Pelvic Venous Insufficiency: Input of Short Tau Inversion Recovery Sequence
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Magnetic Resonance Imaging
2.3. Phlebography
2.4. Imaging Analysis
2.5. Statistical Analysis
3. Results
3.1. Patients and Anatomical Analysis
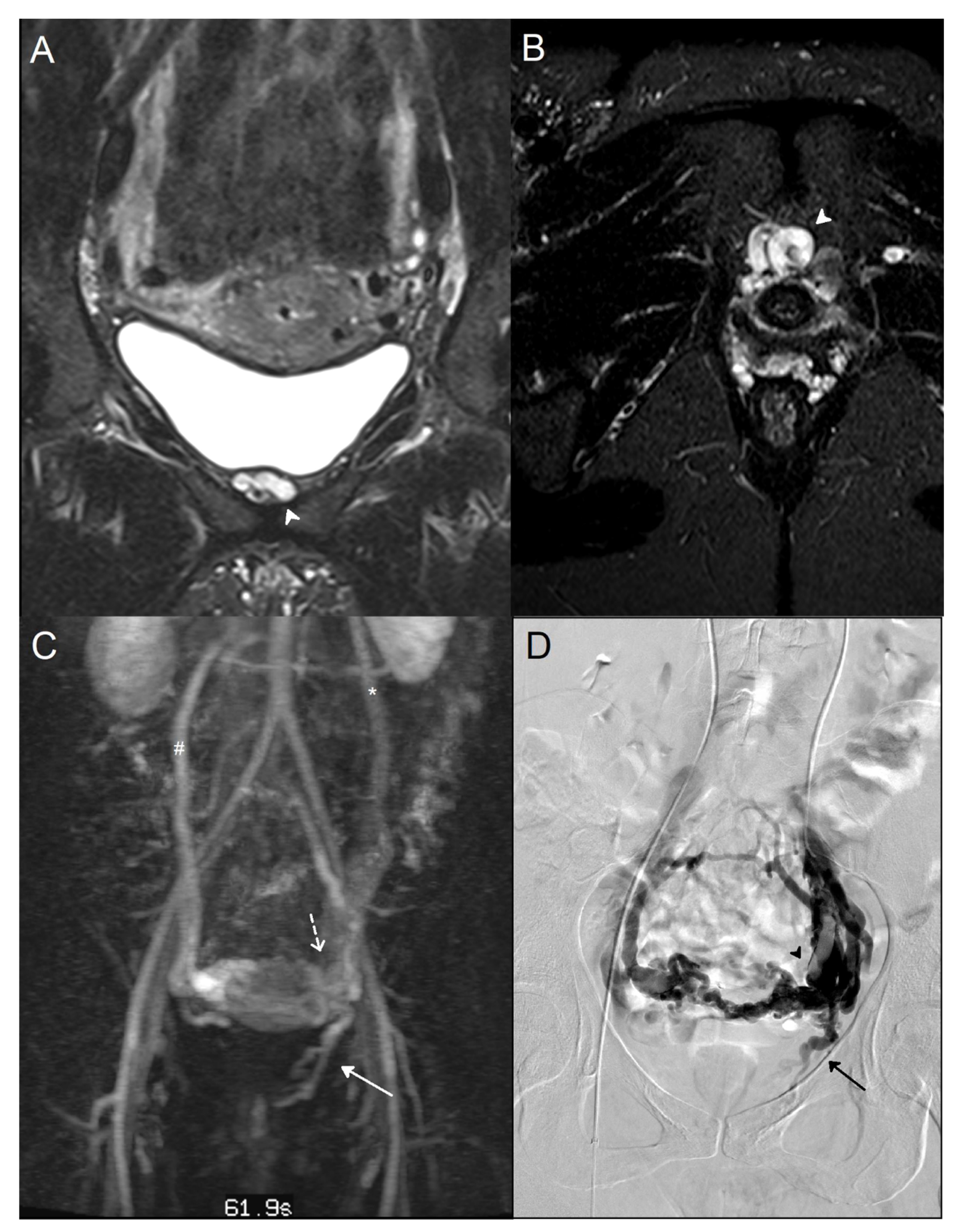
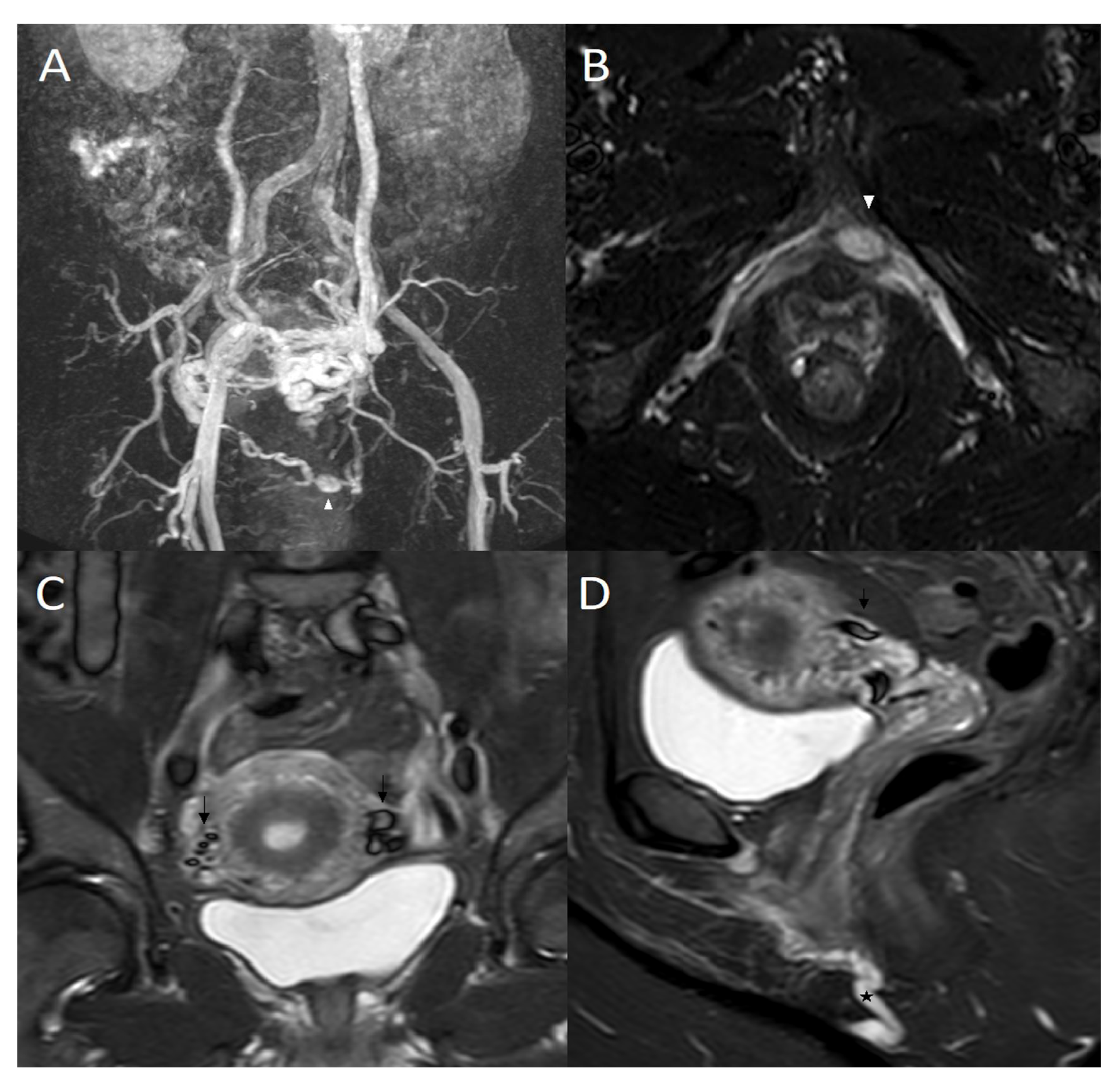
3.2. STIR and Phlebographic Findings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVI | pelvic venous insufficiency |
| MRI | magnetic resonance imaging |
| LOV | left ovarian vein |
| LRV | left renal vein |
| STIR | short tau inversion recovery |
| ROV | right ovarian vein |
| PCS | pelvic congestion syndrome |
| VVLL | varicose veins in the lower limbs |
| CT | computed tomography |
| MIP | maximum intensity projection |
| PPV | positive predictive value |
| NPV | negative predictive value |
References
- Gültaşli, N.Z.; Kurt, A.; Ipek, A.; Gümüş, M.; Yazicioğlu, K.R.; Dilmen, G.; Taş, I. The relation between pelvic varicose veins, chronic pelvic pain and lower extremity venous insufficiency in women. Diagn. Interv. Radiol. 2006, 12, 34–38. [Google Scholar] [PubMed]
- Phillips, D.; Deipolyi, A.R.; Hesketh, R.L.; Midia, M.; Oklu, R. Pelvic Congestion Syndrome: Etiology of Pain, Diagnosis, and Clinical Management. J. Vasc. Interv. Radiol. 2014, 25, 725–733. [Google Scholar] [CrossRef]
- Greiner, M.; Gilling-Smith, G.L. Leg Varices Originating from the Pelvis: Diagnosis and Treatment. Vascular 2007, 15, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bora, A.; Avcu, S.; Arslan, H.; Adali, E.; Bulut, M.D. The relation between pelvic varicose veins and lower extremity venous insufficiency in women with chronic pelvic pain. JBR-BTR 2012, 95, 215. [Google Scholar] [CrossRef] [PubMed]
- Khilnani, N.M.; Meissner, M.H.; Learman, L.A.; Gibson, K.D.; Daniels, J.P.; Winokur, R.S.; Marvel, R.P.; Machan, L.; Venbrux, A.C.; Tu, F.F.; et al. Research Priorities in Pelvic Venous Disorders in Women: Recommendations from a Multidisciplinary Research Consensus Panel. J. Vasc. Interv. Radiol. 2019, 30, 781–789. [Google Scholar] [CrossRef]
- Taylor, H.C. Vascular congestion and hyperemia; their effect on function and structure in the female reproductive organs; the clinical aspects of the congestion-fibrosis syndrome. Am. J. Obstet. Gynecol. 1949, 57, 637–653. [Google Scholar] [CrossRef]
- Champaneria, R.; Shah, L.; Moss, J.; Gupta, J.K.; Birch, J.; Middleton, L.J.; Daniels, J.P. The relationship between pelvic vein incompetence and chronic pelvic pain in women: Systematic reviews of diagnosis and treatment effectiveness. Health Technol. Assess. 2016, 20, 1–108. [Google Scholar] [CrossRef]
- Belenky, A.; Bartal, G.; Atar, E.; Cohen, M.; Bachar, G.N. Ovarian Varices in Healthy Female Kidney Donors: Incidence, Morbidity, and Clinical Outcome. Am. J. Roentgenol. 2002, 179, 625–627. [Google Scholar] [CrossRef]
- Kim, C.Y.; Miller, M.J.; Merkle, E.M. Time-Resolved MR Angiography as a Useful Sequence for Assessment of Ovarian Vein Reflux. Am. J. Roentgenol. 2009, 193, W458–W463. [Google Scholar] [CrossRef]
- Jurga-Karwacka, A.; Karwacki, G.M.; Schoetzau, A.; Zech, C.J.; Heinzelmann-Schwarz, V.; Schwab, F.D. A forgotten disease: Pelvic congestion syndrome as a cause of chronic lower abdominal pain. PLoS ONE 2019, 14, e0213834. [Google Scholar] [CrossRef]
- Ganeshan, A.; Upponi, S.; Hon, L.-Q.; Uthappa, M.C.; Warakaulle, D.R.; Uberoi, R. Chronic Pelvic Pain due to Pelvic Congestion Syndrome: The Role of Diagnostic and Interventional Radiology. Cardiovasc. Interv. Radiol. 2007, 30, 1105–1111. [Google Scholar] [CrossRef]
- Black, C.M.; Thorpe, K.; Venrbux, A.; Kim, H.S.; Millward, S.F.; Clark, T.W.; Kundu, S.; Martin, L.G.; Sacks, D.; York, J.; et al. Research Reporting Standards for Endovascular Treatment of Pelvic Venous Insufficiency. J. Vasc. Interv. Radiol. 2010, 21, 796–803. [Google Scholar] [CrossRef]
- Beard, R.; Pearce, S.; Highman, J.; Reginald, P. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet 1984, 2, 946–949. [Google Scholar] [CrossRef]
- Asciutto, G.; Mumme, A.; Marpe, B.; Köster, O.; Asciutto, K.; Geier, B. MR Venography in the Detection of Pelvic Venous Congestion. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 491–496. [Google Scholar] [CrossRef]
- Steenbeek, M.P.; van der Vleuten, C.J.; Kool, L.J.S.; Nieboer, T.E. Noninvasive diagnostic tools for pelvic congestion syndrome: A systematic review. Acta Obstet. Gynecol. Scand. 2018, 97, 776–786. [Google Scholar] [CrossRef]
- Bookwalter, C.A.; VanBuren, W.M.; Neisen, M.J.; Bjarnason, H. Imaging Appearance and Nonsurgical Management of Pelvic Venous Congestion Syndrome. RadioGraphics 2019, 39, 596–608. [Google Scholar] [CrossRef]
- Jin, K.N.; Lee, W.; Jae, H.J.; Yin, Y.H.; Chung, J.W.; Park, J.H. Venous reflux from the pelvis and vulvoperineal region as a possible cause of lower extremity varicose veins: Diagnosis with computed tomographic and ultrasonographic findings. J. Comput. Assist. Tomogr. 2009, 33, 763–769. [Google Scholar] [CrossRef]
- Arnoldussen, C.W.K.P.; de Wolf, M.A.F.; Wittens, C.H.A. Diagnostic imaging of pelvic congestive syndrome. Phlebology 2015, 30 (Suppl. S1), 67–72. [Google Scholar] [CrossRef]
- Coakley, F.V.; Varghese, S.L.; Hricak, H. CT and MRI of Pelvic Varices in Women. J. Comput. Assist. Tomogr. 1999, 23, 429–434. [Google Scholar] [CrossRef]
- Borghi, C.; Dell’Atti, L. Pelvic congestion syndrome: The current state of the literature. Arch. Gynecol. Obstet. 2015, 293, 291–301. [Google Scholar] [CrossRef]
- Schwartz, L.B.; Panageas, E.; Lange, R.; Rizzo, J.; Comite, F.; McCarthy, S. Female pelvis: Impact of MR imaging on treatment decisions and net cost analysis. Radiology 1994, 192, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Juhan, V. Chronic pelvic pain: An imaging approach. Diagn. Interv. Imaging 2015, 96, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, G.; Rofsky, N.M.; Weinreb, J.C. Nonspecificity of short inversion time inversion recovery (STIR) as a technique of fat suppression: Pitfalls in image interpretation. Am. J. Roentgenol. 1996, 166, 523–526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greiner, M.; Dadon, M.; Lemasle, P.; Cluzel, P. How Does the Pathophysiology Influence the Treatment of Pelvic Congestion Syndrome and is the Result Long-lasting? Phlebology 2012, 27, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hiromura, T.; Nishioka, T.; Nishioka, S.; Ikeda, H.; Tomita, K. Reflux in the Left Ovarian Vein: Analysis of MDCT Findings in Asymptomatic Women. Am. J. Roentgenol. 2004, 183, 1411–1415. [Google Scholar] [CrossRef]
- Giacchetto, C.; Cotroneo, G.B.; Marincolo, F.; Cammisuli, F.; Caruso, G.; Catizone, F. Ovarian varicocele: Ultrasonic and phlebographic evaluation. J. Clin. Ultrasound 1990, 18, 551–555. [Google Scholar] [CrossRef]
- Park, S.J.; Lim, J.W.; Ko, Y.T.; Lee, D.H.; Yoon, Y.; Oh, J.H.; Lee, H.K.; Huh, C.Y. Diagnosis of Pelvic Congestion Syndrome Using Transabdominal and Transvaginal Sonography. Am. J. Roentgenol. 2004, 182, 683–688. [Google Scholar] [CrossRef]
- Pandey, T.; Shaikh, R.; Viswamitra, S.; Jambhekar, K. Use of time resolved magnetic resonance imaging in the diagnosis of pelvic congestion syndrome. J. Magn. Reson. Imaging 2010, 32, 700–704. [Google Scholar] [CrossRef]
- Yang, D.M.; Kim, H.C.; Nam, D.H.; Jahng, G.H.; Huh, C.Y.; Lim, J.W. Time-resolved MR angiography for detecting and grading ovarian venous reflux: Comparison with conventional venography. Br. J. Radiol. 2012, 85, e117–e122. [Google Scholar] [CrossRef]
- Meneses, L.Q.; Uribe, S.; Tejos, C.; Andía, M.E.; Fava, M.; Irarrazaval, P. Using magnetic resonance phase-contrast velocity mapping for diagnosing pelvic congestion syndrome. Phlebology 2011, 26, 157–161. [Google Scholar] [CrossRef]
- Valero, I.; Garcia-Jimenez, R.; Valdevieso, P.; Garcia-Mejido, J.A.; Gonzalez-Herráez, J.V.; Pelayo-Delgado, I.; Fernandez-Palacin, A.; Sainz-Bueno, J.A. Identification of Pelvic Congestion Syndrome Using Transvaginal Ultrasonography. A Useful Tool. Tomography 2022, 8, 89–99. [Google Scholar] [CrossRef]
- Gavrilov, S.G. Vulvar varicosities: Diagnosis, treatment, and prevention. Int. J. Women’s Health 2017, 9, 463–475. [Google Scholar] [CrossRef]
| 164 | |
| Age (years) | 39 ± 9 (21–69)/39 (34–45) |
| Number of pregnancies | 2 ± 1 (0–6)/2 (2–3) |
| PCS | 60 (60/164; 36.59%) |
| VVLL | 45 (45/164; 27.44%) |
| PCS + VVLL | 43 (43/164; 26.22%) |
| Presence of external varicosities * Isolated | 34 (34/164; 20.73%) 16 (16/164; 9.76%) |
| Dysuria | 4 (4/164; 2.44%) |
| Criterion | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Detection of leaks | 0.92 | 0.77 | 0.93 | 0.73 |
| Iliac insufficiency by detection of leaks | 0.91 | 0.27 | 0.68 | 0.63 |
| Left ovarian insufficiency by detection of Periuterine varicosities > 5 mm | 0.82 | 0.53 | 0.93 | 0.26 |
| Internal pudendal or obturator leak by detection of perivaginal varicosities | 0.87 | 0.56 | 0.78 | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jambon, E.; Le Bras, Y.; Cazalas, G.; Grenier, N.; Marcelin, C. Pelvic Venous Insufficiency: Input of Short Tau Inversion Recovery Sequence. J. Pers. Med. 2022, 12, 2055. https://doi.org/10.3390/jpm12122055
Jambon E, Le Bras Y, Cazalas G, Grenier N, Marcelin C. Pelvic Venous Insufficiency: Input of Short Tau Inversion Recovery Sequence. Journal of Personalized Medicine. 2022; 12(12):2055. https://doi.org/10.3390/jpm12122055
Chicago/Turabian StyleJambon, Eva, Yann Le Bras, Gregoire Cazalas, Nicolas Grenier, and Clement Marcelin. 2022. "Pelvic Venous Insufficiency: Input of Short Tau Inversion Recovery Sequence" Journal of Personalized Medicine 12, no. 12: 2055. https://doi.org/10.3390/jpm12122055
APA StyleJambon, E., Le Bras, Y., Cazalas, G., Grenier, N., & Marcelin, C. (2022). Pelvic Venous Insufficiency: Input of Short Tau Inversion Recovery Sequence. Journal of Personalized Medicine, 12(12), 2055. https://doi.org/10.3390/jpm12122055







