registry. Endocr. Pract. 2017, 23, 1201–1209. [Google Scholar] [CrossRef]
- Tucholski, K.; Sokołowska, M.; Tucholska, D.; Kamińska, H.; Jarosz-Chobot, P. Assessment of optimal insulin administration timing for standard carbohydrates-rich meals using continuous glucose monitoring in children with type 1 diabetes: A cross-over randomized study. J. Diabetes Investig. 2019, 10, 1237–1245. [Google Scholar] [CrossRef]
- Datye, K.A.; Boyle, C.T.; Simmons, J.; Moore, D.J.; Jaser, S.S.; Sheanon, N.; Kittelsrud, J.M.; Woerner, S.E.; Miller, K.M.; T1D Exchange. Timing of Meal Insulin and Its Relation to Adherence to Therapy in Type 1 Diabetes. J. Diabetes Sci. Technol. 2018, 12, 349–355. [Google Scholar] [CrossRef] [Green Version]
- De Palma, A.; Giani, E.; Iafusco, D.; Bosetti, A.; Macedoni, M.; Gazzarri, A.; Spiri, D.; Scaramuzza, A.E.; Zuccotti, G.V. Lowering postprandial glycemia in children with type 1 diabetes after Italian pizza “margherita” (TyBoDi2 Study). Diabetes Technol. Ther. 2011, 13, 483–487. [Google Scholar] [CrossRef]
- Bode, B.W.; Iotova, V.; Kovarenko, M.; Laffel, L.M.; Rao, P.V.; Deenadayalan, S.; Ekelund, M.; Larsen, S.F.; Danne, T. Efficacy and Safety of Fast-Acting Insulin Aspart Compared With Insulin Aspart, Both in Combination With Insulin Degludec, in Children and Adolescents With Type 1 Diabetes: The onset 7 Trial. Diabetes Care 2019, 42, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Kikuchi, T.; Horio, H.; Rathor, N.; Ekelund, M. Efficacy and safety of fast-acting insulin aspart versus insulin aspart in children and adolescents with type 1 diabetes from Japan. Endocr. J. 2021, 28, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Wadwa, R.P.; Laffel, L.M.; Franco, D.R.; Dellva, M.A.; Knights, A.W.; Pollom, R.K. Efficacy and safety of ultra-rapid lispro versus lispro in children and adolescents with type 1 diabetes: The PRONTO-Peds trial. Diabetes Obes. Metab. 2023, 25, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzza, A.E.; Iafusco, D.; Santoro, L.; Bosetti, A.; De Palma, A.; Spiri, D.; Mameli, C.; Zuccotti, G.V. Timing of bolus in children with type 1 diabetes using continuous subcutaneous insulin infusion (TiBoDi Study). Diabetes Technol. Ther. 2010, 12, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, J.S.; Barrett, T.G.; Dias, R.P.; Kershaw, M.; Krone, R.; Uday, S. An effective and cost-saving structured education program teaching dynamic glucose management strategies to a socio-economically deprived cohort with type 1 diabetes in a VIRTUAL setting. Pediatr. Diabetes 2022, 23, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Hövelmann, U.; Brøndsted, L.; Adrian, C.L.; Nosek, L.; Haahr, H. Faster-acting insulin aspart: Earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes. Metab. 2015, 17, 682–688. [Google Scholar] [CrossRef]
| Rapid-Acting Analogs | |||||||
|---|---|---|---|---|---|---|---|
| References | Main Objective | Study Design | Population and Comparator, Setting | Methods | Bolus Timing | Results | Study Limitations and Level of Evidence |
| Scaramuzza AE et al. [27] | Effect of different timing of bolus dose | cross-sectional | 30 T1D Age: 6–20 yrs (15.2 ± 3.9) Treatment: CSII, Aspart Setting: hospital for 3 days Period: 2009 Region: Italy | Meal: standard lunch (55% CHO) for 3 days, lasted 15–20 min Pre-prandial BG: 80–140 mg/dL Capillary BG monitor: −15, 0, 30, 60, 90, 120, 180 min Outcome: 1 h- and 3 h-PBG, AUC; number of hypoglycemic events | −15 (PRE), immediately before (START) and immediately after the meal (POST) randomly assigned to each patient | 1 h-PBG was lower when bolus PRE or START vs. POST l (p = 0.024), not significant at 3 h-PBG No difference in PBG at any time, when bolus was administered PRE vs. START Lower AUC for glycemia with bolus PRE, but NS Hypoglycemia: 12 patients experienced 1 episode each of mild hypoglycemia | -Moderate- |
| Cobry E et al. (2010) [16] | Determine the optimal timing of insulin bolus delivery | cross-over | 23 T1D Age: 12–30 yrs (18.3 ± 4.4; 11 pediatric) Treatment: CSII, Glulisine Setting: 3 clinical visits Period: 2009 Region: Colorado | Meal: frozen prepackaged breakfast Pre-prandial BG: 100–180 mg/dL Capillary BG monitor: 30, 60, 90, 120, 150, 180, 210, 240 min Outcome: 1–2 h PBG, BG peak, TAR, AUC, hypoglycemia | −20 (PRE) immediately before (START) and +20 min (POST), randomized | Lower 1 h- and 2 h-PBG with PRE vs. START (0.0029 and 0.0294) vs. POST (p = 0.001 and 0.0408) bolus. No differences between START and POST Lower BG readings above 180 mg/dL in PRE vs. START bolus (p < 0.0001) and POST bolus (p < 0.0001) Lower AUC with PRE vs. START bolus (p = 0.0297) Lower peak BG with PRE vs. START bolus (p = 0.0039) and POST bolus(p = 0.0027). Hypoglycemia: no significant difference among the different treatment groups | Small pediatric sample -Moderate- |
| Danne T et al. (2003) [17] | Compare PBG after pre-prandial vs. post-prandial insulin injection | Randomized, open-labeled, cross-over trial 6 weeks period | 42 T1D 6–12 yrs 34 T1D 13–17 yrs (12.2 ± 2.8 yrs) Treatment: MDI (long-acting basal insulin: NPH, lente, or ultralente) Aspart Setting: 3 visits in 6 week period Period: 2003 Region: Germany, Austria, Sweden | Meal: all Capillary BG profiles (before, 120 min after meal and at 10:00 p.m. ± 1 h) Treatment Satisfaction Questionnaire (DTSQ) completed by adolescents and parents of the children at the clinic before and after each treatment period. Outcome: 2 h-PBG, Fructosamine (+6 weeks), HbA1c (+6 weeks), hypoglycemia, DTSQ score | Immediately before (PRE), immediately after (0–30′) meal start (POST) | Lower PBG 120 min after breakfast for IAsp PRE vs. POST (p = 0.016) Fructosamine and HbA1c: no difference in IAsp PRE vs. IAsp POST The relative risk of hypoglycemia was not significantly different (p = 0.31) No clinically relevant differences were found between the two age groups in any of the parameters Treatment satisfaction was equally high for both regimens with both patients and parents | NPH use -Moderate- |
| Rohilla L et al. (2021) [18] | Real world data on post-prandial bolusing in young children with T1D | Retrospective study | 44 T1D Age: 2–7 yrs (4.1 ± 1.3) Treatment: MDI in basal bolus Period: 2015–2021 Region: North India | Meal: all Capillary BG 2 years f/up Outcome: hypoglycemia, DKA, HbA1c | 10–20′ before (PRE). during or within 10′ after meal (POST) | HbA1c: no difference during f/up between Group 1 and Group 2 DKA, number of hypoglycemic episodes: not different | PBG not detected. The only study with age <6 y -Low- |
| Lane W et al. (2021) [19] | Review of the burden associated with pre-meal insulin administration | Prospective online survey | 350 parents of children ≤15 yrs Treatment: 70% MDI Aspart and Lispro Period: 2019–2020 Region: USA, Canada, UK, Japan, Spain, and France | Meal: all Online survey Outcome: burden, quality of life | 15–20′ before (PRE) 0–2′ before (START) after the start of the meal (POST) | 93% of parents felt that PRE bolus has a negative impact on the child’s day to day life Having the freedom to administer insulin at START or POST would have a positive impact | Online survey -Low- |
| Peters A et al. (2017) [20] | Assess prevalence and characteristics of children and adolescents with T1D using pre-prandial vs. post-prandial bolus | Cross sectional study, data from T1D Exchange registry | 21533 T1D (12450 < 18 yrs) Treatment: 99% used rapid-acting insulin. Pump users 48% Period: 2010–2012 Region: USA | Meal: all Capillary BG Survey: when do you usually give an insulin bolus? Outcomes: HbA1c, total daily insulin dose/Kg, hypoglycemia, DKA, BMI | Insulin several minutes before or immediately before meal (PRE) vs. during meal or after meal (POST) = 32% | Children dosing POST (32%) were characterized by higher HbA1c (p < 0.0001), larger total daily insulin dose/Kg (p < 0.0001), greater prevalence of history of hypoglycemia (p = 0.0071) and DKA (p = 0.02) vs. PRE BMI was significantly increased in the POST group versus PRE for ages 12–18 yrs only (p 0.078) | Cross-sectional design -Moderate- |
| Tucholski K et al. (2019) [21] | Assess PBG in children and adolescents using CSII after carbohydrate-rich meals | Cross over RCT | 29 T1D Age: 9.6–15.2 yrs Treatment: CSII, rapid-acting insulin Period: 2009–2010 Region: Poland | Meal: over a period of 3 days, consumption of carbohydrate-rich meal (60–65%) at breakfast Outcomes: CGM: PBG at 0, 120, 180′, glucose peak, AUC, hypoglycemia | Insulin 20 min before (PRE) vs. 10′ before (PRE) vs. 0′ (START) | Patients who administered bolus 20 min PRE vs. at START had longer median time to reach peak glucose (p = 0.01) PBG and peak differences were NS Hypoglycemia: NS | -Moderate- |
| Datye KA et al. (2018) [22] | Explore the association between timing of insulin bolus and missed bolus | Cross sectional study, data from T1D exchange registry | 3608 T1D < 18 yrs Treatment: CSII (60%) Period: 2010–2012 Region: USA | Meal: all Capillary BG Survey on timing of bolus at meal, frequency of missed meal insulin doses Outcomes: prevalence of bolus before meal, population characteristics, missed bolus, HbA1c, hypoglycemia | Several minutes before (PRE), immediately before (START), during-after meal (POST), and “I do not give a mealtime bolus”. Frequency of missed meal insulin doses (from never to once a day) | Prevalence: Insulin PRE (21%), at START (44%), or POST (during 10%, after 24%) Giving insulin PRE or at START was reported by 61% of participants/parents <6 yrs of age, 72% of those 6–13 yrs, 68% of those 13–18 yrs Insulin PRE: usually younger patient, shorter DT1 duration, more likely to use pump therapy, monitored BG more frequently Insulin PRE was associated with lower HbA1c and fewer missed meal insulin doses (p < 0.01) (vs. during or after meal). No association between timing of meal insulin and occurrence of severe hypoglycemia events | -Moderate- |
| Rapid-Acting Analogs and a Pizza Meal | |||||||
| De Palma A et al. (2011) [23] | Evaluate the most effective type and timing of a pump-delivered, pre-prandial bolus for a pizza ‘‘margherita’’ meal | Longitudinal study | 38 T1D Age: 6–19 yrs Treatment: CSII, rapid-acting insulin Period: 2010 Setting: hospital Region: Italy | Meal based on pizza Margherita, at lunch Capillary BG −15, 0, +30, 60, 90, 120, 180, 240, 300 Outcomes: BG, hypoglycemia, AUC 0–6 h | (a) a dual-wave bolus 30%/70% over a 6-h period, administered 15 min PRE(b) a dual-wave bolus 30/70% given over a 6-h period, at START; (c) a standard bolus 15 min PRE(d) a standard bolus at START | The simple bolus 15 min PRE, rather than at START or delivered as a double-wave bolus, is better to control the glycemic rise (AUC 0–6 h) usually observed (p < 0.01) No difference in hypoglycemia | -Moderate- |
| Fast-Acting Analogs | |||||||
| Bode B et al. (2019) [24] | Assess the efficacy and safety of faster aspart vs. IAsp | RCT | 777 patients with DT1 < 18 yrs Treatment: FAsp vs. IAsp for 26 weekswith Degludec Period: 2016–2018 Region: 150 sites across 17 countries | Meal: a standardized liquid meal at main meals Capillary BG. CGM in a subgroup of 135 patients Outcomes: HbA1c, hypoglycemia, PBG at 1 h, TDI | 260 mealtime FAsp 258 mealtime IAsp 269 post-meal FAsp | HbA1c: Mealtime and post-meal FAsp performed better than IAsp (p = 0.014) Lower 1-h PBG increment with FAsp versus IAsp over all meals (p < 0.01 for all) No significant differences in the overall rate of hypoglycemia, severe hypoglycemia, insulin dose and BMI | Home-sampling kit to measure FPG -Moderate- |
| Kawamura T et al. 2021 [25] | Assess the efficacy and safety of faster aspart vs. IAsp | Post-hoc subgroup analysis based on data from the RCT onset 7 trial | 66 T1D < 18 yrs Treatment: FAsp vs. IAsp for 26 weeks with Degludec Period: 2013–2015 Region: Japan | Meal: all Capillary BG profiles at baseline and week 26 Follow-up on day 7 and day 30 Pre-prandial target BG: 71–145 mg/dL;Bedtime 120–180 mg/dL Outcomes: HbA1c, PBG, hypogycemia, insulin dose, body weight | 24 mealtime FAsp 19 post-meal FAsp 23 mealtime IAsp | HbA1c: the post-prandial FAsp performed better (with a change from baseline of 0.74%) than the meal FAsp (0.23%) and IAsp (0.39%) Lower 1-h PBG increment with mealtime FIAsp versus IAsp over all meals No differences in the overall rate of hypoglycemia, severe hypoglycemia, insulin dose | Low sample size, which precluded statistical analysis between the treatment groups -Low - |
| Fath M et al. 2017 [10] | Assess FIASP exposure and action in children and adolescents vs. IAsp | RCT | 12 children with T1D (6–11 yrs) 13 adolescents with T1D (12–17 yrs) Treatment: MDI and CSII; FiAsp vs. IAsp Period: 2014 Region: Hanover (Germany) | Meal: a standardized liquid meal (BOOST, Nestlé S.A) consumed within 8 min. The volume of the liquid meal was adjusted according to the subject’s body weight Two dosing visits and a follow up visit. At each dosing visit, a stable glucose level was achieved overnight using an established protocol of variable intravenous infusion Capillary BG Outcomes: PBG from 0 to 2 h | Subjects received 0.2 U/kg subcutaneous dosing immediately prior to a standardized meal | Onset of appearance occurred 5–7 min earlier and exposure was greater for FIASP vs. IAsp in children and adolescents PG excursion appeared to be reduced for faster aspart compared with IAsp at 0–1 h (p = 0.05) and at 0–2 h (p = 0.028) and as peak (p = 0.044) in children but not in adolescents | Low sample size, standardized liquid meal -Moderate- |
| Wadwa RP et al. 2022 [26] | Assess the efficacy of ultra-rapid lispro (URLi) versus lispro | RCT prospective, double-blind | 716 T1D Age 12.26 ± 3.39 yrs Treatment: MDI Period: 2019–2021 Region: USA, 96 sites | Meal: all 26-week treatment period: randomized to double-blind and pre-study basal insulin Capillary BG and CGM systems Outcomes: HBA1c, PBG, insulin dose, hypoglycemia | URLi (n = 280) or lispro (n = 298) Injection 0–2 min prior to meals (mealtime) vs. open-label URLi (n = 138) injected up to 20 min after start of meals (post-meal) | HbA1c: no significant differences among the treatment groups after 26 weeks When dosed at the beginning of meals, URLi reduced 1-h PBG (p = 0.001) and PPG excursions versus lispro (p < 0.001) Hypoglycemia: mealtime URLli vs. Lispro presented higher rate of hypoglycemia (<54 mg/dL) at ≤2 h (p = 0.034) CGM group (n = 79): no difference in AUC 0–2 h | Poor CGM data –high |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozzillo, E.; Franceschi, R.; Di Candia, F.; Ricci, A.; Leonardi, L.; Girardi, M.; Rosanio, F.M.; Marcovecchio, M.L. Optimal Prandial Timing of Insulin Bolus in Youths with Type 1 Diabetes: A Systematic Review. J. Pers. Med. 2022, 12, 2058. https://doi.org/10.3390/jpm12122058
Mozzillo E, Franceschi R, Di Candia F, Ricci A, Leonardi L, Girardi M, Rosanio FM, Marcovecchio ML. Optimal Prandial Timing of Insulin Bolus in Youths with Type 1 Diabetes: A Systematic Review. Journal of Personalized Medicine. 2022; 12(12):2058. https://doi.org/10.3390/jpm12122058
Chicago/Turabian StyleMozzillo, Enza, Roberto Franceschi, Francesca Di Candia, Alessia Ricci, Letizia Leonardi, Martina Girardi, Francesco Maria Rosanio, and Maria Loredana Marcovecchio. 2022. "Optimal Prandial Timing of Insulin Bolus in Youths with Type 1 Diabetes: A Systematic Review" Journal of Personalized Medicine 12, no. 12: 2058. https://doi.org/10.3390/jpm12122058
APA StyleMozzillo, E., Franceschi, R., Di Candia, F., Ricci, A., Leonardi, L., Girardi, M., Rosanio, F. M., & Marcovecchio, M. L. (2022). Optimal Prandial Timing of Insulin Bolus in Youths with Type 1 Diabetes: A Systematic Review. Journal of Personalized Medicine, 12(12), 2058. https://doi.org/10.3390/jpm12122058








