Hemodynamic Monitoring by Smartphone—Preliminary Report from a Comparative Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Measurement Methodology
2.3. Statistical Analysis
2.4. Conflict of Interest
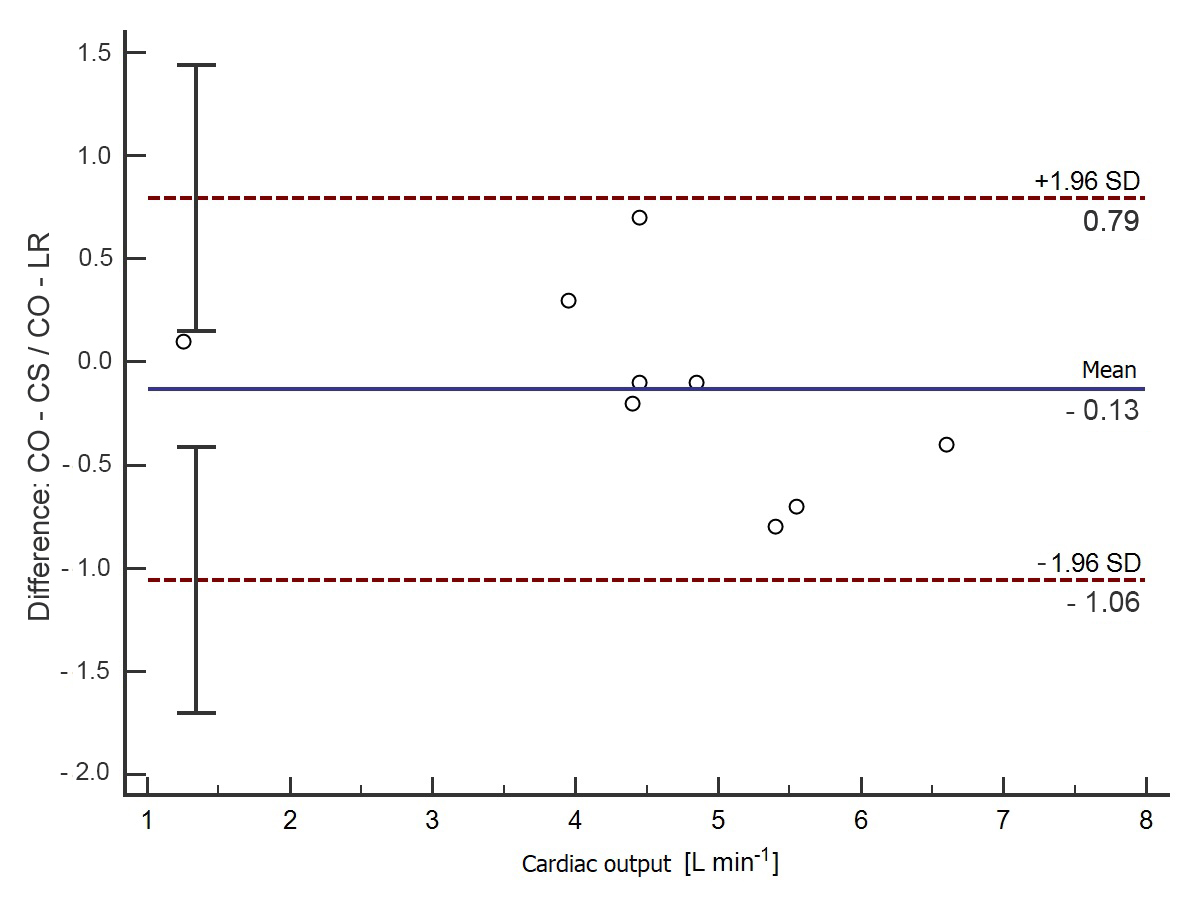
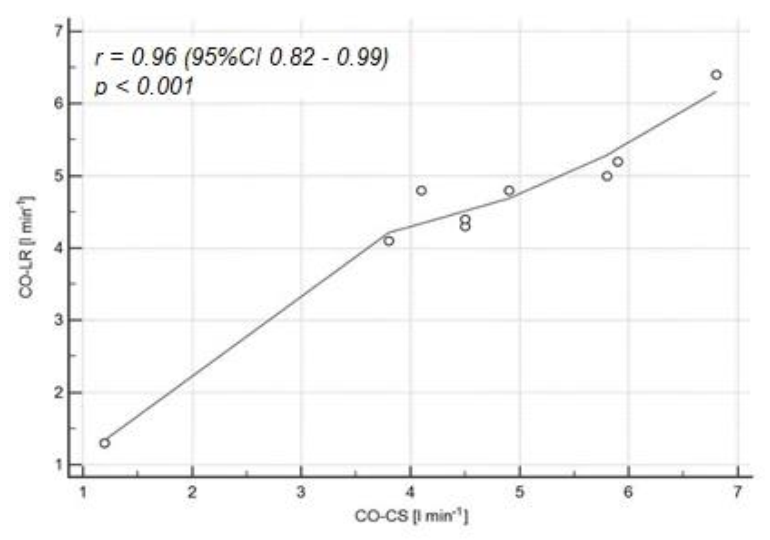
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kucewicz-Czech, E.; Krzych, Ł.J.; Ligowski, M. Perioperative haemodynamic optimisation in patients undergoing non-cardiac surgery—A position statement from the Cardiac and Thoracic Anaesthesia Section of the Polish Society of Anaesthesiology and Intensive Therapy. Part 1. Anaesthesiol. Intensive Ther. 2017, 49, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saugel, B.; Vincent, J.L.; Wagner, J.Y. Personalized hemodynamic management. Curr. Opin. Crit. Care. 2017, 23, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, A.; Pluta, M.; Krzych, Ł. Clinical practice in intraoperative haemodynamic monitoring in Poland: A point prevalence study in 31 Polish hospitals. Anaesthesiol. Intensive Ther. 2020, 52, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Bridges, E.J. Arterial pressure-based stroke volume and functional hemodynamic monitoring. J. Cardiovasc. Nurs. 2008, 23, 105–112. [Google Scholar] [CrossRef]
- Kucewicz-Czech, E.; Krzych, Ł.J.; Ligowski, M. Perioperative haemodynamic optimisation in patients undergoing non-cardiac surgery—A position statement from the Cardiac and Thoracic Anaesthesia Section of the Polish Society of Anaesthesiology and Intensive Therapy. Part 2. Anaesthesiol. Intensive Ther. 2017, 49, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Desebbe, O.; El Hilali, M.; Kouz, K.; Alexander, B.; Karam, L.; Chirnoaga, D.; Knebel, J.-F.; Degott, J.; Schoettker, P.; Michard, F.; et al. Evaluation of a new smartphone optical blood pressure application (OptiBP™) in the post-anesthesia care unit: A method comparison study against the non-invasive automatic oscillometric brachial cuff as the reference method. Int. J. Clin. Monit. Comput. 2022. [Google Scholar] [CrossRef]
- Sanders, J.L.; Noble, V.E.; Raja, A.S.; Sullivan, A.F.; Camargo, J.C.A. Access to and Use of Point-of-Care Ultrasound in the Emergency Department. West. J. Emerg. Med. 2015, 16, 747–752. [Google Scholar] [CrossRef] [Green Version]
- Bhansali, R.; Armstrong, J. Smartphone applications for pediatric anesthesia. Pediatr. Anesthesia 2012, 22, 400–404. [Google Scholar] [CrossRef]
- Czempik, P.F.; Jarosińska, A.; Machlowska, K.; Pluta, M. Impact of Light Intensity on Sleep of Patients in the Intensive Care Unit: A Prospective Observational Study. Indian J. Crit. Care Med. 2020, 24, 33–37. [Google Scholar] [CrossRef]
- Larraza, B.B.; Guerras, O.A.; Lopez-Picado, A. Capstesia, una nueva APP para la monitorización hemodinámica avanzada [Capstesia, a new APP for advanced hemodynamic monitoring]. Rev. Esp Anestesiol Reanim. 2014, 61, 535–536. [Google Scholar] [CrossRef]
- Mohammed, H.M.E.-H.S.; El Halafaway, Y.M.H.; Saad, A.; Mahran, E. Hypertonic saline for goal-directed therapy guided by Capstesia in gastrointestinal surgery: A randomized controlled study. Anaesthesiol. Intensiv. Ther. 2021, 53, 296–303. [Google Scholar] [CrossRef]
- Joosten, A.; Jacobs, A.; Desebbe, O.; Vincent, J.-L.; Sarah, S.; Rinehart, J.; Van Obbergh, L.; Hapfelmeier, A.; Saugel, B. Monitoring of pulse pressure variation using a new smartphone application (Capstesia) versus stroke volume variation using an uncalibrated pulse wave analysis monitor: A clinical decision making study during major abdominal surgery. Int. J. Clin. Monit. Comput. 2019, 33, 787–793. [Google Scholar] [CrossRef]
- Joosten, A.; Boudart, C.; Vincent, J.-L.; Eynden, F.V.; Barvais, L.; Van Obbergh, L.; Rinehart, J.; Desebbe, O. Ability of a New Smartphone Pulse Pressure Variation and Cardiac Output Application to Predict Fluid Responsiveness in Patients Undergoing Cardiac Surgery. Anesthesia Analg. 2019, 128, 1145–1151. [Google Scholar] [CrossRef]
- Linton, N.W.F.; Linton, R.A.F. Estimation of changes in cardiac output from the arterial blood pressure waveform in the upper limb. Br. J. Anaesth. 2001, 86, 486–496. [Google Scholar] [CrossRef] [Green Version]
- Peyton, P.J.; Chong, S.W. Minimally invasive measurement of cardiac output during surgery and critical care: A meta-analysis of accuracy and precision. Anesthesiology 2010, 113, 1220–1235. [Google Scholar] [CrossRef] [Green Version]
- Desebbe, O.; Joosten, A.; Suehiro, K.; Lahham, S.; Essiet, M.; Rinehart, J.; Cannesson, M. A Novel Mobile Phone Application for Pulse Pressure Variation Monitoring Based on Feature Extraction Technology: A Method Comparison Study in a Simulated Environment. Anesthesia Analg. 2016, 123, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Santiago-López, J.; León-Ramírez, V.; Hernández-Ramírez, S.; Vásquez-Márquez, P.I.; Castellanos-Olivares, A. Concordancia en la medición del gasto cardiaco. Vigileo vs. Capstesia [Concordance in the measurement of cardiac output. Vigileo vs. Capstesia]. Rev. Méd. Inst. Mex. Seguro Soc. 2018, 56, 136–142. [Google Scholar]
- Shah, S.B.; Bhargava, A.K.; Hariharan, U.; Vishvakarma, G.; Jain, C.; Kansal, A.; Jain, C.R. Cardiac output monitoring: A comparative prospective observational study of the conventional cardiac output monitor Vigileo™ and the new smartphone-based application Capstesia™. Indian J. Anaesth. 2018, 62, 584–591. [Google Scholar] [CrossRef]
- Desebbe, O.; Vincent, J.-L.; Saugel, B.; Rinehart, J.; Joosten, A. Pulse pressure variation using a novel smartphone application (Capstesia) versus invasive pulse contour analysis in patients undergoing cardiac surgery: A secondary analysis focusing on clinical decision making. Int. J. Clin. Monit. Comput. 2020, 34, 379–380. [Google Scholar] [CrossRef]
- Teboul, J.-L.; Saugel, B.; Cecconi, M.; De Backer, D.; Hofer, C.K.; Monnet, X.; Perel, A.; Pinsky, M.R.; Reuter, D.A.; Rhodes, A.; et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med. 2016, 42, 1350–1359. [Google Scholar] [CrossRef]
- Vignon, P.; Evrard, B.; Asfar, P.; Busana, M.; Calfee, C.S.; Coppola, S.; Demiselle, J.; Geri, G.; Jozwiak, M.; Martin, G.S.; et al. Fluid administration and monitoring in ARDS: Which management? Intensive Care Med. 2020, 46, 2252–2264. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Zhang, Z.-Z.; Li, J.-X.; Wang, Y.-H.; Zhang, W.-L.; Tian, X.-L.; Han, Y.-F.; Yang, M.; Liu, Y. Application of pulse index continuous cardiac output system in elderly patients with acute myocardial infarction complicated by cardiogenic shock: A prospective randomized study. World J. Clin. Cases 2019, 7, 1291–1301. [Google Scholar] [CrossRef]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.K.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.-L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]


| Variable | Value |
|---|---|
| Female (n, %) | 9 (56%) |
| Age (years) | 66 (60–70) |
| Height (m) | 1.65 (1.60–1.75) |
| Body weight (kg) | 70.5 (67.5–80) |
| BMI (kg m−2) | 26 (24–28) |
| BSA (m2) | 1.83 (1.67–1.96) |
| Heart Rate (1 min−1) | 77 (72–93) |
| Systolic blood pressure (mmHg) | 120 (102–134) |
| Diastolic blood pressure (mmHg) | 58 (52–67) |
| Mean Arterial Pressure (mmHg) | 76 (71–91) |
| Pharmacological support of the cardiovascular system | |
| Norepinephrine, n (%) Dose (ug kg−1 min−1) | 10 (63%) 0.1 (0.1–0.2) |
| Epinephrine, n (%) | 2 (13%) |
| Dose (ug kg−1 min−1) | 0.13 (0.05–0.2) |
| Argipressin, n (%) | 1 (6%) |
| Dose (units min−1) | 0.02 |
| Main diagnosis | |
| Septic shock (n, %) | 8 (50%) |
| Hypovolemic shock (n, %) | 1 (6%) |
| Subarachnoid hemorrhage (n, %) | 5 (32%) |
| ARDS (n, %) | 2 (12%) |
| Variable | Value | p | |
|---|---|---|---|
| Dicrotic Notch (+) | Dicrotic Notch (−) | ||
| n (%) | 9 (56%) | 7 (44%) | 0.6 |
| Age (years) | 66 (62–73) | 64 (60–76) | 0.7 |
| Height (m) | 1.65 (1.60–1.75) | 1.75 (1.61–1.79) | 0.4 |
| Body weight (kg) | 70 (68–76) | 80 (66–88) | 0.4 |
| BMI (kg m−2) | 25.7 (22.0–28.2) | 25.7 (24.9–28.1) | 0.7 |
| BSA (m2) | 1.77 (1.66–1.90) | 1.96 (1.70–2.05) | 0.2 |
| Heart Rate (1 min−1) | 74 (68–83) | 92 (77–94) | 0.3 |
| Systolic blood pressure (mmHg) | 120 (100–133) | 120 (106–134) | 0.9 |
| Diastolic blood pressure (mmHg) | 66 (55–84) | 52 (48–60) | 0.1 |
| Mean Arterial Pressure (mmHg) | 85 (73–99) | 72 (68–85) | 0.2 |
| Pharmacological support of the cardiovascular system | |||
| Noradrenaline, n (%) Dose (ug kg−1 min−1) | 5 (31%) 0.1 (0.08–0.2) | 5 (31%) 0.1 (0.2–0.3) | 0.5 0.9 |
| Adrenaline, n (%) | 2 (13%) | - | - |
| Dose (ug kg−1 min−1) | 0.13 (0.05–0.2) | - | - |
| Argipressin, n (%) | 1 (6%) | - | - |
| Dose (units min−1) | 0.02 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluta, M.P.; Dziech, M.; Zachura, M.N.; Szczepańska, A.J.; Czempik, P.F.; Liberski, P.S.; Krzych, Ł.J. Hemodynamic Monitoring by Smartphone—Preliminary Report from a Comparative Prospective Observational Study. J. Pers. Med. 2022, 12, 200. https://doi.org/10.3390/jpm12020200
Pluta MP, Dziech M, Zachura MN, Szczepańska AJ, Czempik PF, Liberski PS, Krzych ŁJ. Hemodynamic Monitoring by Smartphone—Preliminary Report from a Comparative Prospective Observational Study. Journal of Personalized Medicine. 2022; 12(2):200. https://doi.org/10.3390/jpm12020200
Chicago/Turabian StylePluta, Michał P., Magdalena Dziech, Mateusz N. Zachura, Anna J. Szczepańska, Piotr F. Czempik, Piotr S. Liberski, and Łukasz J. Krzych. 2022. "Hemodynamic Monitoring by Smartphone—Preliminary Report from a Comparative Prospective Observational Study" Journal of Personalized Medicine 12, no. 2: 200. https://doi.org/10.3390/jpm12020200
APA StylePluta, M. P., Dziech, M., Zachura, M. N., Szczepańska, A. J., Czempik, P. F., Liberski, P. S., & Krzych, Ł. J. (2022). Hemodynamic Monitoring by Smartphone—Preliminary Report from a Comparative Prospective Observational Study. Journal of Personalized Medicine, 12(2), 200. https://doi.org/10.3390/jpm12020200








