A Revised Stem Cell Theory for the Pathogenesis of Endometriosis
Abstract
:1. Introduction
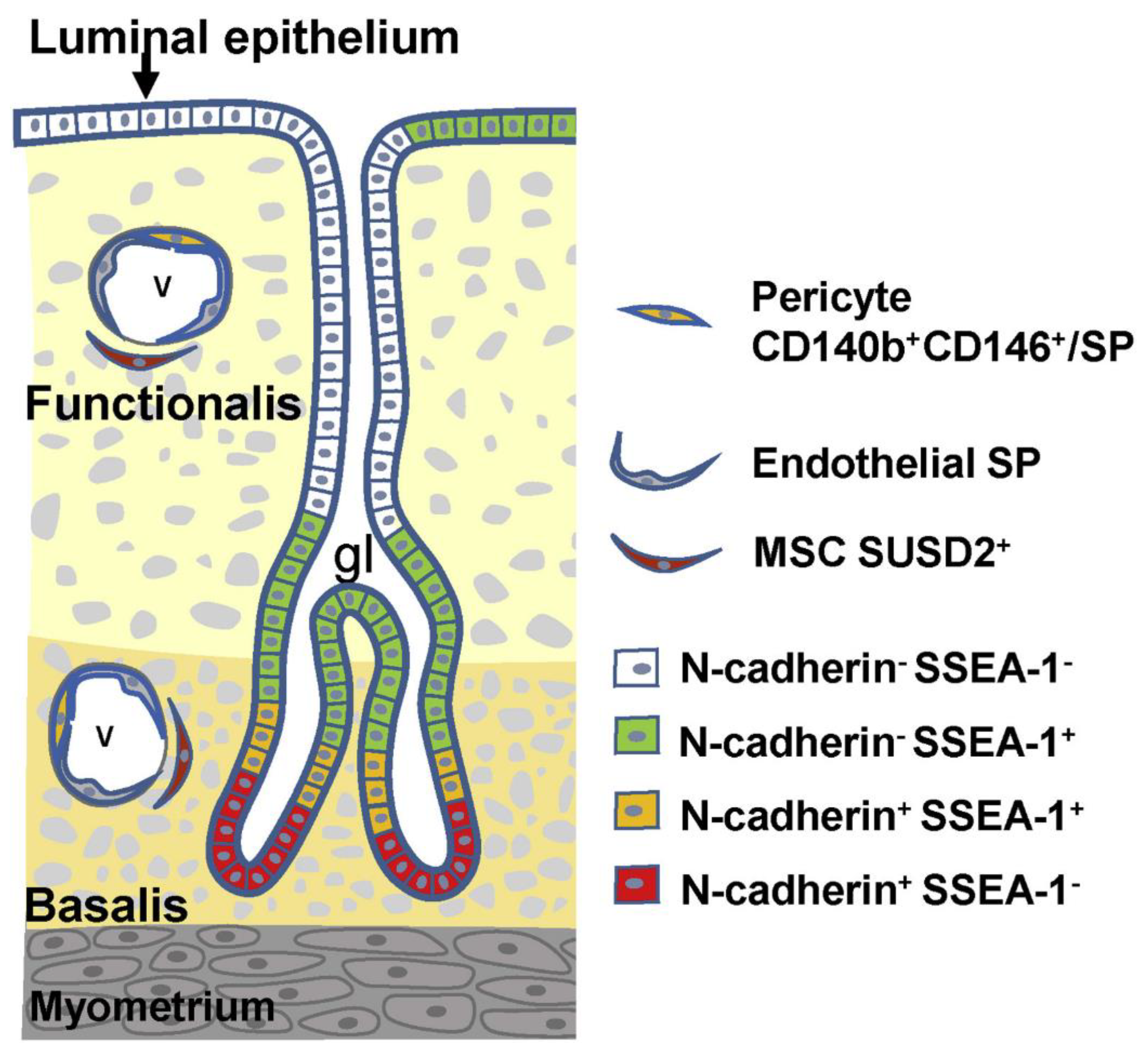
2. Endometrial Stem/Progenitor Cells
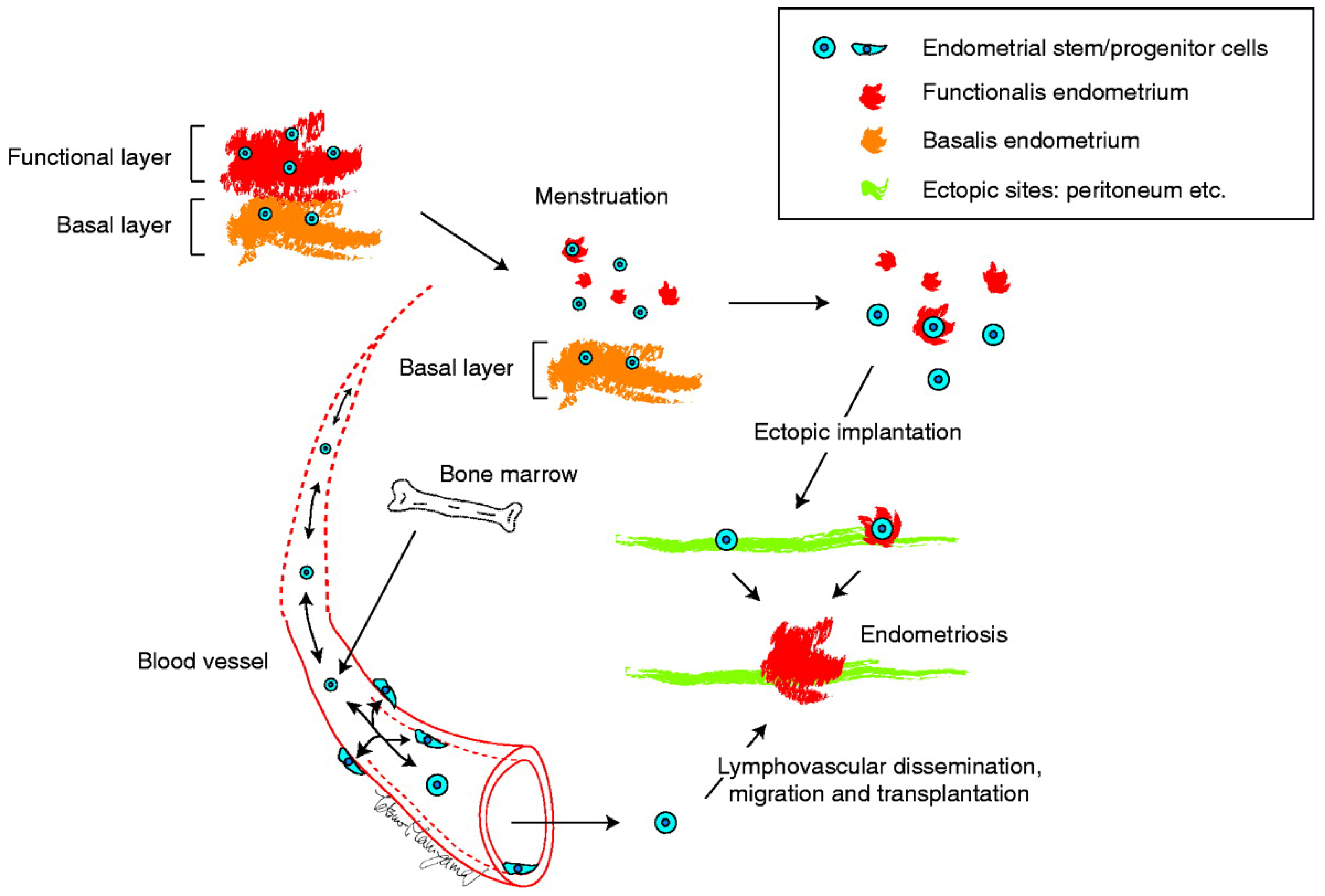
3. The Initial Stem Cell Theory for the Pathogenesis of Endometriosis
3.1. Summary of the Initial Stem Cell Theory
3.2. Supportive Evidence for the Stem Cell Theory
3.3. The Strength and Weakness of Stem Cell Theory
3.4. Candidate Stem/Progenitor Cells Responsible for Genesis of Endometriosis
4. Recent Somatic Mutation Analyses Using Next-Generation Sequencing of Deep Infiltrating Endometriosis, Endometrioma, and Endometrium
5. Modification/Revision of the Stem Cell Theory
5.1. Dual Cell Origin Hypothesis
5.2. Possible Evolutionary Paths Based on the Dual Cell Origin Theory
5.3. Robustness of the Dual Stem Cell Theory for the Pathogenesis of Endometriosis
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seli, E.; Berkkanoglu, M.; Arici, A. Pathogenesis of endometriosis. Obstet. Gynecol. Clin. N. Am. 2003, 30, 41–61. [Google Scholar] [CrossRef]
- Nap, A.W.; Groothuis, P.G.; Demir, A.Y.; Evers, J.L.; Dunselman, G.A. Pathogenesis of endometriosis. Best. Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Yoshimura, Y. Stem cell theory for the pathogenesis of endometriosis. Front. Biosci. (Elite Ed.) 2012, 4, 2754–2763. [Google Scholar] [CrossRef] [Green Version]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Ebert, A.D.; Fuhr, N.; David, M.; Schneppel, L.; Papadopoulos, T. Histological Confirmation of Endometriosis in a 9-Year-Old Girl Suffering from Unexplained Cyclic Pelvic Pain since Her Eighth Year of Life. Gynecol. Obstet. Investig. 2008, 67, 158–161. [Google Scholar] [CrossRef]
- Marsh, E.E.; Laufer, M.R. Endometriosis in premenarcheal girls who do not have an associated obstructive anomaly. Fertil. Steril. 2005, 83, 758–760. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, F.; Bussani, R.; Viceconte, R.; Bulzomi, P.; D’Armiento, M.; D’Avino, A.; Baldi, A. Embryologic origin of endometriosis: Analysis of 101 human female fetuses. J. Cell. Physiol. 2012, 227, 1653–1656. [Google Scholar] [CrossRef]
- I Jabr, F.; Mani, V. An unusual cause of abdominal pain in a male patient: Endometriosis. Avicenna J. Med. 2014, 4, 99–101. [Google Scholar] [CrossRef]
- Giannarini, G.; Scott, C.A.; Moro, U.; Grossetti, B.; Pomara, G.; Selli, C. Cystic endometriosis of the epididymis. Urology 2006, 68, 203.e1–203.e3. [Google Scholar] [CrossRef]
- Redwine, D.B. Was Sampson wrong? Fertil. Steril. 2002, 78, 686–693. [Google Scholar] [CrossRef]
- D’Hooghe, T. Invisible Microscopic Endometriosis: How Wrong Is the Sampson Hypothesis of Retrograde Menstruation to Explain the Pathogenesis of Endometriosis? Gynecol. Obstet. Investig. 2003, 55, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Gargett, C.E.; Guo, S.-W.; Puttemans, P.; Gordts, S.; Brosens, J.; Benagiano, G. Origins and Progression of Adolescent Endometriosis. Reprod. Sci. 2016, 23, 1282–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Gargett, C.E. Uterine stem cells: What is the evidence? Hum. Reprod. Update 2007, 13, 87–101. [Google Scholar] [CrossRef]
- Maruyama, T.; Masuda, H.; Ono, M.; Kajitani, T.; Yoshimura, Y. Human uterine stem/progenitor cells: Their possible role in uterine physiology and pathology. Reproduction 2010, 140, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Masuda, H.; Maruyama, T.; Gargett, C.E.; Miyazaki, K.; Matsuzaki, Y.; Okano, H.; Tanaka, M. Endometrial Side Population Cells: Potential Adult Stem/Progenitor Cells in Endometrium1. Biol. Reprod. 2015, 93, 84. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial stem/progenitor cells: The first 10 years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef] [Green Version]
- Cousins, F.L.; Dorien, F.O.; Gargett, C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 27–38. [Google Scholar] [CrossRef]
- Cousins, F.L.; Pandoy, R.; Jin, S.; Gargett, C.E. The Elusive Endometrial Epithelial Stem/Progenitor Cells. Front. Cell Dev. Biol. 2021, 9, 640319. [Google Scholar] [CrossRef]
- Sasson, I.E.; Taylor, H.S. Stem Cells and the Pathogenesis of Endometriosis. Ann. N. Y. Acad. Sci. 2008, 1127, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Maruyama, T.; Masuda, H.; Kajitani, T.; Nagashima, T.; Arase, T.; Ito, M.; Ohta, K.; Uchida, H.; Asada, H.; et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 18700–18705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, M.; Maruyama, T. Stem Cells in Myometrial Physiology. Semin. Reprod. Med. 2015, 33, 350–356. [Google Scholar] [CrossRef] [PubMed]
- van der Kooy, D.; Weiss, S. Why stem cells? Science 2000, 287, 1439–1441. [Google Scholar] [CrossRef]
- Weissman, I.L. Stem Cells: Units of Development, Units of Regeneration, and Units in Evolution. Cell 2000, 100, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Clevers, H. Coexistence of Quiescent and Active Adult Stem Cells in Mammals. Science 2010, 327, 542–545. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, T.; Yoshimura, Y. Molecular and Cellular Mechanisms for Differentiation and Regeneration of the Uterine Endometrium. Endocr. J. 2008, 55, 795–810. [Google Scholar] [CrossRef] [Green Version]
- Masuda, H.; Maruyama, T.; Hiratsu, E.; Yamane, J.; Iwanami, A.; Nagashima, T.; Ono, M.; Miyoshi, H.; Okano, H.J.; Ito, M.; et al. Noninvasive and real-time assessment of reconstructed functional human endometrium in NOD/SCID/gamma c(null) immunodeficient mice. Proc. Natl. Acad. Sci. USA 2007, 104, 1925–1930. [Google Scholar] [CrossRef] [Green Version]
- Prianishnikov, V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception 1978, 18, 213–223. [Google Scholar] [CrossRef]
- Padykula, H.A.; Coles, L.G.; Okulicz, W.C.; Rapaport, S.I.; McCracken, J.A.; King, N.W., Jr.; Longcope, C.; Kaiserman-Abramof, I.R. The Basalis of the Primate Endometrium: A Bifunctional Germinal Compartment. Biol. Reprod. 1989, 40, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaria, X.; Mas, A.; Cervelló, I.; Taylor, H.; Simón, C. Uterine stem cells: From basic research to advanced cell therapies. Hum. Reprod. Update 2018, 24, 673–693. [Google Scholar] [CrossRef]
- Santamaria, X.; Mas, A.; Cervelló, I.; Taylor, H.S.; Simon, C. Reply: Bone marrow-derived endometrial cells: What you see is what you get. Hum. Reprod. Update 2018, 25, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Yoshimoto, M.; Kato, K.; Adachi, S.; Yamayoshi, A.; Arima, T.; Asanoma, K.; Kyo, S.; Nakahata, T.; Wake, N. Characterization of side-population cells in human normal endometrium. Hum. Reprod. 2007, 22, 1214–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, S.; Yoshimoto, M.; Takahashi, K.; Noda, Y.; Nakahata, T.; Heike, T. Side population cells contribute to the genesis of human endometrium. Fertil. Steril. 2008, 90, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Matsuzaki, Y.; Hiratsu, E.; Ono, M.; Nagashima, T.; Kajitani, T.; Arase, T.; Oda, H.; Uchida, H.; Asada, H.; et al. Stem Cell-Like Properties of the Endometrial Side Population: Implication in Endometrial Regeneration. PLoS ONE 2010, 5, e10387. [Google Scholar] [CrossRef]
- Cervelló, I.; Gil-Sanchis, C.; Mas, A.; Delgado-Rosas, F.; Martínez-Conejero, J.A.; Galán, A.; Martínez-Romero, A.; Martínez, S.; Navarro, I.; Ferro, J.; et al. Human Endometrial Side Population Cells Exhibit Genotypic, Phenotypic and Functional Features of Somatic Stem Cells. PLoS ONE 2010, 5, e10964. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, K.; Maruyama, T.; Masuda, H.; Yamasaki, A.; Uchida, S.; Oda, H.; Uchida, H.; Yoshimura, Y. Stem Cell-Like Differentiation Potentials of Endometrial Side Population Cells as Revealed by a Newly Developed In Vivo Endometrial Stem Cell Assay. PLoS ONE 2012, 7, e50749. [Google Scholar] [CrossRef] [Green Version]
- Challen, G.A.; Little, M.H. A Side Order of Stem Cells: The SP Phenotype. Stem Cells 2006, 24, 3–12. [Google Scholar] [CrossRef]
- Goodell, M.A.; Brose, K.; Paradis, G.; Conner, A.S.; Mulligan, R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996, 183, 1797–1806. [Google Scholar] [CrossRef] [Green Version]
- Huls, M.; Russel, F.G.M.; Masereeuw, R. The Role of ATP Binding Cassette Transporters in Tissue Defense and Organ Regeneration. J. Pharmacol. Exp. Ther. 2009, 328, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Critchley, H.O.D.; Brenner, R.M.; Henderson, T.A.; Williams, K.; Nayak, N.R.; Slayden, O.D.; Millar, M.R.; Saunders, P.T.K. Estrogen Receptor β, But Not Estrogen Receptor α, Is Present in the Vascular Endothelium of the Human and Nonhuman Primate Endometrium1. J. Clin. Endocrinol. Metab. 2001, 86, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Taylor, H.S. Contribution of Bone Marrow-Derived Stem Cells to Endometrium and Endometriosis. Stem Cells 2007, 25, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, D.; Li, F.; Cosar, E.; Krikun, G.; Taylor, H.S. The Role of Stem Cells in the Etiology and Pathophysiology of Endometriosis. Semin. Reprod. Med. 2015, 33, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Götte, M.; Wolf, M.; Staebler, A.; Buchweitz, O.; Kelsch, R.; Schüring, A.; Kiesel, L. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J. Pathol. 2008, 215, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Forte, A.; Schettino, M.T.; Finicelli, M.; Cipollaro, M.; Colacurci, N.; Cobellis, L.; Galderisi, U. Expression Pattern of Stemness-Related Genes in Human Endometrial and Endometriotic Tissues. Mol. Med. 2009, 15, 392–401. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Adenosine triphosphate-binding cassette transporter G2 expression in endometriosis and in endometrium from patients with and without endometriosis. Fertil. Steril. 2012, 98, 1512–1520.e3. [Google Scholar] [CrossRef]
- Chang, J.-H.; Au, H.-K.; Lee, W.-C.; Chi, C.-C.; Ling, T.-Y.; Wang, L.-M.; Kao, S.-H.; Huang, Y.-H.; Tzeng, C.-R. Expression of the pluripotent transcription factor OCT4 promotes cell migration in endometriosis. Fertil. Steril. 2013, 99, 1332–1339.e5. [Google Scholar] [CrossRef]
- Silveira, C.G.; Abrão, M.S.; Dias, J.J.A.; Coudry, R.A.; Soares, F.A.; Drigo, S.A.; Domingues, M.A.; Rogatto, S.R. Common chromosomal imbalances and stemness-related protein expression markers in endometriotic lesions from different anatomical sites: The potential role of stem cells. Hum. Reprod. 2012, 27, 3187–3197. [Google Scholar] [CrossRef]
- Chan, R.; Ng, E.H.Y.; Yeung, W.S.B. Identification of Cells with Colony-Forming Activity, Self-Renewal Capacity, and Multipotency in Ovarian Endometriosis. Am. J. Pathol. 2011, 178, 2832–2844. [Google Scholar] [CrossRef] [Green Version]
- Jimbo, H.; Hitomi, Y.; Yoshikawa, H.; Yano, T.; Momoeda, M.; Sakamoto, A.; Tsutsumi, O.; Taketani, Y.; Esumi, H. Evidence for monoclonal expansion of epithelial cells in ovarian endometrial cysts. Am. J. Pathol. 1997, 150, 1173–1178. [Google Scholar] [PubMed]
- Tamura, M.; Fukaya, T.; Murakami, T.; Uehara, S.; Yajima, A. Analysis of clonality in human endometriotic cysts based on evaluation of X chromosome inactivation in archival formalin-fixed, paraffin-embedded tissue. Lab. Investig. 1998, 78, 213–218. [Google Scholar] [PubMed]
- Wu, Y.; Basir, Z.; Kajdacsy-Balla, A.; Strawn, E.; Macias, V.; Montgomery, K.; Guo, S.W. Resolution of clonal origins for endometriotic lesions using laser capture microdissection and the human androgen receptor (HUMARA) assay. Fertil. Steril. 2003, 79 (Suppl. 1), 710–717. [Google Scholar] [CrossRef]
- Nabeshima, H.; Murakami, T.; Yoshinaga, K.; Sato, K.; Terada, Y.; Okamura, K. Analysis of the clonality of ectopic glands in peritoneal endometriosis using laser microdissection. Fertil. Steril. 2003, 80, 1144–1150. [Google Scholar] [CrossRef]
- Mayr, D.; Amann, G.; Siefert, C.; Diebold, J.; Anderegg, B. Does endometriosis really have premalignant potential? A clonal analysis of laser-microdissected tissue. FASEB J. 2003, 17, 693–695. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Tahlak, M.; Keckstein, J.; Wattiez, A.; Martin, D.C. The epidemiology of endometriosis is poorly known as the pathophysiology and diagnosis are unclear. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 14–26. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noë, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef] [Green Version]
- Salk, J.J.; Horwitz, M.S. Passenger mutations as a marker of clonal cell lineages in emerging neoplasia. Semin. Cancer Biol. 2010, 20, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Noë, M.; Ayhan, A.; Wang, T.-L.; Shih, I.-M. Independent development of endometrial epithelium and stroma within the same endometriosis. J. Pathol. 2018, 245, 265–269. [Google Scholar] [CrossRef]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef] [Green Version]
- Suda, K.; Diaz, L.A.C.; Yoshihara, K.; Nakaoka, H.; Yachida, N.; Motoyama, T.; Inoue, I.; Enomoto, T. Clonal lineage from normal endometrium to ovarian clear cell carcinoma through ovarian endometriosis. Cancer Sci. 2020, 111, 3000–3009. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nicholes, K.; Shih, I.-M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 71–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Adachi, S.; Kase, H.; Motoyama, T.; Inoue, I.; Enomoto, T. Different mutation profiles between epithelium and stroma in endometriosis and normal endometrium. Hum. Reprod. 2019, 34, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wu, J.; Wang, W.; Xie, H.; Yao, S. Pro-endometriotic niche in endometriosis. Reprod. Biomed. Online 2019, 38, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Tal, A.; Tal, R.; Pluchino, N.; Taylor, H.S. Endometrial cells contribute to preexisting endometriosis lesions in a mouse model of retrograde menstruationdagger. Biol. Reprod. 2019, 100, 1453–1460. [Google Scholar] [CrossRef]
- Moore, L.; Leongamornlert, D.; Coorens, T.H.H.; Sanders, M.A.; Ellis, P.; Dentro, S.C.; Dawson, K.J.; Butler, T.; Rahbari, R.; Mitchell, T.J.; et al. The mutational landscape of normal human endometrial epithelium. Nature 2020, 580, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Fujishita, A.; Kitajima, M.; Hiraki, K.; Nakashima, M.; Masuzaki, H. Occult microscopic endometriosis: Undetectable by laparoscopy in normal peritoneum. Hum. Reprod. 2014, 29, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Bentin-Ley, U.; Pedersen, B.; Lindenberg, S.; Larsen, J.F.; Hamberger, L.; Horn, T. Isolation and culture of human endometrial cells in a three-dimensional culture system. Reproduction 1994, 101, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Bläuer, M.; Heinonen, P.; Martikainen, P.; Tomás, E.; Ylikomi, T. A novel organotypic culture model for normal human endometrium: Regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum. Reprod. 2005, 20, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef] [Green Version]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Gamperl, M.; Burkard, T.R.; Kunihs, V.; Kaindl, U.; Junttila, S.; Fiala, C.; Schmidt, K.; Mendjan, S.; Knöfler, M.; et al. Estrogen Signaling Drives Ciliogenesis in Human Endometrial Organoids. Endocrinology 2019, 160, 2282–2297. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef]
- Fitzgerald, H.C.; Dhakal, P.; Behura, S.K.; Schust, D.J.; Spencer, T.E. Self-renewing endometrial epithelial organoids of the human uterus. Proc. Natl. Acad. Sci. USA 2019, 116, 23132–23142. [Google Scholar] [CrossRef]
- Clement, P.B. The pathology of endometriosis: A survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv. Anat. Pathol. 2007, 14, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.P.; McCluggage, W.G. Peritoneal stromal endometriosis: A detailed morphological analysis of a large series of cases of a common and under-recognised form of endometriosis. J. Clin. Pathol. 2009, 62, 530–533. [Google Scholar] [CrossRef]
- Tsujioka, H.; Hachisuga, T.; Fukuoka, M.; Ueda, T.; Miyahara, D.; Horiuchi, S.; Shirota, K.; Yoshizato, T.; Emoto, M.; Miyamoto, S.; et al. Monitoring of Endometrial K-ras Mutation in Tamoxifen-Treated Patients With Breast Cancer. Int. J. Gynecol. Cancer 2009, 19, 1052–1056. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, T. A Revised Stem Cell Theory for the Pathogenesis of Endometriosis. J. Pers. Med. 2022, 12, 216. https://doi.org/10.3390/jpm12020216
Maruyama T. A Revised Stem Cell Theory for the Pathogenesis of Endometriosis. Journal of Personalized Medicine. 2022; 12(2):216. https://doi.org/10.3390/jpm12020216
Chicago/Turabian StyleMaruyama, Tetsuo. 2022. "A Revised Stem Cell Theory for the Pathogenesis of Endometriosis" Journal of Personalized Medicine 12, no. 2: 216. https://doi.org/10.3390/jpm12020216
APA StyleMaruyama, T. (2022). A Revised Stem Cell Theory for the Pathogenesis of Endometriosis. Journal of Personalized Medicine, 12(2), 216. https://doi.org/10.3390/jpm12020216





