Adalimumab and ABP 501 in the Treatment of a Large Cohort of Patients with Inflammatory Arthritis: A Real Life Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics
3.1.1. RA Cohort
3.1.2. PsA Cohort
3.1.3. AxSpA Cohort
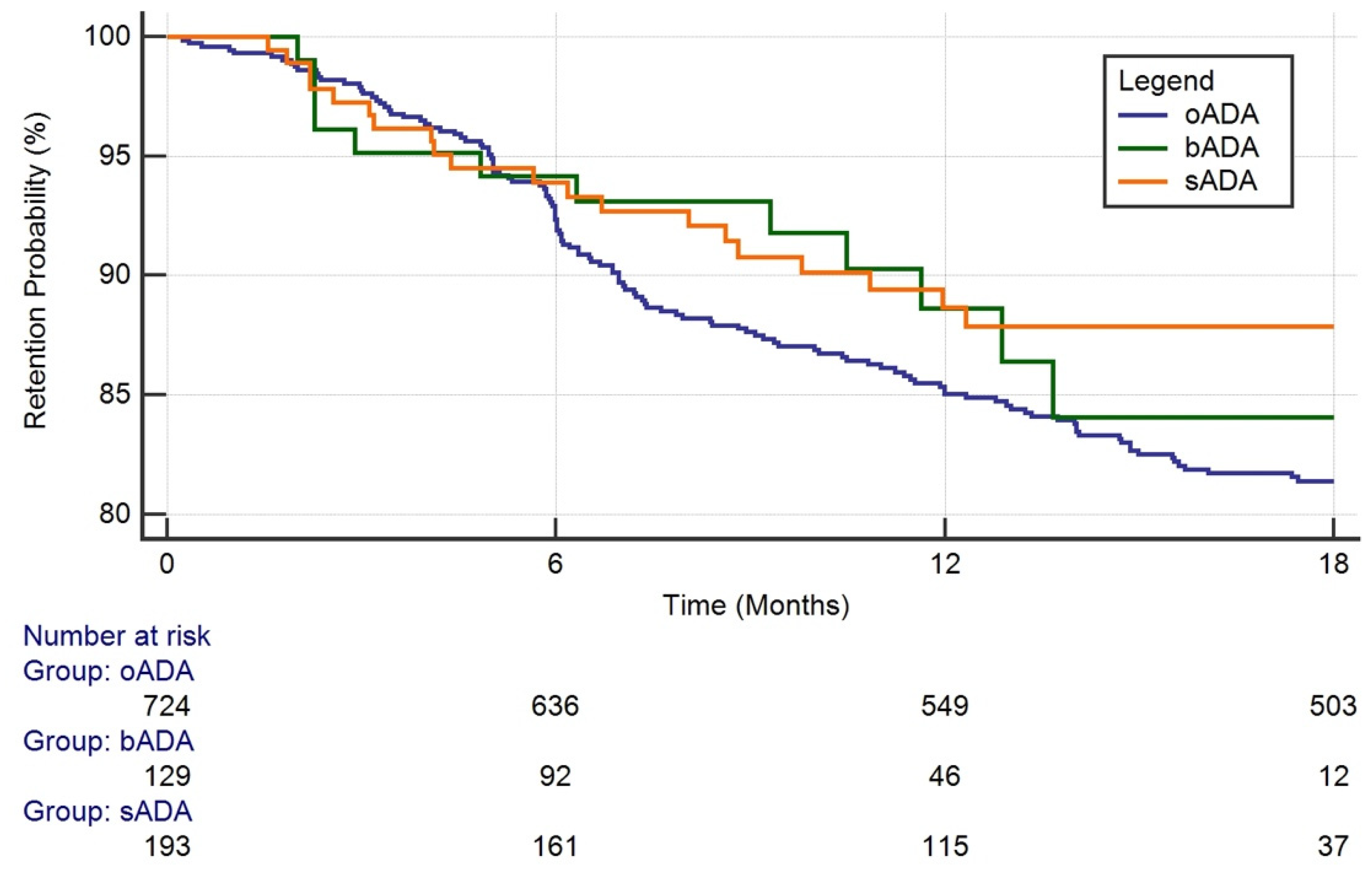
3.2. Drug Survival
3.3. Reasons for Discontinuation
3.4. Predictors of ADA Discontinuation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dörner, T.; Strand, V.; Castañeda-Hernández, G.; Ferraccioli, G.; Isaacs, J.D.; Kvien, T.K.; Martin-Mola, E.; Mittendorf, T.; Smolen, J.S.; Burmester, G.R. The role of biosimilars in the treatment of rheumatic diseases. Ann. Rheum. Dis. 2013, 72, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luchetti, M.M.; Benfaremo, D.; Gabrielli, A. Biologics in inflammatory and immunomediated arthritis. Curr. Pharm. Biotechnol. 2018, 18, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Velayudhan, J.; Chen, Y.; Rohrbach, A.; Pastula, C.; Maher, G.; Thomas, H.; Brown, R.; Born, T.L. Demonstration of functional similarity of proposed biosimilar ABP 501 to adalimumab. BioDrugs 2016, 30, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Eris, T.; Li, C.; Cao, S.; Kuhns, S. Assessing analytical similarity of proposed amgen biosimilar ABP 501 to adalimumab. BioDrugs 2016, 30, 321–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, P.; Chow, V.; Zhang, N.; Moxness, M.; Kaliyaperumal, A.; Markus, R. A Randomised, single-blind, single-dose, three-arm, parallel-group study in healthy subjects to demonstrate pharmacokinetic equivalence of ABP 501 and adalimumab. Ann. Rheum. Dis. 2017, 76, 526–533. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Genovese, M.C.; Choy, E.; Perez-Ruiz, F.; Matsumoto, A.; Pavelka, K.; Pablos, J.L.; Rizzo, W.; Hrycaj, P.; Zhang, N.; et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: A randomised, double-blind, phase III equivalence study. Ann. Rheum. Dis. 2017, 76, 1679–1687. [Google Scholar] [CrossRef]
- Cohen, S.; Pablos, J.L.; Pavelka, K.; Müller, G.A.; Matsumoto, A.; Kivitz, A.; Wang, H.; Krishnan, E. An open-label extension study to demonstrate long-term safety and efficacy of ABP 501 in patients with rheumatoid arthritis. Arthritis. Res. Ther. 2019, 21, 84. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F. Why results of clinical trials and observational studies of antitumour necrosis factor (Anti-TNF) therapy differ: Methodological and interpretive issues. Ann. Rheum. Dis. 2004, 63, ii13–ii17. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Tribocco, E.; Rosso, C.; Armandi, A.; Vernero, M.; Bugianesi, E.; Astegiano, M.; Saracco, G.M.; Caviglia, G.P. Switching from biosimilar to biosimilar adalimumab, including multiple switching, in Crohn’s disease: A prospective study. J. Clin. Med. 2021, 10, 3387. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Cappello, M.; Busacca, A.; Fries, W.; Viola, A.; Costantino, G.; Magnano, A.; Vinci, E.; Ferracane, C.; Privitera, A.C.; et al. SPOSAB ABP 501: A sicilian prospective observational study of patients with inflammatory Bowel disease treated with adalimumab biosimilar ABP 501. J. Gastroenterol. Hepatol. 2021, 36, 3041–3049. [Google Scholar] [CrossRef]
- Giunta, A.; Zangrilli, A.; Bavetta, M.; Manfreda, V.; Pensa, C.; Bianchi, L. A Single-centre, observational, retrospective, real-life study evaluating adalimumab biosimilar ABP 501 in the Treatment of plaque-type psoriasis and psoriatic arthritis in originator-naïve patients and in patients undergoing non-medical switch from originator. Curr. Med. Res. Opin. 2021, 37, 1099–1102. [Google Scholar] [CrossRef]
- Cingolani, L.; Barberio, B.; Zingone, F.; Ferronato, A.; Bertani, L.; Costa, F.; Bodini, G.; Demarzo, M.G.; Melatti, P.; Gubbiotti, A.; et al. Adalimumab biosimilars, ABP501 and SB5, are equally effective and safe as adalimumab originator. Sci. Rep. 2021, 11, 10368. [Google Scholar] [CrossRef] [PubMed]
- Ribaldone, D.G.; Caviglia, G.P.; Pellicano, R.; Vernero, M.; Saracco, G.M.; Morino, M.; Astegiano, M. Effectiveness and safety of adalimumab biosimilar ABP 501 in Crohn’s disease: An observational study. Rev. Esp. Enferm. Dig. 2020, 112, 195–200. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, S.; Cantini, F.; Ramonda, R.; Cantarini, L.; Carletto, A.; Chimenti, M.S.; Delle Sedie, A.; Foti, R.; Gerli, R.; Lomater, C.; et al. Effectiveness of adalimumab for the treatment of psoriatic arthritis: An italian real-life retrospective study. Front. Pharmacol. 2019, 10, 1497. [Google Scholar] [CrossRef] [Green Version]
- Favalli, E.G.; Selmi, C.; Becciolini, A.; Biggioggero, M.; Ariani, A.; Santilli, D.; Fusaro, E.; Parisi, S.; Massarotti, M.; Marchesoni, A.; et al. Eight-year retention rate of first-line tumor necrosis factor inhibitors in spondyloarthritis: A multicenter retrospective analysis: Survival of TNF inhibitors over eight years in SpA. Arthritis Care Res. 2017, 69, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Favalli, E.G.; Pregnolato, F.; Biggioggero, M.; Becciolini, A.; Penatti, A.E.; Marchesoni, A.; Meroni, P.L. Twelve-year retention rate of first-line tumor necrosis factor inhibitors in rheumatoid arthritis: Real-life data from a local registry: Long-term persistence on infliximab, etanercept, and adalimumab. Arthritis Care Res. 2016, 68, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Nabi, H.; Georgiadis, S.; Loft, A.G.; Hendricks, O.; Andersen, M.; Chrysidis, S.; Colic, A.; Danebod, K.; Hussein, M.R.; Kalisz, M.H.; et al. Comparative effectiveness of two adalimumab biosimilars in 1318 real-world patients with inflammatory rheumatic disease mandated to switch from originator adalimumab: Nationwide observational study emulating a randomised clinical trial. Ann. Rheum. Dis. 2021, 80, 1400–1409. [Google Scholar] [CrossRef]
- Favalli, E.G.; Becciolini, A.; Biggioggero, M.; Bertoldi, I.; Crotti, C.; Raimondo, M.G.; Marchesoni, A. The role of concomitant methotrexate dosage and maintenance over time in the therapy of rheumatoid arthritis patients treated with adalimumab or etanercept: Retrospective analysis of a local registry. Drug Des. Dev. Ther. 2018, 12, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Neuenschwander, R.; Hebeisen, M.; Micheroli, R.; Bürki, K.; Exer, P.; Niedermann, K.; Nissen, M.J.; Scherer, A.; Ciurea, A. Differences between men and women with nonradiographic axial spondyloarthritis: Clinical characteristics and treatment effectiveness in a real-life prospective cohort. Arthritis Res. Ther. 2020, 22, 233. [Google Scholar] [CrossRef]
- Stober, C.; Ye, W.; Guruparan, T.; Htut, E.; Clunie, G.; Jadon, D. Prevalence and predictors of tumour necrosis factor inhibitor persistence in psoriatic arthritis. Rheumatology 2018, 57, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-W.; Kang, J.-H.; Yim, Y.-R.; Kim, J.-E.; Wen, L.; Lee, K.-E.; Park, D.-J.; Kim, T.-J.; Park, Y.-W.; Lee, S.-S. Predictors of switching anti-tumor necrosis factor therapy in patients with ankylosing spondylitis. PLoS ONE 2015, 10, e0131864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neovius, M.; Arkema, E.V.; Olsson, H.; Eriksson, J.K.; Kristensen, L.E.; Simard, J.F.; Askling, J. ARTIS study group drug survival on tnf inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann. Rheum. Dis. 2015, 74, 354–360. [Google Scholar] [CrossRef]
- Scirè, C.A.; Caporali, R.; Sarzi-Puttini, P.; Frediani, B.; Di Franco, M.; Tincani, A.; Sinigaglia, L.; Sfriso, P.; Tirri, R.; Bellis, E.; et al. Drug survival of the first course of Anti-TNF agents in patients with rheumatoid arthritis and seronegative spondyloarthritis: Analysis from the MonitorNet Database. Clin. Exp. Rheumatol. 2013, 31, 857–863. [Google Scholar] [PubMed]
- Heiberg, M.S.; Koldingsnes, W.; Mikkelsen, K.; Rødevand, E.; Kaufmann, C.; Mowinckel, P.; Kvien, T.K. The comparative one-year performance of anti–tumor necrosis factor α drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: Results from a longitudinal, observational, multicenter study. Arthritis Rheum. 2008, 59, 234–240. [Google Scholar] [CrossRef]
- Bruni, C.; Bitti, R.; Nacci, F.; Cometi, L.; Tofani, L.; Bartoli, F.; Fiori, G.; Matucci-Cerinic, M. Efficacy and safety of switching from reference adalimumab to SB5 in a real-life cohort of inflammatory rheumatic joint diseases. Clin. Rheumatol. 2021, 40, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ditto, M.C.; Parisi, S.; Priora, M.; Sanna, S.; Peroni, C.L.; Laganà, A.; D’Avolio, A.; Fusaro, E. Efficacy and safety of a single switch from etanercept originator to etanercept biosimilar in a cohort of inflammatory arthritis. Sci. Rep. 2020, 10, 16178. [Google Scholar] [CrossRef]
- Abdalla, A.; Byrne, N.; Conway, R.; Walsh, T.; Mannion, G.; Hanly, M.; O’Sullivan, M.; Curran, A.M.; Carey, J.J. Long-term safety and efficacy of biosimilar infliximab among patients with inflammatory arthritis switched from reference product. Open Access Rheumatol. Res. Rev. 2017, 9, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Glintborg, B.; Ibsen, R.; Bilbo, R.E.Q.; Lund Hetland, M.; Kjellberg, J. Does a mandatory non-medical switch from originator to biosimilar etanercept lead to increase in healthcare use and costs? A danish register-based study of patients with inflammatory arthritis. RMD Open 2019, 5, e001016. [Google Scholar] [CrossRef] [Green Version]
- Glintborg, B.; Sørensen, I.J.; Loft, A.G.; Lindegaard, H.; Linauskas, A.; Hendricks, O.; Hansen, I.M.J.; Jensen, D.V.; Manilo, N.; Espesen, J.; et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 Patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann. Rheum. Dis. 2017, 76, 1426–1431. [Google Scholar] [CrossRef]
- Glintborg, B.; Loft, A.G.; Omerovic, E.; Hendricks, O.; Linauskas, A.; Espesen, J.; Danebod, K.; Jensen, D.V.; Nordin, H.; Dalgaard, E.B.; et al. To switch or not to switch: Results of a nationwide guideline of mandatory switching from originator to biosimilar etanercept. One-year treatment outcomes in 2061 patients with inflammatory arthritis from the DANBIO registry. Ann. Rheum. Dis. 2019, 78, 192–200. [Google Scholar] [CrossRef]
- Becciolini, A.; Lumetti, F.; Di Donato, E.; Giordano, S.; Santilli, D.; Mozzani, F.; Riva, M.; Lucchini, G.; Ariani, A. bDMARDs Retention Rate in the Biosimilar Era: A Real-Life Monocentric Study. Eur. J. Rheumatol. 2021, 8, 109–110. [Google Scholar] [CrossRef] [PubMed]
| oADA Group | bADA Group | sADA Group | p Value | |
|---|---|---|---|---|
| N | 724 | 129 | 193 | - |
| M:F | 300:424 | 42:87 | 88:105 | >0.05 ° |
| Age, median (IQR) (years) | 53.9 (43.6–63.5) | 56.0 (45.7–67.0) | 57.7 (50.3–69.7) | <0.05 + |
| Disease duration, median (IQR) (years) | 4.3 (1.4–11.0) | 3.6 (1.4–9.8) | 12.6 (7.5–19.6) | <0.05 + |
| Diagnosis, n (%) - RA - PsA - AxSpA | 311 (43.0%) 216 (29.8%) 197 (27.2%) | 40 (31.0%) 52 (40.3 %) 37 (28.7%) | 79 (40.9%) 48 (24.9%) 66 (34.2%) | <0.05 ° |
| Line, n (%) - 1 - 2 - 3 + | 521 (71.9%) 146 (20.2%) 57 (7.9%) | 89 (69.0%) 28 (21.7%) 12 (9.3%) | 0 (0) 138 (71.5%) 55 (28.4%) | <0.05 ° |
| oADA Group | bADA Group | sADA Group | p Value | |
|---|---|---|---|---|
| N | 311 | 40 | 79 | - |
| M:F | 77:234 | 5:35 | 21:58 | >0.05 ° |
| Age, median (IQR) (years) | 59.3 (49.6–67.6) | 59.9 (51.5–67.2) | 66.5 (56.5–78.5) | <0.05 + |
| Disease duration, median (IQR) (years) | 6.4 (2.4–15.5) | 5.7 (2.4–11.9) | 14.5 (9.4–23.8) | <0.05 + |
| RF positive, % | 50.2% | 50.0% | 39.2% | >0.05 ° |
| ACPA positive, % | 54.7% | 57.5% | 40.5% | <0.05 ° |
| Line, n (%) - 1 - 2 - 3 + | 208 (66.9%) 75 (24.1%) 28 (9.0%) | 26 (65.0%) 9 (22.5%) 5 (12.5%) | 0 (0%) 50 (63.3%) 29 (36.7%) | <0.05 ° |
| csDMARDs association, % | 60.5% | 72.5% | 53.2% | <0.05 ° |
| steroid association, % | 54.7% | 72.5% | 39.2% | <0.05 ° |
| oADA Group | bADA Group | sADA Group | p Value | |
|---|---|---|---|---|
| N | 216 | 52 | 48 | - |
| M:F | 108:108 | 18:34 | 30:18 | <0.05 ° |
| Age, median (IQR) (years) | 53.4 (43.0–60.1) | 61.3 (50.3–68.9) | 55.7 (46.9–65.2) | <0.05 + |
| Disease duration, median (IQR) (years) | 3.5 (1.2–8.0) | 4.5 (1.2–7.6) | 12.6 (5.8–19.1) | <0.05 + |
| Line, n (%) - 1 - 2 - 3 + | 163 (75.5%) 40 (18.5%) 13 (6.0%) | 34 (65.4%) 12 (23.1%) 6 (11.5%) | 0 (0%) 38 (79.2%) 10 (20.2%) | <0.05 ° |
| csDMARDs association, % | 35.2% | 42.3% | 41.7% | >0.05 ° |
| oADA Group | bADA Group | sADA Group | p Value | |
|---|---|---|---|---|
| N | 197 | 37 | 66 | - |
| M:F | 115:82 | 19:18 | 37:29 | >0.05 ° |
| Age, median (IQR) (years) | 48.4 (39.7–56) | 46.0 (41.1–56.1) | 53.2 (46.1–61.6) | <0.05 + |
| Disease duration, median (IQR) (years) | 2.2 (0.4–8.1) | 3.0 (1.1–8.1) | 10.3 (6.5–13.6) | <0.05 + |
| HLAB27 positive, % | 73.1% | 54.0% | 74.2% | >0.05 ° |
| Line, n (%) - 1 - 2 - 3 + | 150 (76.1%) 31 (15.8%) 16 (8.1%) | 29 (78.4%) 7 (18.9%) 1 (2.7%) | 0 (0%) 50 (75.8%) 16 (24.2%) | <0.05 ° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becciolini, A.; Parisi, S.; Caccavale, R.; Bravi, E.; Lumetti, F.; Andracco, R.; Volpe, A.; Gardelli, L.; Girelli, F.; Di Donato, E.; et al. Adalimumab and ABP 501 in the Treatment of a Large Cohort of Patients with Inflammatory Arthritis: A Real Life Retrospective Analysis. J. Pers. Med. 2022, 12, 335. https://doi.org/10.3390/jpm12030335
Becciolini A, Parisi S, Caccavale R, Bravi E, Lumetti F, Andracco R, Volpe A, Gardelli L, Girelli F, Di Donato E, et al. Adalimumab and ABP 501 in the Treatment of a Large Cohort of Patients with Inflammatory Arthritis: A Real Life Retrospective Analysis. Journal of Personalized Medicine. 2022; 12(3):335. https://doi.org/10.3390/jpm12030335
Chicago/Turabian StyleBecciolini, Andrea, Simone Parisi, Rosalba Caccavale, Elena Bravi, Federica Lumetti, Romina Andracco, Alessandro Volpe, Lucia Gardelli, Francesco Girelli, Eleonora Di Donato, and et al. 2022. "Adalimumab and ABP 501 in the Treatment of a Large Cohort of Patients with Inflammatory Arthritis: A Real Life Retrospective Analysis" Journal of Personalized Medicine 12, no. 3: 335. https://doi.org/10.3390/jpm12030335







