Resveratrol Inhibits Oxidative Stress and Regulates M1/M2-Type Polarization of Microglia via Mediation of the Nrf2/Shh Signaling Cascade after OGD/R Injury In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. OGD/R Model
2.3. Establishment of Co-Culture Model by Condition Medium
2.4. Drug Treatment
2.5. Cell Viability Assay
2.6. Measurements of SOD Activity and MDA Levels
2.7. Immunocytochemistry
2.8. Quantitative Real-Time PCR (RT-qPCR)
2.9. Western Blot Analysis
2.10. Annexin V-FITC Flow Cytometric Analysis of HT22 Neuronal Apoptosis
2.11. Statistical Analysis
3. Results
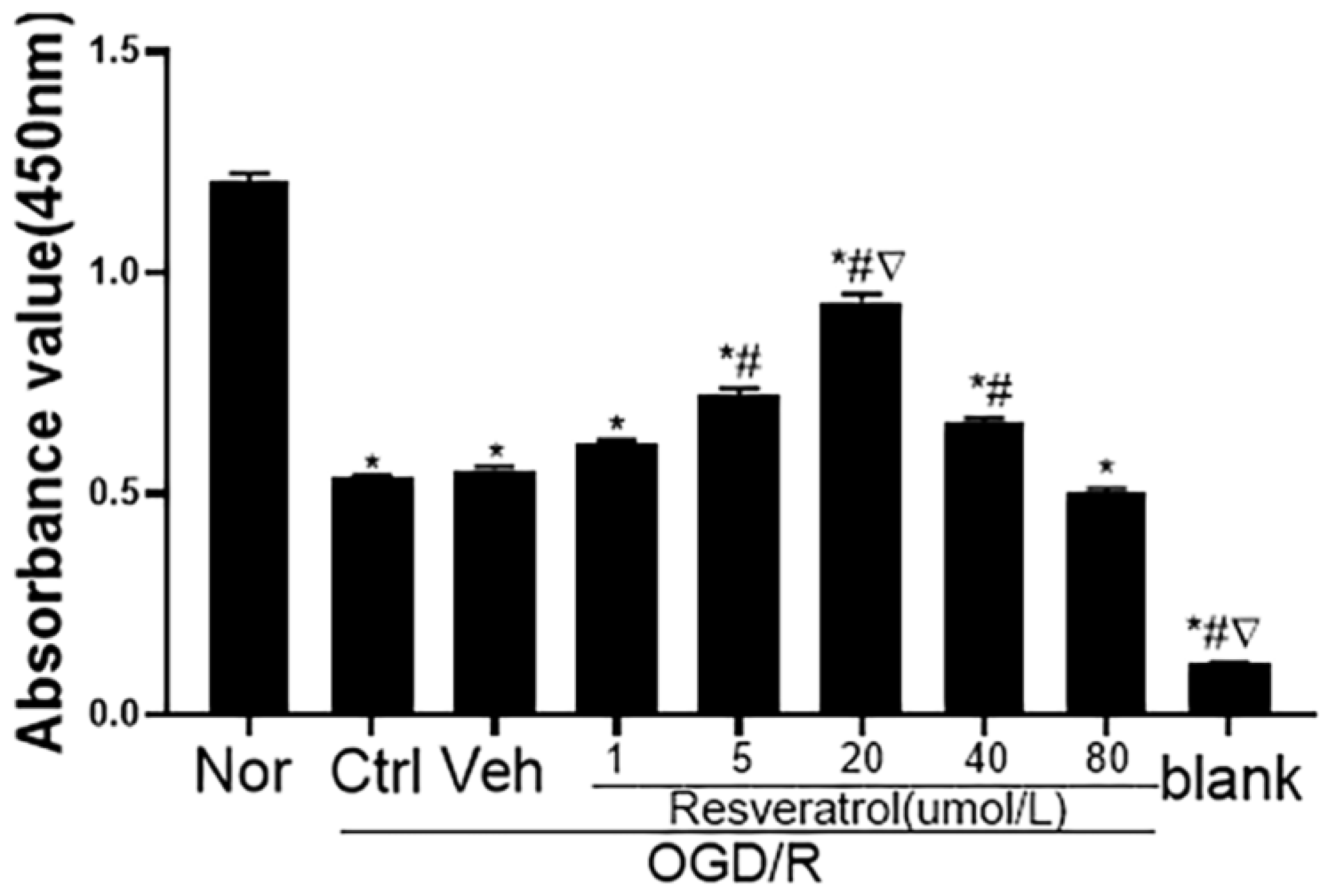
3.1. Concentration Effect of Resveratrol on Viability of N9 Microglia Following OGD/R Injury
3.2. Resveratrol Pretreatment Ameliorates Oxidative Damage and Regulates M1/M2 Polarization of N9 Microglia Following OGD/R Injury In Vitro
3.3. Resveratrol Pretreatment Upregulates Expression of Nrf2, HO 1 and NQO 1 Proteins in N9 Microglia Following OGD/R Injury In Vitro
3.4. Nrf2 Signaling Mediates the Effects of Resveratrol to Inhibit Oxidative Stress and Regulate M1/M2 Phenotype Polarization of Microglia Following OGD/R Injury In Vitro
3.5. Nrf2 Signaling Mediates Resveratrol to Affect Shh Signaling Pathway in N9 Microglia Following OGD/R Injury
3.6. Nrf2 Signaling Mediated Resveratrol to Regulate Neuronal Apoptosis and Viability in Neuron-Microglia Co-Culture
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and Monocytes/Macrophages Polarization Reveal Novel Therapeutic Mechanism against Stroke. Int. J. Mol. Sci. 2017, 18, 2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R.M. Targeting Mitochondrial Dysfunction and Oxidative Stress in Activated Microglia using Dendrimer-Based Therapeutics. Theranostics 2018, 8, 5529–5547. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Trojanowski, P.J.; Villanueva, E.; Navarro, E.; Godbout, J.P. Sequential activation of microglia and astrocyte cytokine expression precedes increased iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 2015, 64, 300–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, M.S.; Dempsey, R.J.; Vemuganti, R. Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem. Int. 2015, 89, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; Guo, S.; Liao, H.; Yu, P.; Wang, L.; Song, X.; Chen, J.; Yang, Q. Resveratrol Enhances Neurite Outgrowth and Synaptogenesis Via Sonic Hedgehog Signaling Following Oxygen-Glucose Deprivation/Reoxygenation Injury. Cell. Physiol. Biochem. 2017, 43, 852–869. [Google Scholar] [CrossRef]
- Yu, P.; Wang, L.; Tang, F.; Zeng, L.; Zhou, L.; Song, X.; Jia, W.; Chen, J.; Yang, Q. Resveratrol Pretreatment Decreases Ischemic Injury and Improves Neurological Function Via Sonic Hedgehog Signaling After Stroke in Rats. Mol. Neurobiol. 2016, 54, 212–226. [Google Scholar] [CrossRef]
- Yang, X.; Xu, S.; Qian, Y.; Xiao, Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017, 64, 162–172. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, M.; Wang, Y.; Xie, F.; Zhang, G.; Qin, X. Nrf2—A Promising Therapeutic Target for Defensing Against Oxidative Stress in Stroke. Mol. Neurobiol. 2016, 54, 6006–6017. [Google Scholar] [CrossRef]
- Cui, B.; Zhang, S.; Wang, Y.; Guo, Y. Farrerol attenuates β-amyloid-induced oxidative stress and inflammation through Nrf2/Keap1 pathway in a microglia cell line. Biomed. Pharmacother. 2019, 109, 112–119. [Google Scholar] [CrossRef]
- Waza, A.A.; Hamid, Z.; Ali, S.; Bhat, S.A.; Bhat, M.A. A review on heme oxygenase-1 induction: Is it a necessary evil. Inflamm. Res. 2018, 67, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. 2018, 115, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.-Q.; Jiang, P.-F.; Gao, Y.-Z. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch. Med. Sci. 2018, 14, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Fan, C.; Chen, N.; Huang, J.; Yang, Q. Resveratrol Pretreatment Attenuates Cerebral Ischemic Injury by Upregulating Expression of Transcription Factor Nrf2 and HO-1 in Rats. Neurochem. Res. 2011, 36, 2352–2362. [Google Scholar] [CrossRef]
- Ingham, P.W.; Placzek, M. Orchestrating ontogenesis: Variations on a theme by sonic hedgehog. Nat. Rev. Genet. 2006, 7, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, T.K.; Carpenter, R.L.; Qasem, S.; Chan, M.; Lo, H.-W. Targeting the Sonic Hedgehog Signaling Pathway: Review of Smoothened and GLI Inhibitors. Cancers 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.; Yu, P.; Wang, L.; Shen, C.; Song, X.; Chen, J.; Tang, F.; Yang, Q. Sonic Hedgehog Signaling Mediates Resveratrol to Increase Proliferation of Neural Stem Cells After Oxygen-Glucose Deprivation/Reoxygenation Injury in Vitro. Cell. Physiol. Biochem. 2015, 35, 2019–2032. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liao, H.; Liu, J.; Liu, J.; Tang, F.; He, Z.; Li, Y.; Yang, Q. Resveratrol Activated Sonic Hedgehog Signaling to Enhance Viability of NIH3T3 Cells in Vitro via Regulation of Sirt1. Cell. Physiol. Biochem. 2018, 50, 1346–1360. [Google Scholar] [CrossRef]
- Fu, J.; Shrivastava, A.; Shrivastava, S.K.; Srivastava, R.K.; Shankar, S. Triacetyl resveratrol upregulates miRNA-200 and suppresses the Shh pathway in pancreatic cancer: A potential therapeutic agent. Int. J. Oncol. 2019, 54, 1306–1316. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.-X.; Yao, P.-S.; Chen, P.-P.; Guan, J.-H.; Zhuang, J.-H.; Zhu, J.-B.; Wu, G.; Yang, J.-S. Neuronal EphA4 Regulates OGD/R-Induced Apoptosis by Promoting Alternative Activation of Microglia. Inflammation 2018, 42, 572–585. [Google Scholar] [CrossRef]
- Wachholz, S.; Eßlinger, M.; Plümper, J.; Manitz, M.-P.; Juckel, G.; Friebe, A. Microglia activation is associated with IFN-α induced depressive-like behavior. Brain Behav. Immun. 2016, 55, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Zhou, L.; Zeng, L.; Yang, Q. Resveratrol pretreatment attenuates injury and promotes proliferation of neural stem cells after oxygen-glucose deprivation/reoxygenation by upregulating expressions of Nrf2, HO-1 and NQO1 in vitro. Mol. Med. Rep. 2016, 14, 3646–3654. [Google Scholar] [CrossRef] [Green Version]
- Leung, H.W.; Lau, E.Y.T.; Leung, C.O.N.; Lei, M.M.L.; Mok, E.H.K.; Ma, V.W.S.; Cho, W.C.S.; Ng, I.O.L.; Yun, J.P.; Cai, S.H.; et al. NRF2/SHH signaling cascade promotes tumor-initiating cell lineage and drug resistance in hepatocellular carcinoma. Cancer Lett. 2020, 476, 48–56. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Q.; Cheng, X.; Li, X.; Li, N.; Liu, T.; Li, J.; Yang, Q.; Dong, R.; Zhang, Y.; et al. Inhibitive Effect of Resveratrol on the Inflammation in Cultured Astrocytes and Microglia Induced by Aβ1–42. Neuroscience 2018, 379, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, J.; Rashid, K.; Langmann, T. Resveratrol induces dynamic changes to the microglia transcriptome, inhibiting inflammatory pathways and protecting against microglia-mediated photoreceptor apoptosis. Biochem. Biophys. Res. Commun. 2018, 501, 239–245. [Google Scholar] [CrossRef]
- Subedi, L.; Baek, S.-H.; Kim, S.Y. Genetically Engineered Resveratrol-Enriched Rice Inhibits Neuroinflammation in Lipopolysaccharide-Activated BV2 Microglia Via Downregulating Mitogen-Activated Protein Kinase-Nuclear Factor Kappa B Signaling Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Wang, H.; Wu, Q.; Lu, Y.; Nie, J.; Xie, X.; Shi, J. Resveratrol Protects Cortical Neurons against Microglia-mediated Neuroinflammation. Phytotherapy Res. 2012, 27, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Yan, X.; Yu, A.; Zheng, H.; Wang, S.; He, Y.; Wang, L. Calycosin-7-O-β-D-glucoside Attenuates OGD/R-Induced Damage by Preventing Oxidative Stress and Neuronal Apoptosis via the SIRT1/FOXO1/PGC-1α Pathway in HT22 Cells. Neural Plast. 2019, 2019, 8798069. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Zhu, L.; Liu, J.; Zhu, T.; Xie, Z.; Sun, X.; Zhang, H. Metformin Protects against Oxidative Stress Injury Induced by Ischemia/Reperfusion via Regulation of the lncRNA-H19/miR-148a-3p/Rock2 Axis. Oxidative Med. Cell. Longev. 2019, 2019, 8768327. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, H.; Zhang, J.; Yan, M. Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem. Biol. Interact. 2018, 284, 32–40. [Google Scholar] [CrossRef] [PubMed]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35 Pt 5, 1156–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, G.-C.; Duh, P.-D.; Lin, C.-W. Effects of resveratrol and 4-hexylresorcinol on hydrogen peroxide-induced oxidative DNA damage in human lymphocytes. Free Radic. Res. 2003, 37, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, S.; Lomnicki, S.; McAvey, K.M.; Cole, R.B.; Dellinger, B.; Cormier, A.S. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Part. Fibre Toxicol. 2009, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Ji, G.; Shen, Y.; Zhao, N.; Liang, Y.; Wang, Z.; Liu, M.; Lin, L. Autophagy Triggered by Oxidative Stress Appears to Be Mediated by the AKT/mTOR Signaling Pathway in the Liver of Sleep-Deprived Rats. Oxidative Med. Cell. Longev. 2020, 2020, 6181630. [Google Scholar] [CrossRef] [Green Version]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Vilhardt, F. Microglia: Phagocyte and glia cell. Int. J. Biochem. Cell Biol. 2005, 37, 17–21. [Google Scholar] [CrossRef]
- Wang, J.; Xing, H.; Wan, L.; Jiang, X.; Wang, C.; Wu, Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed. Pharmacother. 2018, 105, 518–525. [Google Scholar] [CrossRef]
- Chen, C.; Ai, Q.; Chu, S.; Zhang, Z.; Zhou, X.; Luo, P.; Liu, Y.; Chen, N. IMM-H004 protects against oxygen-glucose deprivation/reperfusion injury to BV2 microglia partly by modulating CKLF1 involved in microglia polarization. Int. Immunopharmacol. 2019, 70, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Xu, Y.; Ruan, W.; Wang, H.; Zhang, Y.; Saavedra, J.M.; Zhang, L.; Huang, Z.; Pang, T. A Dual AMPK/Nrf2 Activator Reduces Brain Inflammation After Stroke by Enhancing Microglia M2 Polarization. Antioxid. Redox Signal. 2018, 28, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.V.; Dave, K.R.; Saul, I.; Perez-Pinzon, M.A. Resveratrol Preconditioning Protects Against Cerebral Ischemic Injury via Nuclear Erythroid 2–Related Factor 2. Stroke 2015, 46, 1626–1632. [Google Scholar] [CrossRef] [Green Version]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Cano-Martínez, A.; Díaz-Díaz, E.; Manzano-Pech, L.; Gamas-Magaña, A.; Castrejón-Tellez, V.; Tapia-Cortina, C.; Pérez-Torres, I. Resveratrol and Quercetin Administration Improves Antioxidant DEFENSES and reduces Fatty Liver in Metabolic Syndrome Rats. Molecules 2019, 24, 1297. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chopp, M.; Meier, D.H.; Winter, S.; Wang, L.; Szalad, A.; Lu, M.; Wei, M.; Cui, Y.; Zhang, Z.G. Sonic Hedgehog Signaling Pathway Mediates Cerebrolysin-Improved Neurological Function After Stroke. Stroke 2013, 44, 1965–1972. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Li, Y.; Liu, Z.; Zhang, J.; Cui, Y.; Chen, X.; Chopp, M. The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J. Cereb. Blood Flow Metab. 2013, 33, 1015–1024. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Raviv, N.; Barnett, A.; Bambakidis, N.C.; Filichia, E.; Luo, Y. The Shh Signaling Pathway Is Upregulated in Multiple Cell Types in Cortical Ischemia and Influences the Outcome of Stroke in an Animal Model. PLoS ONE 2015, 10, e0124657. [Google Scholar] [CrossRef]
- Chechneva, O.V.; Mayrhofer, F.; Daugherty, D.J.; Krishnamurty, R.G.; Bannerman, P.; Pleasure, D.E.; Deng, W. A Smoothened receptor activator is neuroprotective and promotes regeneration after ischemic brain injury. Cell Death Dis. 2014, 5, e1481. [Google Scholar] [CrossRef]
- Huang, S.-S.; Cheng, H.; Tang, C.-M.; Nien, M.-W.; Huang, Y.-S.; Lee, I.-H.; Yin, J.-H.; Kuo, T.B.; Yang, C.C.; Tsai, S.-K.; et al. Anti-oxidative, anti-apoptotic, and pro-angiogenic effects mediate functional improvement by sonic hedgehog against focal cerebral ischemia in rats. Exp. Neurol. 2013, 247, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Qin, T.; Qian, W.; Li, X.; Li, W.; Han, L.; Zhang, D.; Wang, Z.; Ma, Q.; Wu, Z.; et al. Cav-1 Ablation in Pancreatic Stellate Cells Promotes Pancreatic Cancer Growth through Nrf2-Induced shh Signaling. Oxidative Med. Cell. Longev. 2020, 2020, 1868764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noman, A.S.M.; Parag, R.R.; Rashid, M.I.; Rahman, M.Z.; Chowdhury, A.A.; Sultana, A.; Jerin, C.; Siddiqua, A.; Rahman, L.; Shirin, A.; et al. Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920911229. [Google Scholar] [CrossRef] [Green Version]
- Roqué, P.J.; Costa, L.G. Co-Culture of Neurons and Microglia. Curr. Protoc. Toxicol. 2017, 74, 11.24.1–11.24.17. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.-S.; Zhang, Z.-H.; Zhou, X.-M.; Gao, Y.-Y.; Liu, G.-J.; Wang, H.; Wu, L.-Y.; Li, W.; Hang, C.-H. Peroxiredoxin 2 activates microglia by interacting with Toll-like receptor 4 after subarachnoid hemorrhage. J. Neuroinflamm. 2018, 15, 87. [Google Scholar] [CrossRef] [PubMed]





| Genes | Forward | Reverse |
|---|---|---|
| CD206 | 5′-GTCAACCCAAGGGCTCTTCTAA-3′ | 5′-AGGTGGCCTCTTGAGGTATGTG-3′ |
| Arg1 | 5′-GGAACTCAACGGGAGGGTAAC-3 | 5′-GAAGGCGTTTGCTTAGTTCTGTC-3′ |
| iNOS | 5′-TTGGCTCCAGCATGTACCCT-3′ | 5′-TCCTGCCCACTGAGTTCGTC-3′ |
| TNF-α | 5′-CCAACGGCAGGATCTCAAAG-3′ | 5′-TGACGGTGTGGGTGAGGAGC-3′ |
| GAPDH | 5′-GACATCAAGAAGGTGGTGAAGC-3′ | 5′-GAAGGTGGAAGAGTGGGAGTT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liao, H.; Chen, Y.; Zhu, H.; Li, X.; Liu, J.; Xiang, Q.; Zeng, F.; Yang, Q. Resveratrol Inhibits Oxidative Stress and Regulates M1/M2-Type Polarization of Microglia via Mediation of the Nrf2/Shh Signaling Cascade after OGD/R Injury In Vitro. J. Pers. Med. 2022, 12, 2087. https://doi.org/10.3390/jpm12122087
Liu J, Liao H, Chen Y, Zhu H, Li X, Liu J, Xiang Q, Zeng F, Yang Q. Resveratrol Inhibits Oxidative Stress and Regulates M1/M2-Type Polarization of Microglia via Mediation of the Nrf2/Shh Signaling Cascade after OGD/R Injury In Vitro. Journal of Personalized Medicine. 2022; 12(12):2087. https://doi.org/10.3390/jpm12122087
Chicago/Turabian StyleLiu, Jie, Hongyan Liao, Yue Chen, Huimin Zhu, Xuemei Li, Jing Liu, Qin Xiang, Fanling Zeng, and Qin Yang. 2022. "Resveratrol Inhibits Oxidative Stress and Regulates M1/M2-Type Polarization of Microglia via Mediation of the Nrf2/Shh Signaling Cascade after OGD/R Injury In Vitro" Journal of Personalized Medicine 12, no. 12: 2087. https://doi.org/10.3390/jpm12122087






