PRLR and CACNA2D1 Impact the Prognosis of Breast Cancer by Regulating Tumor Immunity
Abstract
:1. Introduction
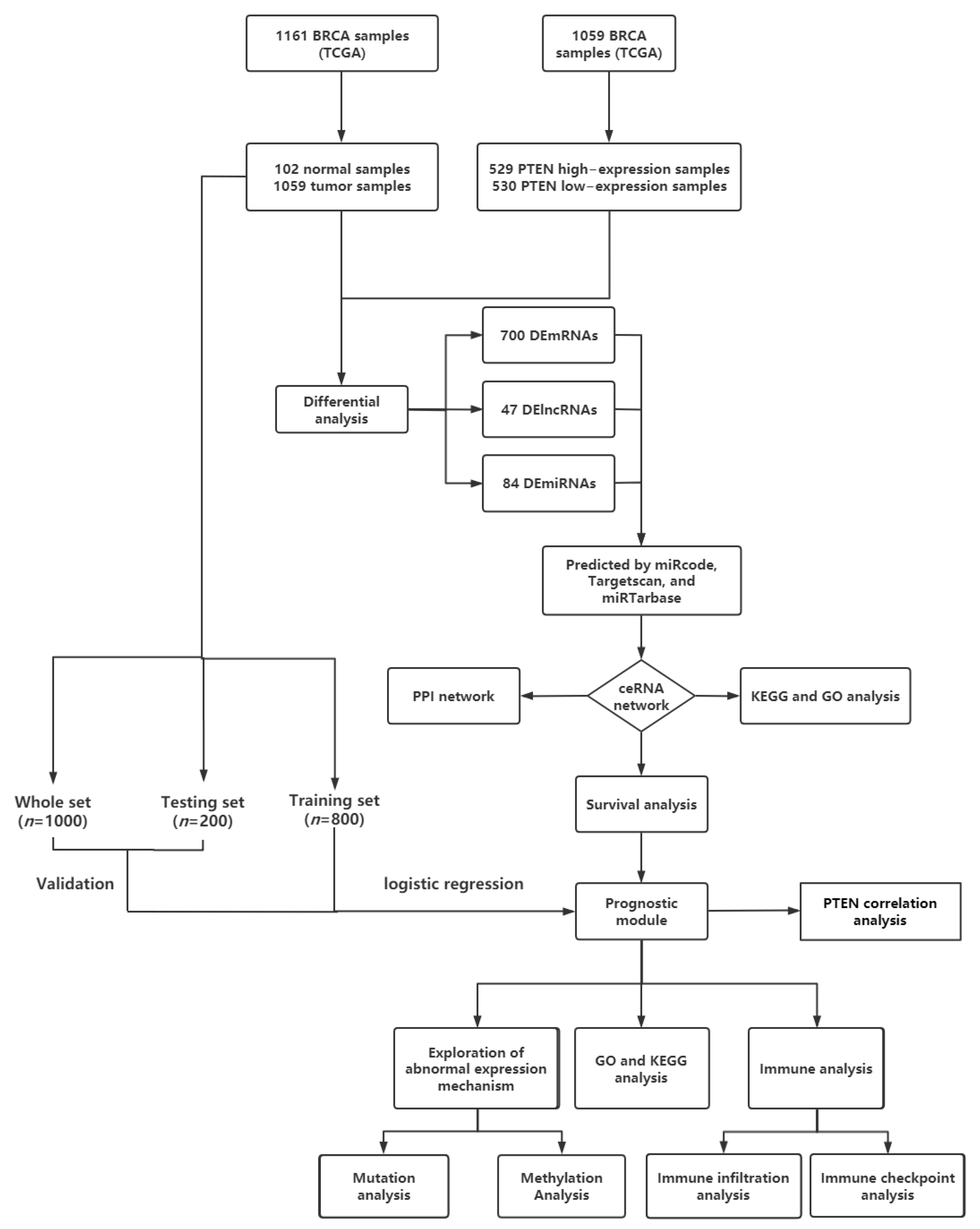
2. Materials and Methods
2.1. Data Acquisition and Processing
2.2. Identification of deRNAs
2.3. Construction of ceRNA Network and PPI Network of BC
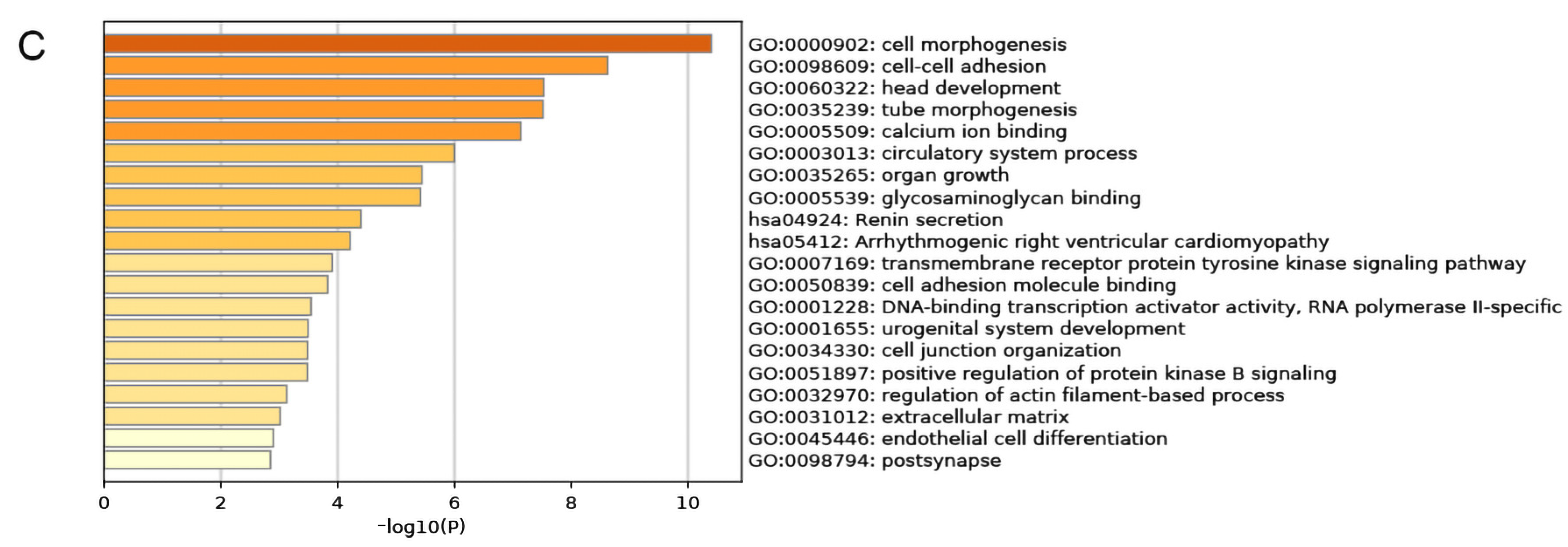
2.4. Functional Enrichment Analysis
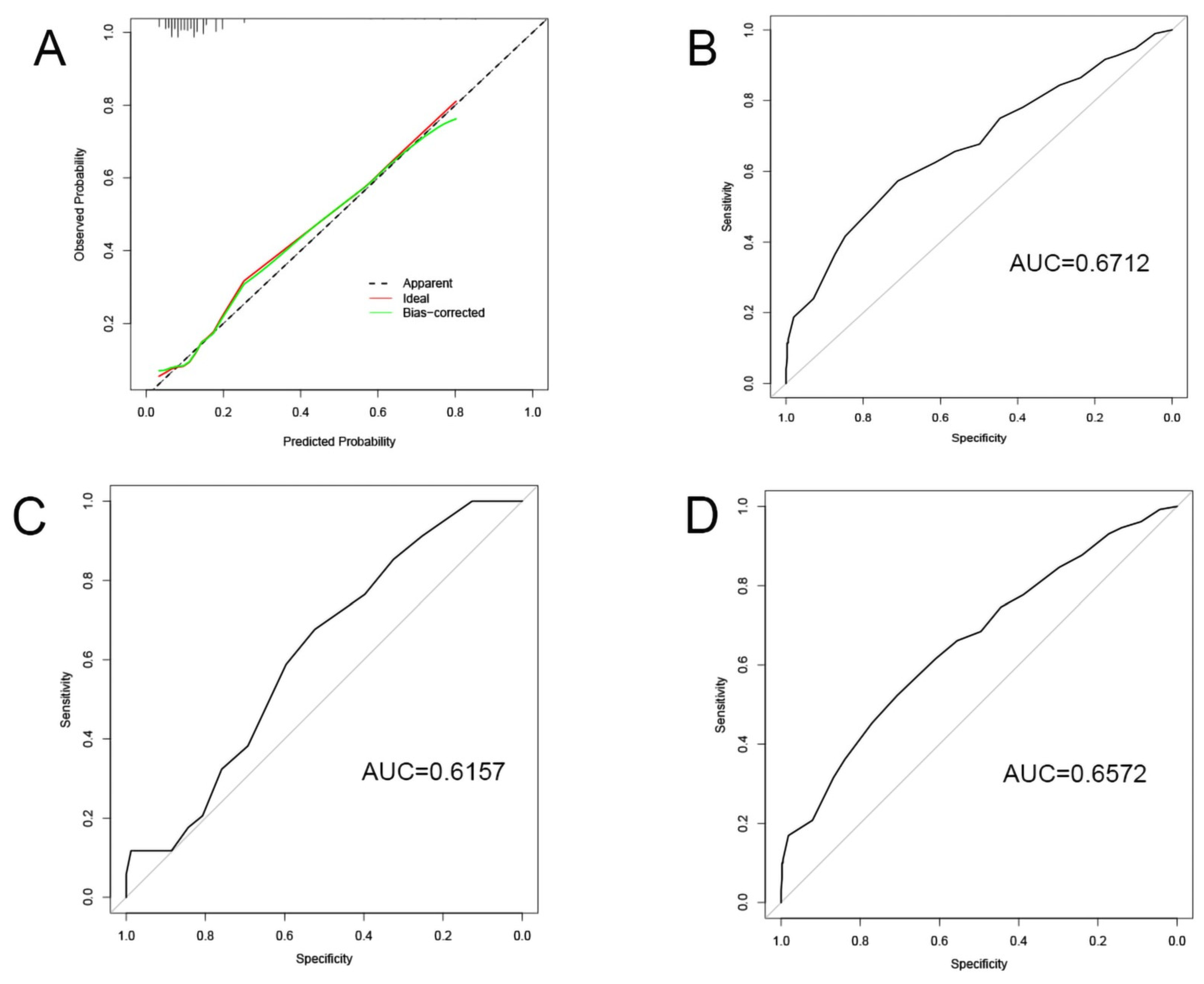
2.5. Survival Analysis and Construction and Verification of Prognosis Model
2.6. Analysis of RNA Mutation, Methylation, and Expression
2.7. Immune Infiltration and Immune Checkpoint Analysis
3. Results
3.1. Inhibitory Effect and Prognostic Value of PTEN Overexpression in BC Cells
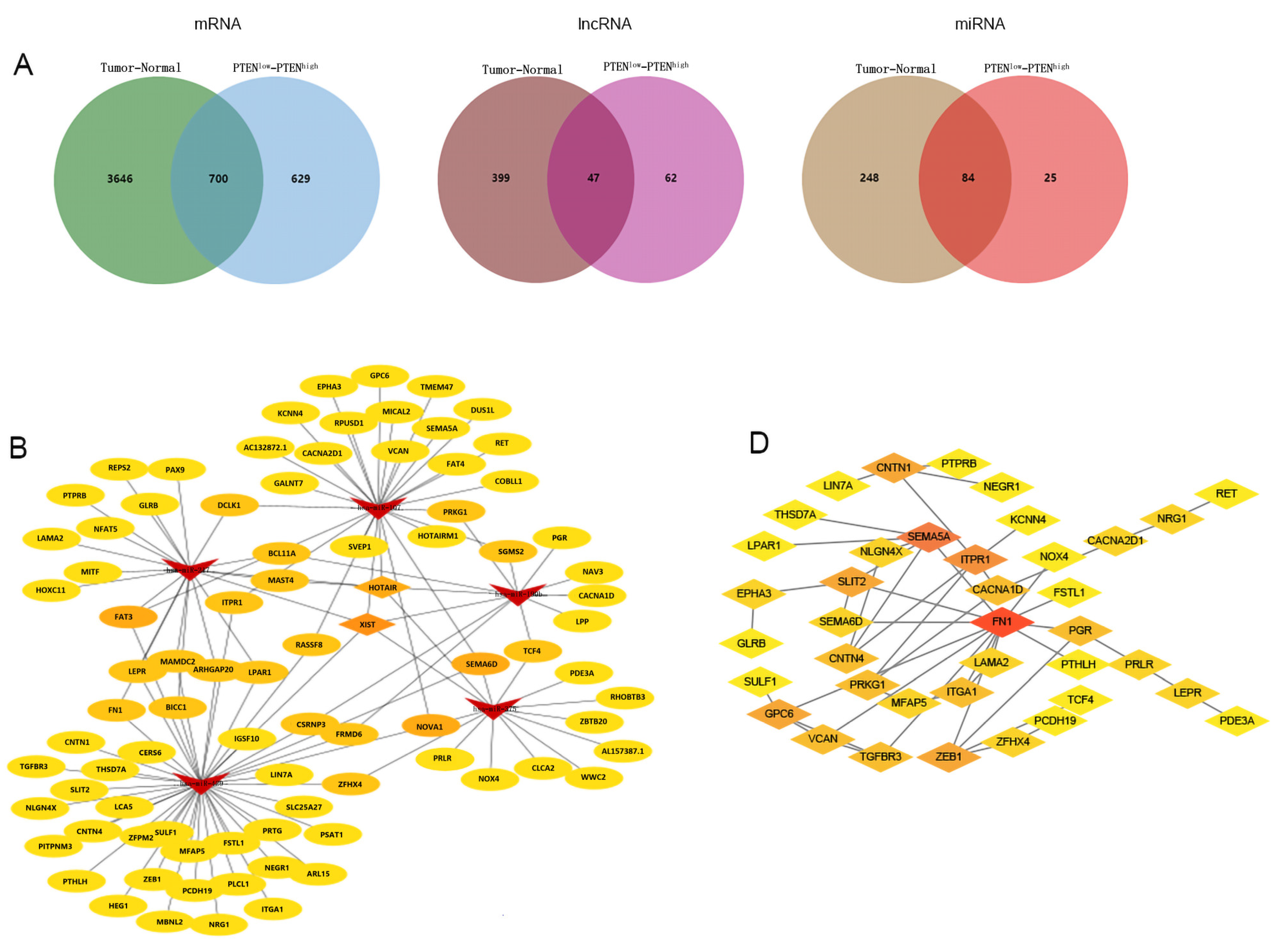
3.2. Screening of deRNAs (demRNAs, delncRNAs, and demiRNAs)
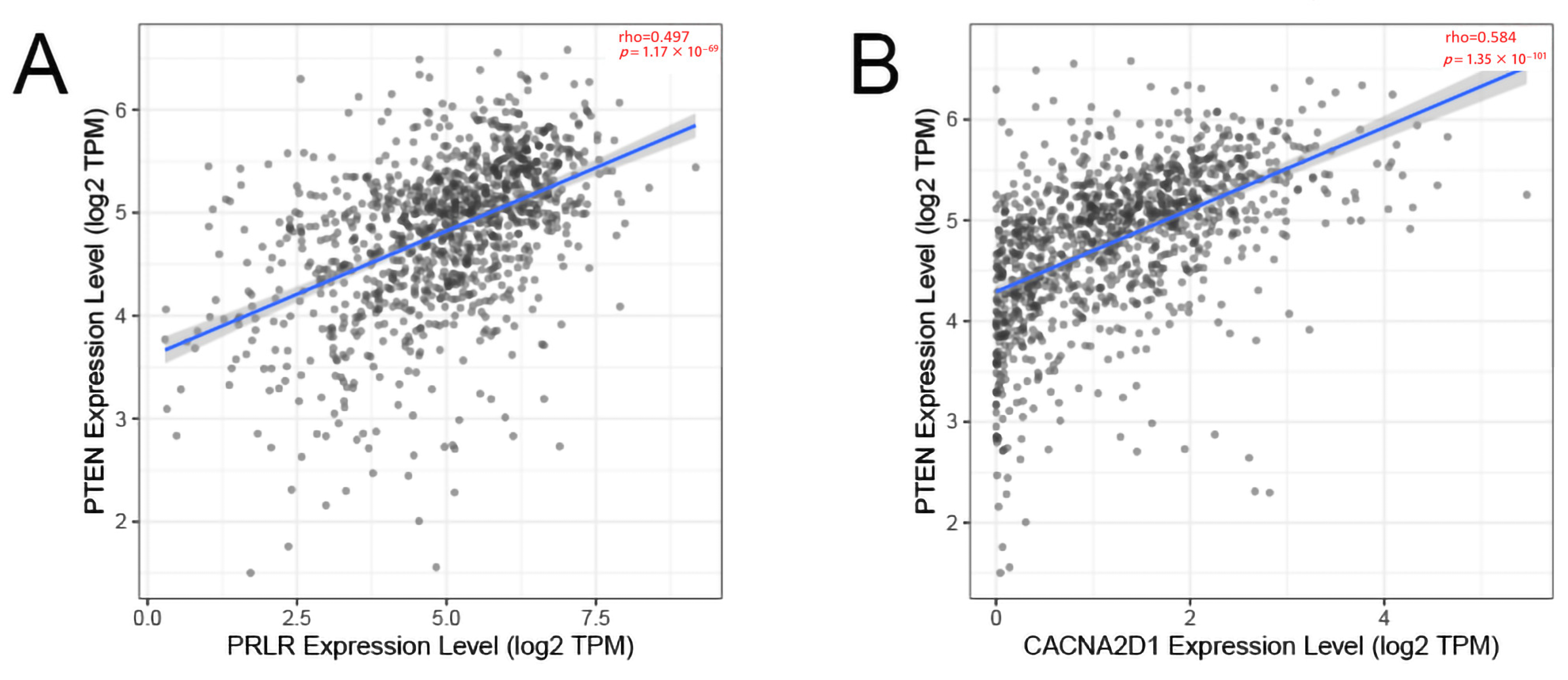
3.3. Construction and Analysis of ceRNA Network Related to PTEN
3.4. Construction and Validation of the Specific BC Prognosis Model
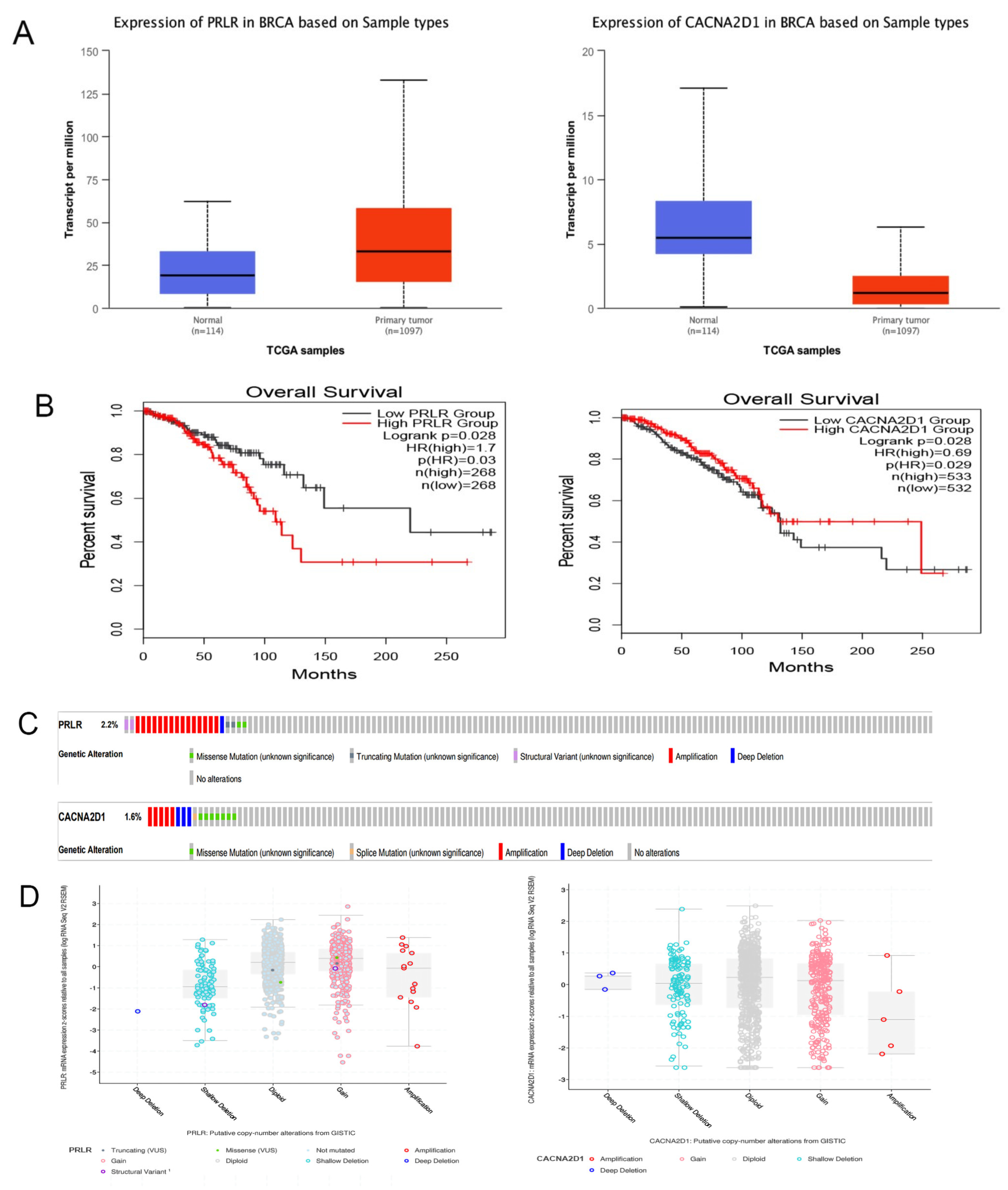
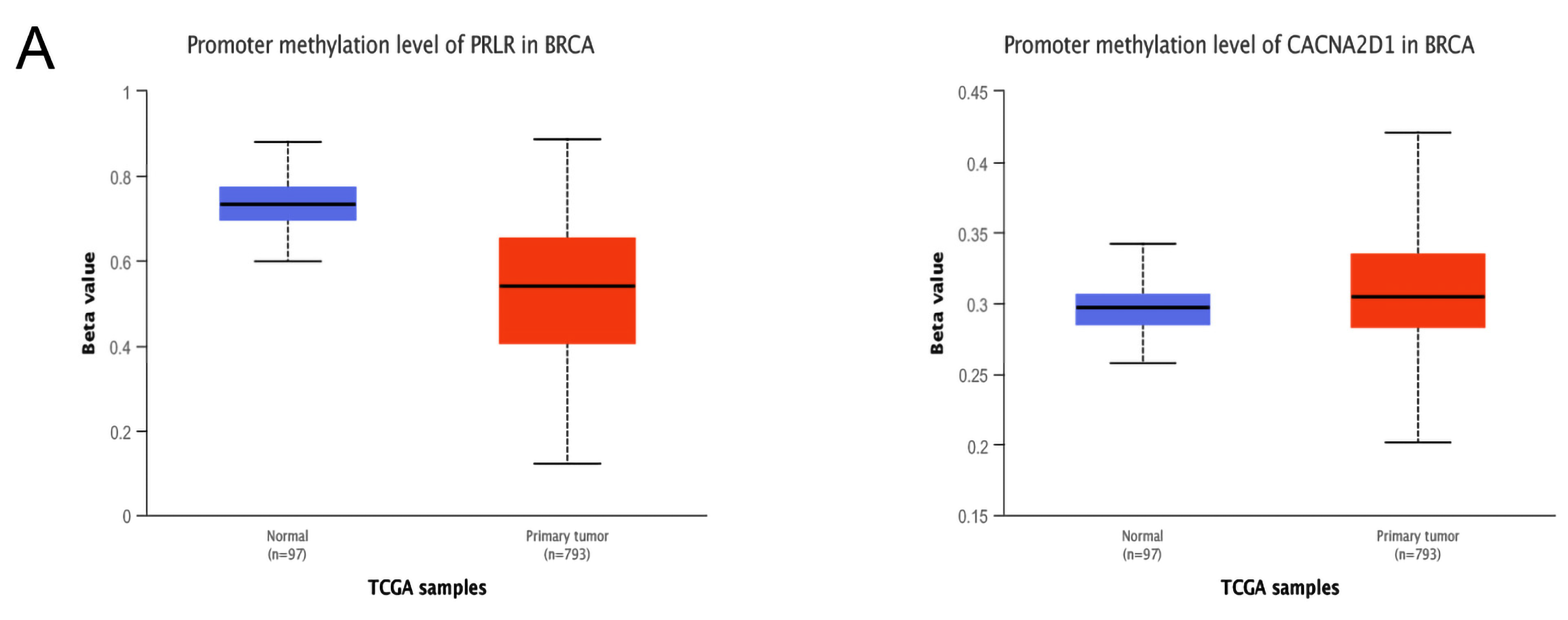
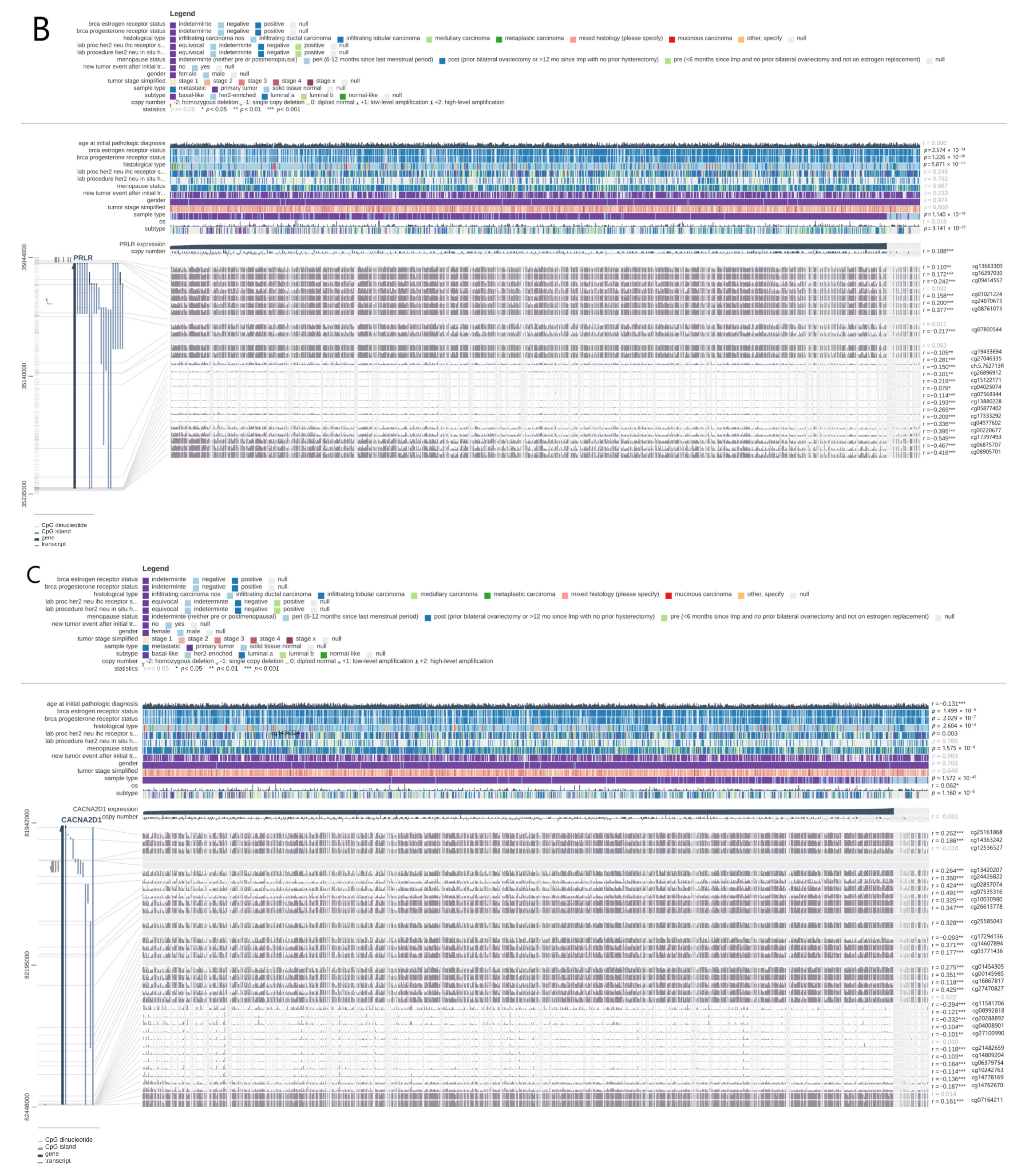
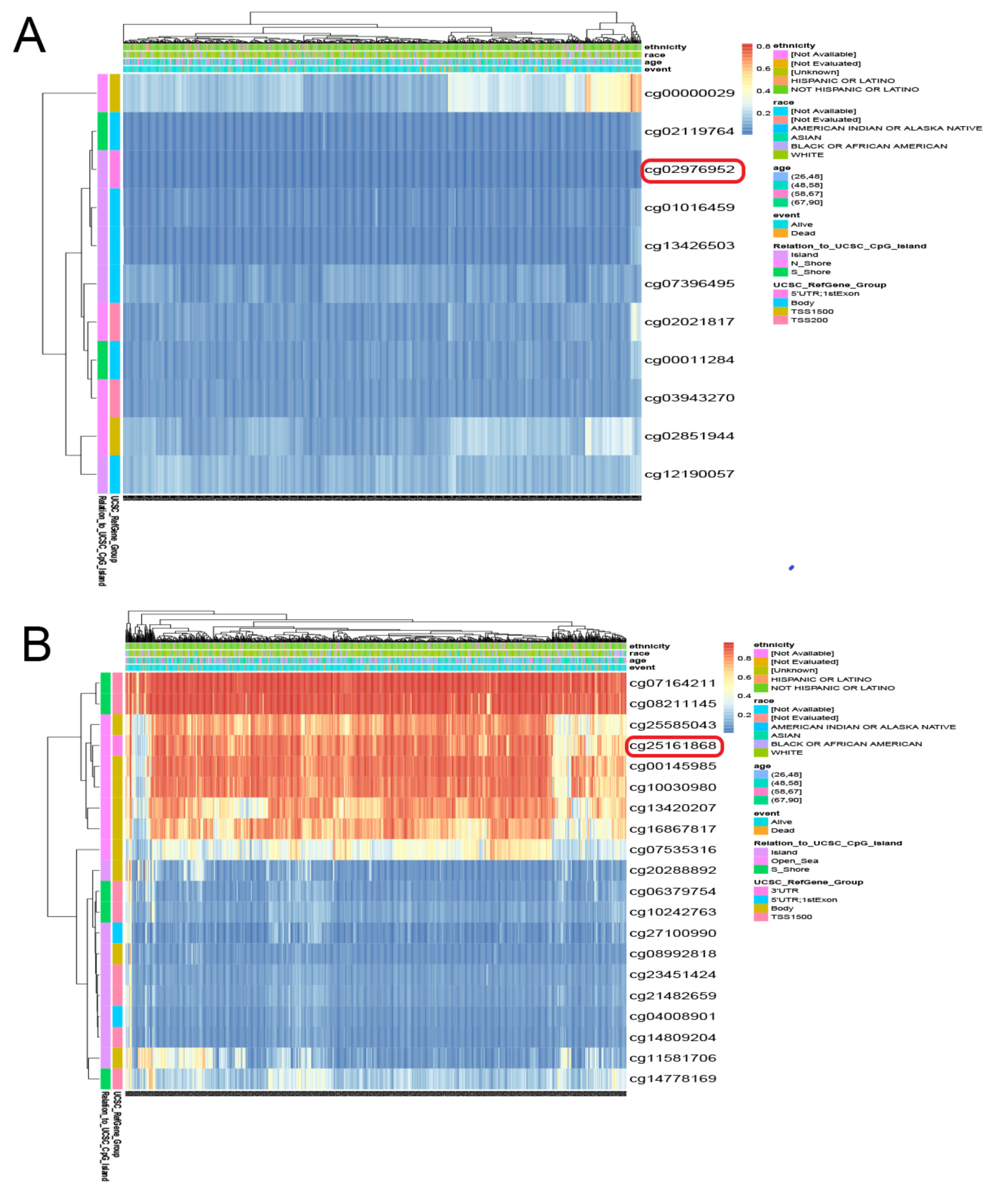
3.5. Verification of PRLR and CACNA2D1 Expression in BC and the Causes of Abnormal Expression
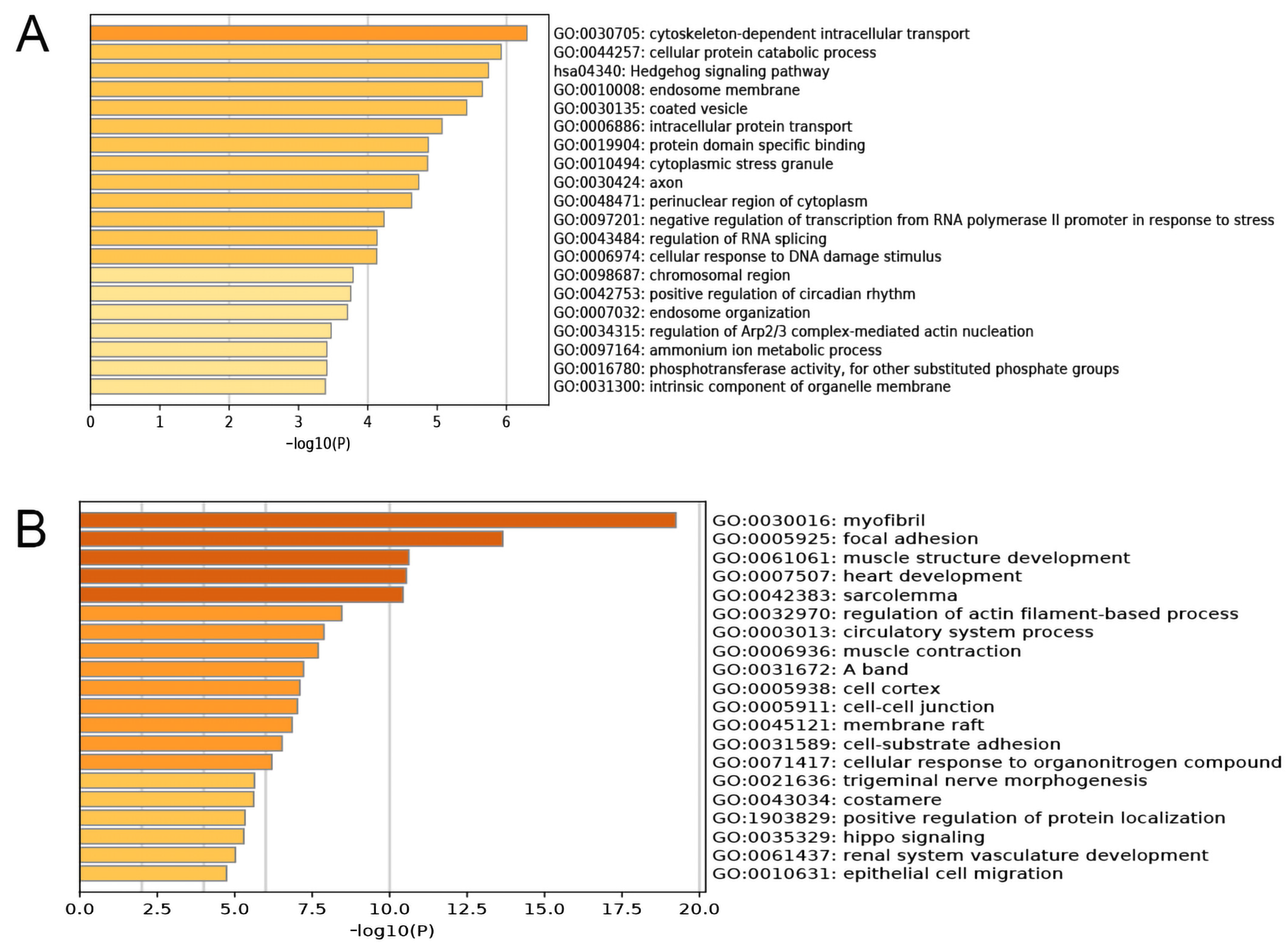
3.6. Functional Enrichment Analysis of PRLR and CACNA2D1
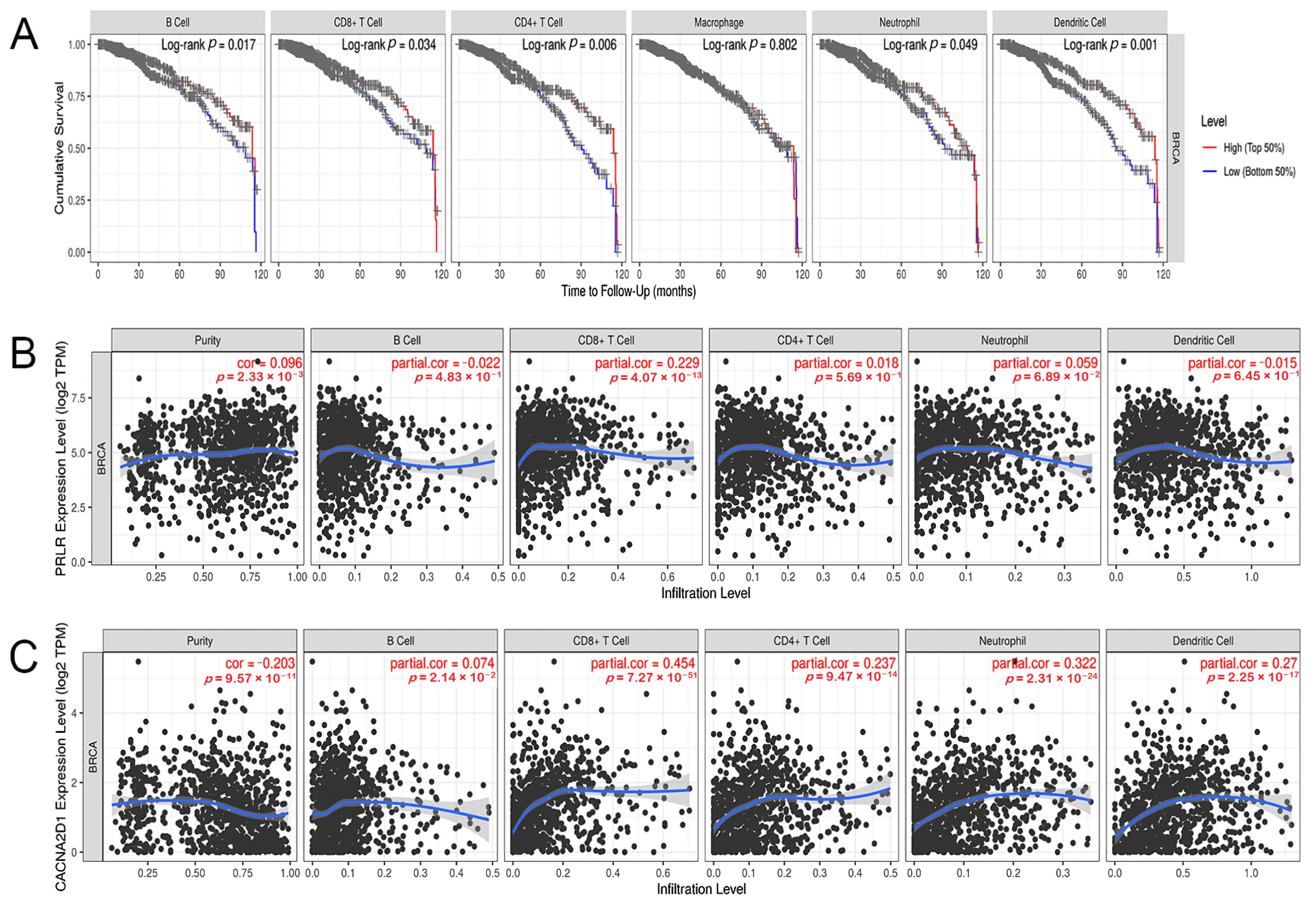
3.7. Correlation between the Expression of PRLR and CACNA2D1 and the Immune Infiltrating Cells in BC
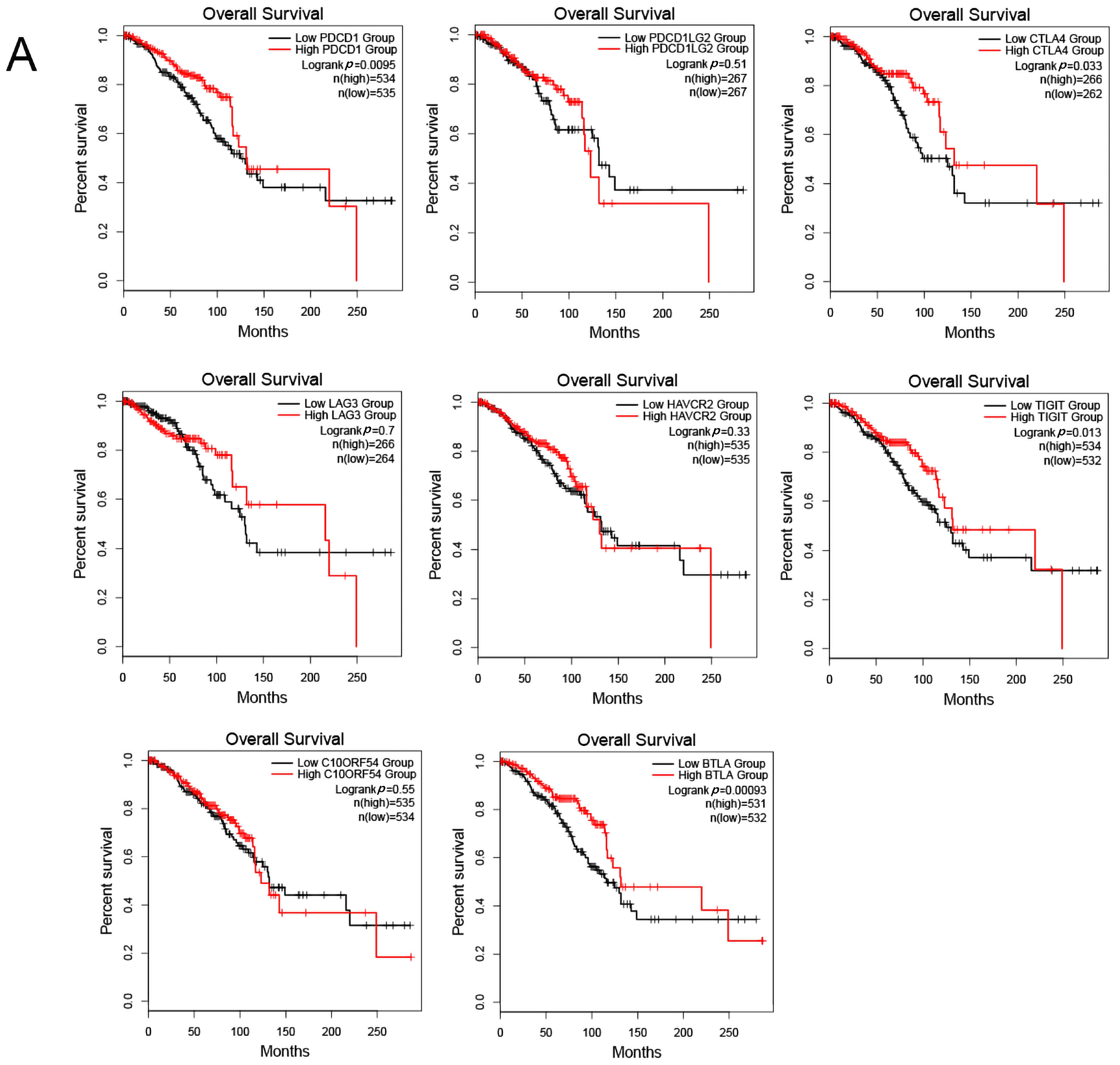
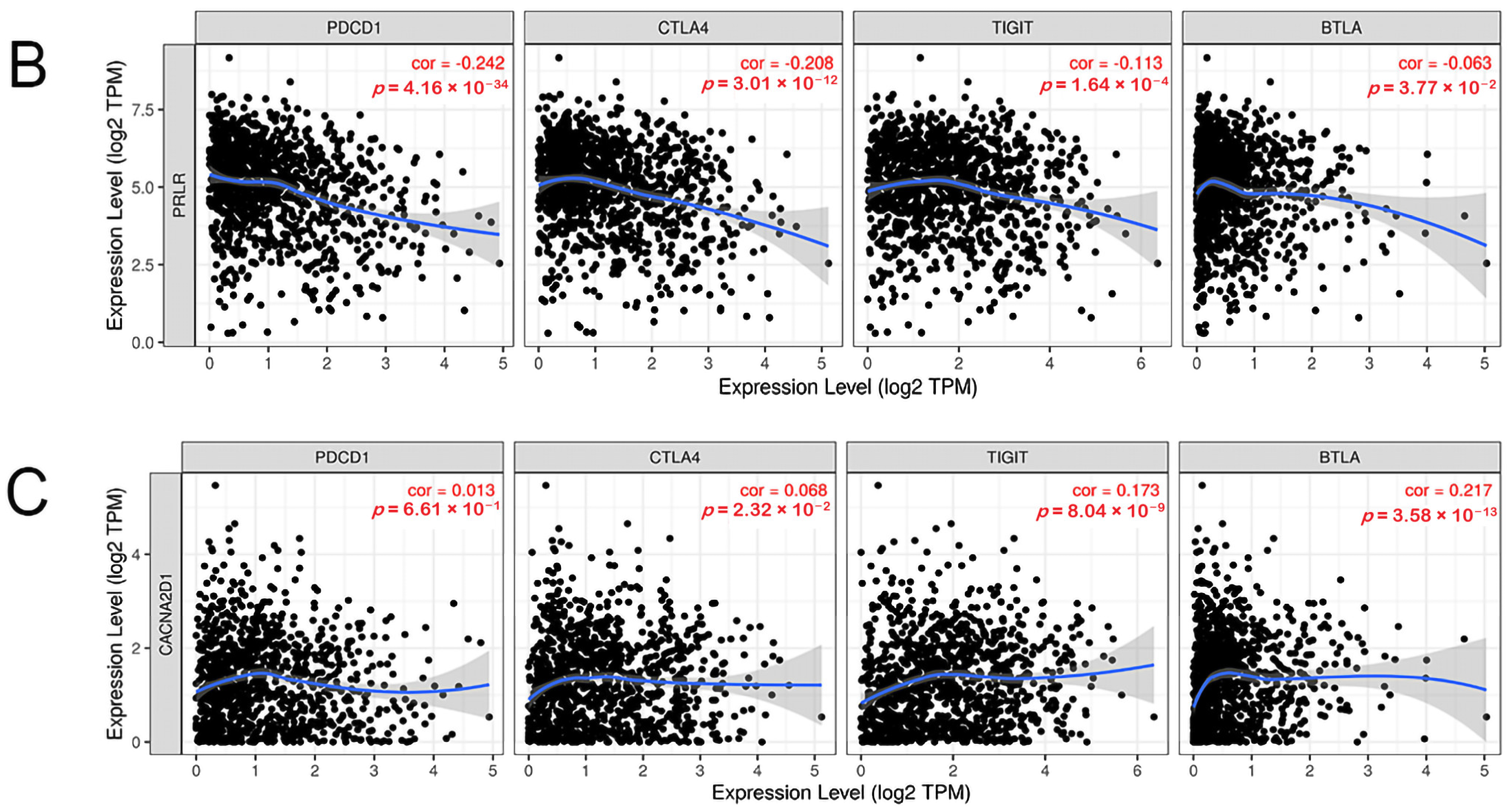
3.8. Correlation between PRLR, CACNA2D1, and Immune Checkpoints
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Rank | Name | Score |
| 1 | FN1 | 13 |
| 2 | SEMA5A | 6 |
| 3 | ITPR1 | 5 |
| 4 | CNTN1 | 4 |
| 4 | SLIT2 | 4 |
| 4 | GPC6 | 4 |
| 4 | ZEB1 | 4 |
| 8 | TGFBR3 | 3 |
| 8 | VCAN | 3 |
| 8 | ITGA1 | 3 |
| 8 | CNTN4 | 3 |
| 8 | PGR | 3 |
| 8 | CACNA1D | 3 |
| 8 | PRKG1 | 3 |
| 15 | ZFHX4 | 2 |
| 15 | MFAP5 | 2 |
| 15 | NLGN4X | 2 |
| 15 | SEMA6D | 2 |
| 15 | LEPR | 2 |
| 15 | EPHA3 | 2 |
| 15 | PRLR | 2 |
| 15 | CACNA2D1 | 2 |
| 15 | NRG1 | 2 |
| 15 | LAMA2 | 2 |
| 25 | RET | 1 |
| 25 | PCDH19 | 1 |
| 25 | PTPRB | 1 |
| 25 | NOX4 | 1 |
| 25 | SULF1 | 1 |
| 25 | NEGR1 | 1 |
| 25 | LIN7A | 1 |
| 25 | FSTL1 | 1 |
| 25 | THSD7A | 1 |
| 25 | KCNN4 | 1 |
| 25 | TCF4 | 1 |
| 25 | GLRB | 1 |
| 25 | PTHLH | 1 |
| 25 | PDE3A | 1 |
| 25 | LPAR1 | 1 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Boyle, P.; Goldhirsch, A.; Orecchia, R.; Viale, G. Breast cancer. Lancet 2005, 365, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, T.; Zhang, G.; Liang, T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol. Cancer 2021, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Lin, F.; Sun, H.; Lin, Y.; Wang, Z.; Wang, X. The lnc-CTSLP8 upregulates CTSL1 as a competitive endogenous RNA and promotes ovarian cancer metastasis. J. Exp. Clin. Cancer Res. CR 2021, 40, 151. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Sun, Q.; Chen, R.; Zhang, C.; Yang, P.; Tan, Y.; Peng, C.; Wang, T.; Jin, C.; et al. Hsa_circ_0001666 suppresses the progression of colorectal cancer through the miR-576-5p/PCDH10 axis. Clin. Transl. Med. 2021, 11, e565. [Google Scholar] [CrossRef]
- Gao, X.; Qin, T.; Mao, J.; Zhang, J.; Fan, S.; Lu, Y.; Sun, Z.; Zhang, Q.; Song, B.; Li, L. PTENP1/miR-20a/PTEN axis contributes to breast cancer progression by regulating PTEN via PI3K/AKT pathway. J. Exp. Clin. Cancer Res. CR 2019, 38, 256. [Google Scholar] [CrossRef] [Green Version]
- Cocco, S.; Piezzo, M.; Calabrese, A.; Cianniello, D.; Caputo, R.; Lauro, V.D.; Fusco, G.; Gioia, G.D.; Licenziato, M.; De Laurentiis, M. Biomarkers in Triple-Negative Breast Cancer: State-of-the-Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 4579. [Google Scholar] [CrossRef]
- Cao, L.Q.; Yang, X.W.; Chen, Y.B.; Zhang, D.W.; Jiang, X.F.; Xue, P. Exosomal miR-21 regulates the TETs/PTENp1/PTEN pathway to promote hepatocellular carcinoma growth. Mol. Cancer 2019, 18, 148. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Tao, T.; Yang, L.; Qin, Q.; Wang, Y.; Liu, H.; Song, R.; Yang, X.; Wang, Q.; Gu, S.; et al. Loss of PDZK1 expression activates PI3K/AKT signaling via PTEN phosphorylation in gastric cancer. Cancer Lett. 2019, 453, 107–121. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Liu, B.; Jin, X.; Wang, X.; Pan, J.; Tu, W.; Shao, Y. IMP3 accelerates the progression of prostate cancer through inhibiting PTEN expression in a SMURF1-dependent way. J. Exp. Clin. Cancer Res. CR 2020, 39, 190. [Google Scholar] [CrossRef]
- Wu, W.; Jing, D.; Meng, Z.; Hu, B.; Zhong, B.; Deng, X.; Jin, X.; Shao, Z. FGD1 promotes tumor progression and regulates tumor immune response in osteosarcoma via inhibiting PTEN activity. Theranostics 2020, 10, 2859–2871. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ye, Y.; Jiang, Q.; Chen, Y.; Lyu, X.; Li, J.; Wang, S.; Liu, T.; Cai, H.; Yao, K.; et al. Epstein-Barr virus-encoded microRNA BART1 induces tumour metastasis by regulating PTEN-dependent pathways in nasopharyngeal carcinoma. Nat. Commun. 2015, 6, 7353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.proteinatlas.org/ENSG00000171862-PTEN/tissue/breast (accessed on 5 September 2022).
- Available online: https://www.proteinatlas.org/ENSG00000171862-PTEN/pathology/breast+cancer#img (accessed on 5 September 2022).
- Joseph, N.; Reicher, B.; Barda-Saad, M. The calcium feedback loop and T cell activation: How cytoskeleton networks control intracellular calcium flux. Biochim. Biophys. Acta 2014, 1838, 557–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giampazolias, E.; Schulz, O.; Lim, K.H.J.; Rogers, N.C.; Chakravarty, P.; Srinivasan, N.; Gordon, O.; Cardoso, A.; Buck, M.D.; Poirier, E.Z.; et al. Secreted gelsolin inhibits DNGR-1-dependent cross-presentation and cancer immunity. Cell 2021, 184, 4016–4031.e4022. [Google Scholar] [CrossRef]
- Aridor, M.; Hannan, L.A. Traffic jam: A compendium of human diseases that affect intracellular transport processes. Traffic 2000, 1, 836–851. [Google Scholar] [CrossRef]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol. Cancer 2020, 19, 165. [Google Scholar] [CrossRef]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef]
- Clevenger, C.V.; Furth, P.A.; Hankinson, S.E.; Schuler, L.A. The role of prolactin in mammary carcinoma. Endocr. Rev. 2003, 24, 1–27. [Google Scholar] [CrossRef]
- Nie, H.; Huang, P.Q.; Jiang, S.H.; Yang, Q.; Hu, L.P.; Yang, X.M.; Li, J.; Wang, Y.H.; Li, Q.; Zhang, Y.F.; et al. The short isoform of PRLR suppresses the pentose phosphate pathway and nucleotide synthesis through the NEK9-Hippo axis in pancreatic cancer. Theranostics 2021, 11, 3898–3915. [Google Scholar] [CrossRef] [PubMed]
- Alkharusi, A.; AlMuslahi, A.; AlBalushi, N.; AlAjmi, R.; AlRawahi, S.; AlFarqani, A.; Norstedt, G.; Zadjali, F. Connections between prolactin and ovarian cancer. PLoS ONE 2021, 16, e0255701. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.C.; Bates, S.E. Targeting Prolactin Receptor (PRLR) Signaling in PRLR-Positive Breast and Prostate Cancer. Oncologist 2016, 21, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Gharbaran, R.; Onwumere, O.; Codrington, N.; Somenarain, L.; Redenti, S. Immunohistochemical localization of prolactin receptor (PRLR) to Hodgkin’s and Reed-Sternberg cells of Hodgkin’s lymphoma. Acta Histochem. 2021, 123, 151657. [Google Scholar] [CrossRef]
- Gorvin, C.M.; Newey, P.J.; Rogers, A.; Stokes, V.; Neville, M.J.; Lines, K.E.; Ntali, G.; Lees, P.; Morrison, P.J.; Singhellakis, P.N.; et al. Association of prolactin receptor (PRLR) variants with prolactinomas. Hum. Mol. Genet. 2019, 28, 1023–1037. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kong, X.; Xuan, L.; Wang, Z.; Huang, Y.H. Prolactin and endocrine therapy resistance in breast cancer: The next potential hope for breast cancer treatment. J. Cell. Mol. Med. 2021, 25, 10327–10348. [Google Scholar] [CrossRef]
- Kavarthapu, R.; Anbazhagan, R.; Dufau, M.L. Crosstalk between PRLR and EGFR/HER2 Signaling Pathways in Breast Cancer. Cancers 2021, 13, 4685. [Google Scholar] [CrossRef]
- Hachim, I.Y.; Hachim, M.Y.; Lopez, V.M.; Lebrun, J.J.; Ali, S. Prolactin Receptor Expression is an Independent Favorable Prognostic Marker in Human Breast Cancer. Appl. Immunohistochem. Mol. Morphol. AIMM 2016, 24, 238–245. [Google Scholar] [CrossRef]
- Vaclavicek, A.; Hemminki, K.; Bartram, C.R.; Wagner, K.; Wappenschmidt, B.; Meindl, A.; Schmutzler, R.K.; Klaes, R.; Untch, M.; Burwinkel, B.; et al. Association of prolactin and its receptor gene regions with familial breast cancer. J. Clin. Endocrinol. Metab. 2006, 91, 1513–1519. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.A.; Haiman, C.A.; Burtt, N.P.; Pooler, L.C.; Cheng, I.; Kolonel, L.N.; Pike, M.C.; Altshuler, D.; Hirschhorn, J.N.; Henderson, B.E.; et al. A comprehensive analysis of common genetic variation in prolactin (PRL) and PRL receptor (PRLR) genes in relation to plasma prolactin levels and breast cancer risk: The multiethnic cohort. BMC Med. Genet. 2007, 8, 72. [Google Scholar] [CrossRef]
- Wang, L.; Yu, L.; Shi, J.; Li, F.; Zhang, C.; Xu, H.; Yin, X.; Wang, L.; Lin, S.; Litvinova, A.; et al. Functional regulations between genetic alteration-driven genes and drug target genes acting as prognostic biomarkers in breast cancer. Sci. Rep. 2022, 12, 10641. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, C.V.; Gadd, S.L.; Zheng, J. New mechanisms for PRLr action in breast cancer. Trends Endocrinol. Metab. TEM 2009, 20, 223–229. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.L.; do Amaral, V.C.; Gabrielli, V.; Montt Guevara, M.M.; Mannella, P.; Baracat, E.C.; Soares, J.M., Jr.; Simoncini, T. Prolactin Promotes Breast Cancer Cell Migration through Actin Cytoskeleton Remodeling. Front. Endocrinol. 2015, 6, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, A.; Forsyth, A.; Cong, Y.; Grant, L.; Juan, T.H.; Lee, J.K.; Klimowicz, A.; Petrillo, S.K.; Hu, J.; Chan, A.; et al. The Role of Prolactin in Bone Metastasis and Breast Cancer Cell-Mediated Osteoclast Differentiation. J. Natl. Cancer Inst. 2016, 108, djv338. [Google Scholar] [CrossRef]
- Goffin, V.; Touraine, P.; Culler, M.D.; Kelly, P.A. Drug Insight: Prolactin-receptor antagonists, a novel approach to treatment of unresolved systemic and local hyperprolactinemia? Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 571–581. [Google Scholar] [CrossRef]
- Kelly, M.P.; Hickey, C.; Makonnen, S.; Coetzee, S.; Jalal, S.; Wang, Y.; Delfino, F.; Shan, J.; Potocky, T.B.; Chatterjee, I.; et al. Preclinical Activity of the Novel Anti-Prolactin Receptor (PRLR) Antibody-Drug Conjugate REGN2878-DM1 in PRLR-Positive Breast Cancers. Mol. Cancer Ther. 2017, 16, 1299–1311. [Google Scholar] [CrossRef] [Green Version]
- Grible, J.M.; Zot, P.; Olex, A.L.; Hedrick, S.E.; Harrell, J.C.; Woock, A.E.; Idowu, M.O.; Clevenger, C.V. The human intermediate prolactin receptor is a mammary proto-oncogene. NPJ Breast Cancer 2021, 7, 37. [Google Scholar] [CrossRef]
- Neo, S.Y.; Yang, Y.; Record, J.; Ma, R.; Chen, X.; Chen, Z.; Tobin, N.P.; Blake, E.; Seitz, C.; Thomas, R.; et al. CD73 immune checkpoint defines regulatory NK cells within the tumor microenvironment. J. Clin. Investig. 2020, 130, 1185–1198. [Google Scholar] [CrossRef] [Green Version]
- Krenz, B.; Gebhardt-Wolf, A.; Ade, C.P.; Gaballa, A.; Roehrig, F.; Vendelova, E.; Baluapuri, A.; Eilers, U.; Gallant, P.; D’Artista, L.; et al. MYC- and MIZ1-Dependent Vesicular Transport of Double-Strand RNA Controls Immune Evasion in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2021, 81, 4242–4256. [Google Scholar] [CrossRef]
- Zhou, Y.; Zong, H.; Han, L.; Xie, Y.; Jiang, H.; Gilly, J.; Zhang, B.; Lu, H.; Chen, J.; Sun, R.; et al. A novel bispecific antibody targeting CD3 and prolactin receptor (PRLR) against PRLR-expression breast cancer. J. Exp. Clin. Cancer Res. CR 2020, 39, 87. [Google Scholar] [CrossRef]
- Yonezawa, T.; Chen, K.H.; Ghosh, M.K.; Rivera, L.; Dill, R.; Ma, L.; Villa, P.A.; Kawaminami, M.; Walker, A.M. Anti-metastatic outcome of isoform-specific prolactin receptor targeting in breast cancer. Cancer Lett. 2015, 366, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safran, M.; Rosen, N.; Twik, M.; BarShir, R.; Stein, T.I.; Dahary, D.; Fishilevich, S.; Lancet, D. The GeneCards Suite. In Practical Guide to Life Science Databases; Abugessaisa, I., Kasukawa, T., Eds.; Springer Nature Singapore: Singapore, 2021; pp. 27–56. [Google Scholar]
- Luo, R.; Huang, M.; Wang, Y. A Transcription Factor-Based Risk Model for Predicting the Prognosis of Prostate Cancer and Potential Therapeutic Drugs. Evid. -Based Complement. Altern. Med. Ecam 2021, 2021, 6894278. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Long, Y.; Jin, Y.; Ya, W.; Meng, D.; Qin, T.; Su, L.; Zhou, W.; Wu, J.; Huang, C.; et al. Comprehensive analysis of the lncRNA-miRNA-mRNA regulatory network for bladder cancer. Transl. Androl. Urol. 2021, 10, 1286–1301. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Holm, R.; Goscinski, M.A.; Trope, C.G.; Nesland, J.M.; Suo, Z. Prognostic and clinicopathological significance of Cacna2d1 expression in epithelial ovarian cancers: A retrospective study. Am. J. Cancer Res. 2016, 6, 2088–2097. [Google Scholar]
- Huang, C.; Wang, Z.; Zhang, K.; Dong, Y.; Zhang, A.; Lu, C.; Liu, L. MicroRNA-107 inhibits proliferation and invasion of laryngeal squamous cell carcinoma cells by targeting CACNA2D1 in vitro. Anti-Cancer Drugs 2020, 31, 260–271. [Google Scholar] [CrossRef]
- Shiozaki, A.; Katsurahara, K.; Kudou, M.; Shimizu, H.; Kosuga, T.; Ito, H.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; et al. Amlodipine and Verapamil, Voltage-Gated Ca(2+) Channel Inhibitors, Suppressed the Growth of Gastric Cancer Stem Cells. Ann. Surg. Oncol. 2021, 28, 5400–5411. [Google Scholar] [CrossRef]
- Sui, X.; Geng, J.H.; Li, Y.H.; Zhu, G.Y.; Wang, W.H. Calcium channel α2δ1 subunit (CACNA2D1) enhances radioresistance in cancer stem-like cells in non-small cell lung cancer cell lines. Cancer Manag. Res. 2018, 10, 5009–5018. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zhang, W.; Yang, X.; An, G.; Zhao, W. The α2δ1 subunit of the voltage-gated calcium channel acts as a potential candidate for breast cancer tumor initial cells biomarker. Cancer Biomark. Sect. A Dis. Mrk. 2021, 31, 295–305. [Google Scholar] [CrossRef]
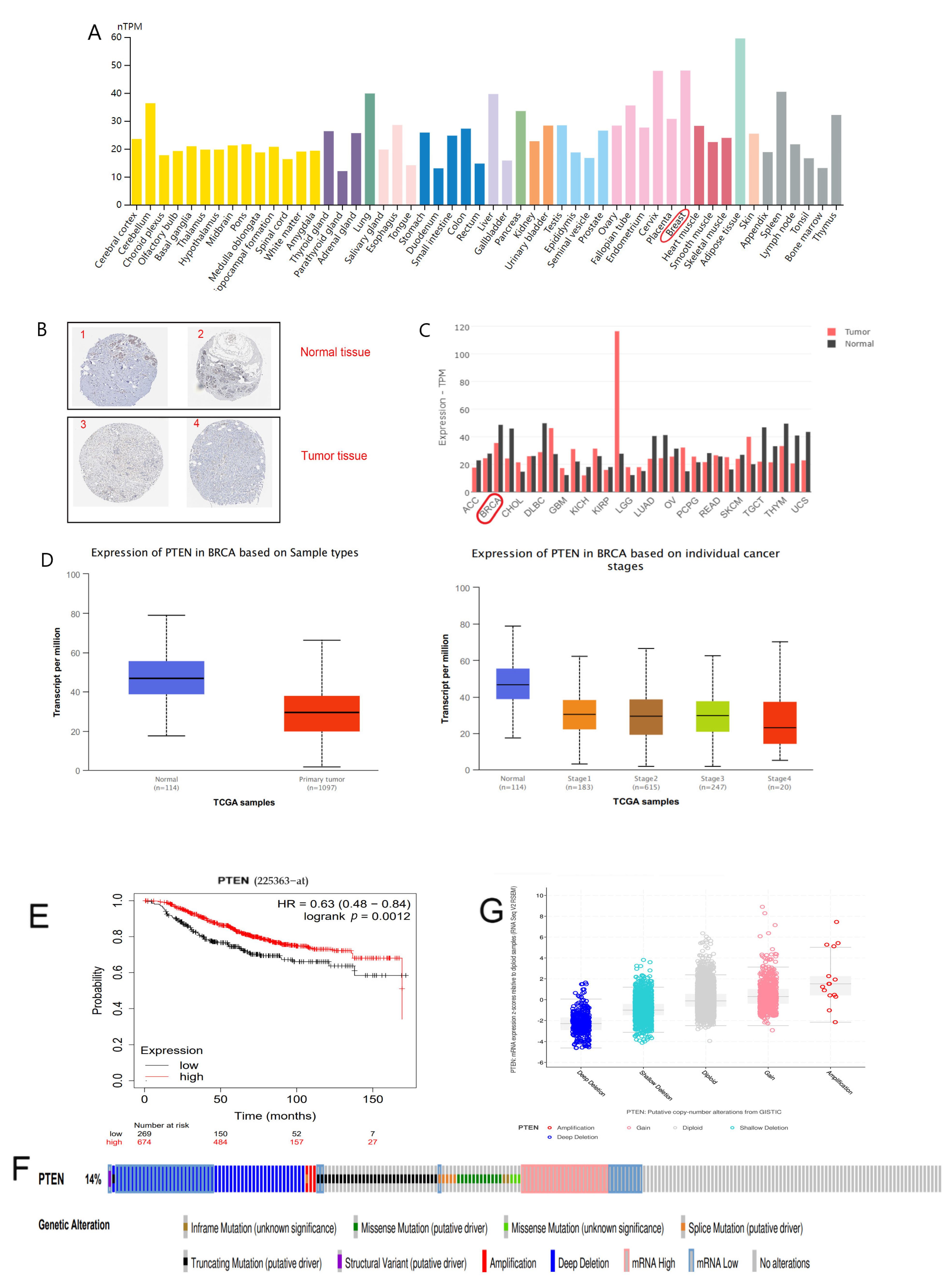


| Number | Tissue Type | ID | Age | Gender | Staining |
|---|---|---|---|---|---|
| 1 | Normal | 3856 | 27 | Female | Low |
| 2 | Normal | 2042 | 75 | Female | Low |
| 3 | Tumor | 2252 | 47 | Female | Not detected |
| 4 | Tumor | 2199 | 60 | Female | Not detected |
| Variable | B | Wald | OR with CI | p Value |
|---|---|---|---|---|
| Intercept | −2.293 | 35.349 | 0.01 (0.046–0.211) | <0.001 |
| M | 3.182 | 27.803 | 24.083 (7.941–89.816) | <0.001 |
| N | 0.627 | 6.869 | 1.871 (1.179–3.019) | 0.009 |
| CACNA2D1 | −0.470 | 9.247 | 0.625 (0.459–0.843) | 0.002 |
| PRLR | 0.352 | 5.469 | 1.421 (1.061–1.915) | 0.019 |
| Variable | Df | Pr (>Chi) |
|---|---|---|
| M | 1 | <0.001 |
| N | 1 | 0.011 |
| CACNA2D1 | 1 | 0.014 |
| PRLR | 1 | 0.018 |
| Data Set | Symbol | LogFC | p Value | Expression |
|---|---|---|---|---|
| GSE21422 | PRLR | 3.34826808 | 1.16 × 10−3 | Up |
| CACNA2D1 | −4.18492567 | 1.34 × 10−4 | Down | |
| TCGA | PRLR | 1.335680424 | 2.52 × 10−21 | Up |
| CACNA2D1 | −1.93331686 | 1.13 × 10−75 | Down |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Deng, Y.; Zhang, Y.; Wu, B.; Zhou, J. PRLR and CACNA2D1 Impact the Prognosis of Breast Cancer by Regulating Tumor Immunity. J. Pers. Med. 2022, 12, 2086. https://doi.org/10.3390/jpm12122086
Liang J, Deng Y, Zhang Y, Wu B, Zhou J. PRLR and CACNA2D1 Impact the Prognosis of Breast Cancer by Regulating Tumor Immunity. Journal of Personalized Medicine. 2022; 12(12):2086. https://doi.org/10.3390/jpm12122086
Chicago/Turabian StyleLiang, Jiamin, Yu Deng, Yubi Zhang, Bin Wu, and Jing Zhou. 2022. "PRLR and CACNA2D1 Impact the Prognosis of Breast Cancer by Regulating Tumor Immunity" Journal of Personalized Medicine 12, no. 12: 2086. https://doi.org/10.3390/jpm12122086