Pathway Phenotypes Underpinning Depression, Anxiety, and Chronic Fatigue Symptoms Due to Acute Rheumatoid Arthritis: A Precision Nomothetic Psychiatry Analysis
Abstract
:1. Introduction
2. Materials and Methods
Participants
3. Assays
3.1. Statistical Analysis
3.2. Precision Nomothetic Network Psychiatry
4. Results
4.1. Sociodemographic Data
4.2. Multivariate GLM Analysis
4.3. Intercorrelation Matrix between Psychiatric Rating Scales and Biomarkers
4.4. Prediction of the Psychopathology Scores Using Biomarkers
4.5. Correlation of Psychiatric Rating Scale Scores and the Indices of Clinical Severity of RA
4.6. Prediction of the PP Scale Scores Using Biomarkers and RA Severity Scores
4.7. Precision Nomothetic Psychiatry Models
PLS Analysis to Optimize the RA Disease Model
4.8. Clustering Analysis to Discover New Endophenotype Clusters
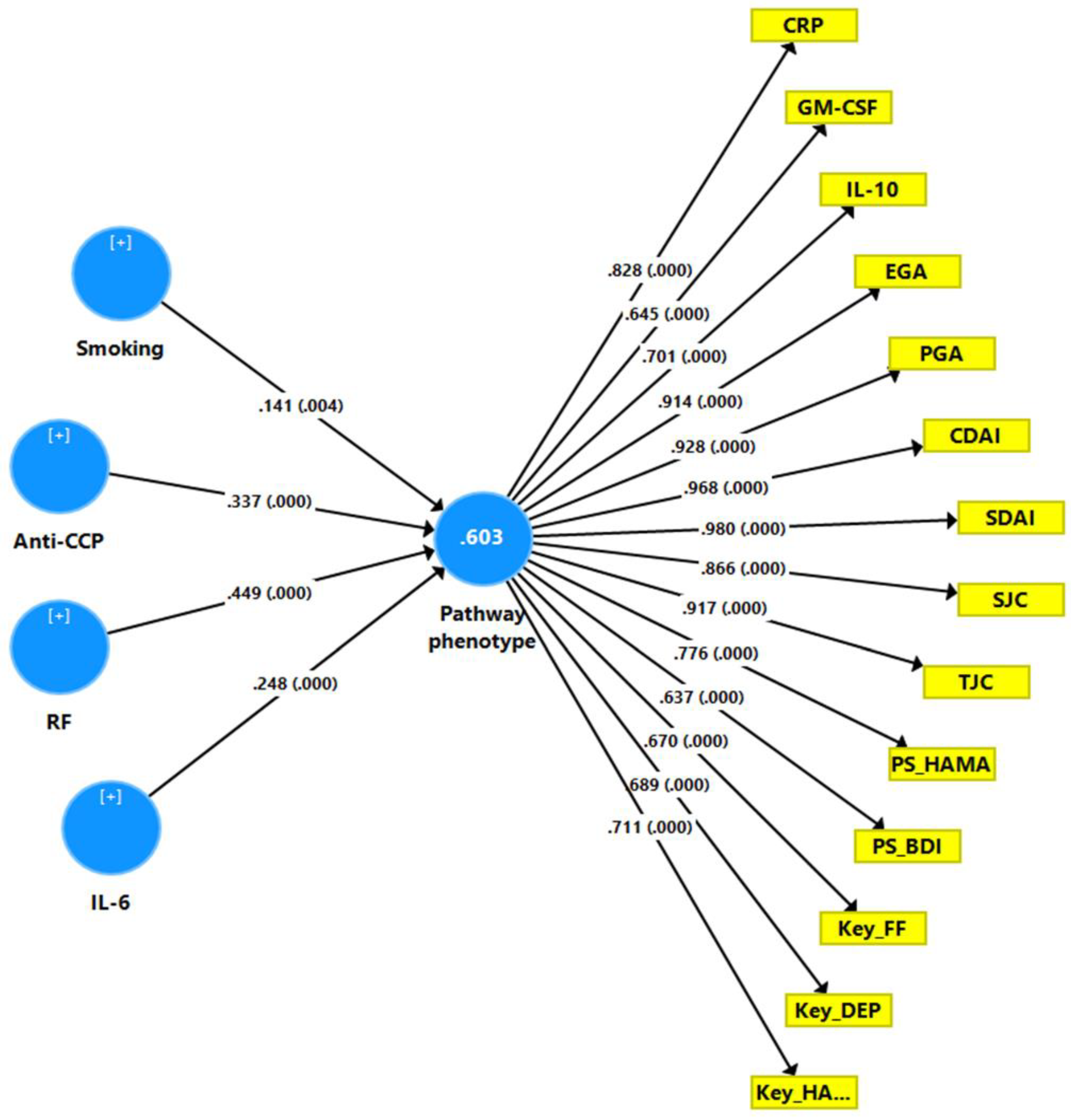
4.9. Construction of a Pathway Phenotype Using PLS Analysis
5. Discussion
5.1. Affective and CFS-Like Symptoms in RA
5.2. Immune–Inflammatory Pathways
5.3. Other Biomarkers of PP Due to RA
5.4. Precision Nomothetic Psychiatry
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amaya-Amaya, J.; Rojas-Villarraga, A.; Mantilla, R.; Anaya, J. Rheumatoid arthritis. In Autoimmunity: From Bench to Bedside; Anaya, J.M., Rojas-Villarraga, S.Y., Levy, A., Cervera, R.A.R., Eds.; El Rosario University Press: Bogota, Columbia, 2013; Chapter 24. [Google Scholar]
- Miossec, P. Rheumatoid arthritis: Still a chronic disease. Lancet 2013, 381, 884–886. [Google Scholar] [CrossRef]
- Bala, A.; Mondal, C.; Haldar, P.K.; Khandelwal, B. Oxidative stress in inflammatory cells of patient with rheumatoid arthritis: Clinical efficacy of dietary antioxidants. Inflammopharmacology 2017, 25, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Van Dartel, S.; Repping-Wuts, J.; Van Hoogmoed, D.; Bleijenberg, G.; Van Riel, P.; Fransen, J. Association between fatigue and pain in rheumatoid arthritis: Does pain precede fatigue or does fatigue precede pain? Arthritis Care Res. 2013, 65, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Anandarajah, A.P. Clinical aspects of rheumatoid arthritis: Highlights from the 2010 ACR conference. Int. J. Clin. Rheumatol. 2011, 6, 267. [Google Scholar] [CrossRef]
- Giannini, D.; Antonucci, M.; Petrelli, F.; Bilia, S.; Alunno, A.; Puxeddu, I. One year in review 2020: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2020, 38, 387–397. [Google Scholar]
- Bala, M. Concerted action of Nrf2-ARE pathway, MRN complex, HMGB1 and inflammatory cytokines-implication in modification of radiation damage. Redox Biol. 2014, 2, 832–846. [Google Scholar]
- Maes, M. A review on the acute phase response in major depression. Rev. Neurosci. 1993, 4, 407–416. [Google Scholar] [CrossRef]
- Narazaki, M.; Tanaka, T.; Kishimoto, T. The role and therapeutic targeting of IL-6 in rheumatoid arthritis. Expert. Rev. Clin. Immunol. 2017, 13, 535–551. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Seitz, M.; Loetscher, P.; Fey, M.F.; Tobler, A. Constitutive mRNA and protein production of macrophage colony-stimulating factor but not of other cytokines by synovial fibroblasts from rheumatoid arthritis and osteoarthritis patients. Br. J. Rheumatol. 1994, 33, 613–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.D.; Firestein, G.S.; Taetle, R.; Kaushansky, K.; Zvaifler, N.J. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J. Clin. Investig. 1989, 83, 876–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neve, A.; Corrado, A.; Cantatore, F.P. Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin. Exp. Med. 2014, 14, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bello, J.; Oregón-Romero, E.; Vázquez-Villamar, M.; García-Arellano, S.; Valle, Y.; Padilla-Gutiérrez, J.R.; Román-Fernández, I.V.; Palafox-Sánchez, C.A.; Martínez-Bonilla, G.E.; Muñoz-Valle, J.F. Aberrant expression of interleukin-10 in rheumatoid arthritis: Relationship with IL10 haplotypes and autoantibodies. Cytokine 2017, 95, 88–96. [Google Scholar] [CrossRef]
- Roelofs, M.F.; Wenink, M.H.; Brentano, F.; Abdollahi-Roodsaz, S.; Oppers-Walgreen, B.; Barrera, P.; van Riel, P.L.; Joosten, L.A.; Kyburz, D.; van den Berg, W.B.; et al. Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4-mediated synovial inflammation in rheumatoid arthritis (RA). Ann. Rheum. Dis. 2009, 68, 1486–1493. [Google Scholar] [CrossRef] [Green Version]
- Gierut, A.; Perlman, H.; Pope, R.M. Innate immunity and rheumatoid arthritis. Rheum. Dis. Clin. 2010, 36, 271–296. [Google Scholar] [CrossRef] [Green Version]
- Pierer, M.; Wagner, U.; Rossol, M.; Ibrahim, S. Toll-like receptor 4 is involved in inflammatory and joint destructive pathways in collagen-induced arthritis in DBA1J mice. PLoS ONE 2011, 6, e23539. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Kohsaka, H.; Miyasaka, N. Ceramide, a mediator of interleukin 1, tumour necrosis factor α, as well as Fas receptor signalling, induces apoptosis of rheumatoid arthritis synovial cells. Ann. Rheum. Dis. 1998, 57, 495–499. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Iwabuchi, K.; Nagaoka, I.; Adachi, Y.; Ohno, N.; Tamura, H.; Seyama, K.; Fukuchi, Y.; Nakayama, H.; Yoshizaki, F.; et al. Induction of human neutrophil chemotaxis by Candida albicans-derived beta-1,6-long glycoside side-chain-branched beta-glucan. J. Leukoc. Biol. 2006, 80, 204–211. [Google Scholar] [CrossRef]
- Iwabuchi, K.; Prinetti, A.; Sonnino, S.; Mauri, L.; Kobayashi, T.; Ishii, K.; Kaga, N.; Murayama, K.; Kurihara, H.; Nakayama, H. Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj. J. 2008, 25, 357–374. [Google Scholar] [CrossRef]
- Yeh, L.H.; Kinsey, A.M.; Chatterjee, S.; Alevriadou, B.R. Lactosylceramide mediates shear-induced endothelial superoxide production and intercellular adhesion molecule-1 expression. J. Vasc. Res. 2001, 38, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Pannu, R.; Won, J.-S.; Khan, M.; Singh, A.K.; Singh, I. A novel role of lactosylceramide in the regulation of lipopolysaccharide/interferon-γ-mediated inducible nitric oxide synthase gene expression: Implications for neuroinflammatory diseases. J. Neurosci. 2004, 24, 5942–5954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbeialy, A.; Elbarbary, M.; Kamel, M. Peripheral beta-endorphin in rheumatoid arthritis. A correlation with the disease activity. Scand. J. Rheumatol. 1997, 26, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Machelska, H.; Schopohl, J.K.; Mousa, S.A.; Labuz, D.; Schäfer, M.; Stein, C. Different mechanisms of intrinsic pain inhibition in early and late inflammation. J. Neuroimmunol. 2003, 141, 30–39. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Zeki Al-Fadhel, S.; Al-Dujaili, A.H.; Maes, M. In major depression, increased kappa and mu opioid receptor levels are associated with immune activation. Acta. Neuropsychiatr. 2020, 32, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Matcham, F.; Rayner, L.; Steer, S.; Hotopf, M. The prevalence of depression in rheumatoid arthritis: A systematic review and meta-analysis. Rheumatology 2013, 52, 2136–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baerwald, C.; Manger, B.; Hueber, A. Depression as comorbidity of rheumatoid arthritis. Z. Rheumatol. 2019, 78, 243–248. [Google Scholar] [CrossRef]
- Fiest, K.M.; Hitchon, C.A.; Bernstein, C.N.; Peschken, C.A.; Walker, J.R.; Graff, L.A.; Zarychanski, R.; Abou-Setta, A.; Patten, S.B.; Sareen, J.; et al. Systematic Review and Meta-Analysis of Interventions for Depression and Anxiety in Persons with Rheumatoid Arthritis. J. Clin. Rheumatol. 2017, 23, 425–434. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Cavanagh, J.; Tindell, A.; Derakhshan, M.; Paterson, C.; Porter, D.; McInnes, I.B.; Siebert, S. Depression and anxiety in an early rheumatoid arthritis inception cohort. associations with demographic, socioeconomic and disease features. RMD Open 2020, 6, e001376. [Google Scholar] [CrossRef]
- Qiu, X.J.; Zhang, X.L.; Cai, L.S.; Yan, C.; Yu, L.; Fan, J.; Zhang, R.W.; Huang, J.W.; Duan, X.W. Rheumatoid arthritis and risk of anxiety: A meta-analysis of cohort studies. Clin. Rheumatol. 2019, 38, 2053–2061. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Baerwald, C.; Ablin, J.; Häuser, W. Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): A systematic review of randomized controlled trials. Schmerz 2016, 30, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.J.; Shahouri, S.H.; Shaver, T.S.; Anderson, J.D.; Weidensaul, D.N.; Busch, R.E.; Wang, S.; Wolfe, F. Is fatigue an inflammatory variable in rheumatoid arthritis (RA)? Analyses of fatigue in RA, osteoarthritis, and fibromyalgia. J. Rheumatol. 2009, 36, 2788–2794. [Google Scholar] [CrossRef] [PubMed]
- Albeltagy, E.S.; Elaziz, S.Y.A.; Abozaid, S.Y.; El Zomor, H.M.; Elhamed, S.S.A. Interleukin 6, interleukin 17, disease-related and contextual factor association with depression, and its severity in patients with rheumatoid arthritis. Clin. Rheumatol. 2021, 40, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Katz, P. Fatigue in rheumatoid arthritis. Curr. Rheumatol. Rep. 2017, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar]
- Maes, M.; Twisk, F.N.; Kubera, M.; Ringel, K. Evidence for inflammation and activation of cell-mediated immunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Increased interleukin-1, tumor necrosis factor-alpha, PMN-elastase, lysozyme and neopterin. J. Affect. Disord. 2012, 136, 933–939. [Google Scholar] [CrossRef]
- Maes, M. Evidence for an immune response in major depression: A review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 1995, 19, 11–38. [Google Scholar] [CrossRef]
- Maes, M.; Carvalho, A.F. The compensatory immune-regulatory reflex system (CIRS) in depression and bipolar disorder. Mol. Neurobiol. 2018, 55, 8885–8903. [Google Scholar] [CrossRef]
- Maes, M.; Twisk, F.N. Chronic fatigue syndrome: Harvey and Wessely’s (bio)psychosocial model versus a bio(psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways. BMC Med. 2010, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Maes, M.; Ringel, K.; Kubera, M.; Anderson, G.; Morris, G.; Galecki, P.; Geffard, M.J.J. In myalgic encephalomyelitis/chronic fatigue syndrome, increased autoimmune activity against 5-HT is associated with immuno-inflammatory pathways and bacterial translocation. J. Affect. Disord. 2013, 150, 223–230. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Curr. Neuropharmacol. 2014, 12, 168–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Kubera, M.; Stoyanova, K.; Leunis, J.C. The Reification of the Clinical Diagnosis of Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome (ME/CFS) as an Immune and Oxidative Stress Disorder: Construction of a Data-driven Nomothethic Network and Exposure of ME/CFS Subgroups. Curr. Top. Med. Chem. 2021, 21, 1488–1499. [Google Scholar] [CrossRef] [PubMed]
- van Steenbergen, H.W.; Tsonaka, R.; Huizinga, T.W.; Boonen, A.; van der Helm-van Mil, A.H. Fatigue in rheumatoid arthritis; a persistent problem: A large longitudinal study. RMD Open 2015, 1, e000041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Druce, K.L.; Basu, N. Predictors of fatigue in rheumatoid arthritis. Rheumatology 2019, 58, v29–v34. [Google Scholar] [CrossRef] [Green Version]
- Lucas, K.; Maes, M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: Possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Brunkhorst-Kanaan, N.; Klatt-Schreiner, K.; Hackel, J.; Schröter, K.; Trautmann, S.; Hahnefeld, L.; Wicker, S.; Reif, A.; Thomas, D.; Geisslinger, G.; et al. Targeted lipidomics reveal derangement of ceramides in major depression and bipolar disorder. Metabolism 2019, 95, 65–76. [Google Scholar] [CrossRef]
- Kornhuber, J.; Medlin, A.; Bleich, S.; Jendrossek, V.; Henkel, A.; Wiltfang, J.; Gulbins, E. High activity of acid sphingomyelinase in major depression. J. Neural. Transm. Suppl. 2005, 112, 1583–1590. [Google Scholar] [CrossRef]
- Gracia-Garcia, P.; Rao, V.; Haughey, N.J.; Ratnam Banduru, V.V.; Smith, G.; Rosenberg, P.B.; Lobo, A.; Lyketsos, C.G.; Mielke, M.M. Elevated plasma ceramides in depression. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 215–218. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Hadi, H.H.; Jawad, G.A.; Maes, M. Intersections between copper, β-arrestin-1, calcium, FBXW7, CD17, insulin resistance and atherogenicity mediate depression and anxiety due to type 2 diabetes mellitus: A nomothetic network approach. MedRixiv 2021, 12, 23. [Google Scholar] [CrossRef]
- Al-Fadhel, S.Z.; Al-Hakeim, H.K.; Al-Dujaili, A.H.; Maes, M. IL-10 is associated with increased mu-opioid receptor levels in major depressive disorder. Eur. Psychiatry 2019, 57, 46–51. [Google Scholar] [CrossRef]
- Browne, C.A.; Lucki, I. Targeting opioid dysregulation in depression for the development of novel therapeutics. Pharmacol. Ther. 2019, 201, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, C.K.; Rouine, J.; O’Mara, S.M. Potential roles for opioid receptors in motivation and major depressive disorder. Prog. Brain Res. 2018, 239, 89–119. [Google Scholar] [PubMed]
- Bengoechea-Alonso, M.T.; Ericsson, J. The ubiquitin ligase Fbxw7 controls adipocyte differentiation by targeting C/EBPα for degradation. Proc. Nat. Acad. Sci. USA 2010, 107, 11817–11822. [Google Scholar] [CrossRef] [Green Version]
- Alberi, L.; Liu, S.; Wang, Y.; Badie, R.; Smith-Hicks, C.; Wu, J.; Pierfelice, T.J.; Abazyan, B.; Mattson, M.P.; Kuhl, D.; et al. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron 2011, 69, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M. Precision Nomothetic Medicine in Depression Research: New Depression Models, Endophenotype Classes, Pathway Phenotypes, and a Digital Self. 2021. Available online: https://www.preprints.org/ (accessed on 25 January 2022).
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Nikiphorou, E.; Radner, H.; Chatzidionysiou, K.; Desthieux, C.; Zabalan, C.; van Eijk-Hustings, Y.; Dixon, W.G.; Hyrich, K.L.; Askling, J.; Gossec, L. Patient global assessment in measuring disease activity in rheumatoid arthritis: A review of the literature. Arthritis Res. 2016, 18, 251. [Google Scholar] [CrossRef]
- Turk, M.; Pope, J.E. Physician global assessments for disease activity in rheumatoid arthritis are all over the map! RMD Open 2018, 4, e000578. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory (BDI-II); Pearson: San Antonio, TX, USA, 1996; Volume 10. [Google Scholar]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol 1959, 32, 50–55. [Google Scholar] [CrossRef]
- Zachrisson, O.; Regland, B.; Jahreskog, M.; Kron, M.; Gottfries, C.G. A rating scale for fibromyalgia and chronic fatigue syndrome (the FibroFatigue scale). J. Psychosom. Res. 2002, 52, 501–509. [Google Scholar] [CrossRef]
- Ringle, C.; Da Silva, D.; Bido, D. Structural equation modeling with the SmartPLS. Braz. J. Market. 2015, 13, e000578. [Google Scholar]
- Kuriya, B.; Joshi, R.; Movahedi, M.; Rampakakis, E.; Sampalis, J.S.; Bombardier, C.; Ontario Best Practices Research Initiative Investigators. High disease activity is associated with self-reported depression and predicts persistent depression in early rheumatoid arthritis: Results from the ontario best practices research initiative. J. Rheumatol. 2018, 45, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Pezzato, S.; Bonetto, C.; Caimmi, C.; Tomassi, S.; Montanari, I.; Gnatta, M.G.; Fracassi, E.; Cristofalo, D.; Rossini, M.; Carletto, A.; et al. Depression is associated with increased disease activity and higher disability in a large Italian cohort of patients with rheumatoid arthritis. Adv. Rheumatol. 2021, 61, 57. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, A.M.; Harrold, L.R.; Reed, G.W. A Prospective Evaluation of the Effects of Prevalent Depressive Symptoms on Disease Activity in Rheumatoid Arthritis Patients Treated With Biologic Response Modifiers. Clin. Ther. 2016, 38, 1759–1772.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayant, D.S. Depression and Its Association with Disease Activity and Quality of Life In Patients with Rheumatoid Arthritis at the Kenyatta National Hospital. Master’s Thesis, Department of Clinical Medicine and Therapeutics, University of Nairobi, Nairobi, Kenya, 2020; pp. 1–55. [Google Scholar]
- Tiosano, S.; Yavne, Y.; Watad, A.; Langevitz, P.; Lidar, M.; Feld, J.; Tishler, M.; Aamar, S.; Elkayam, O.; Balbir-Gurman, A.; et al. The impact of tocilizumab on anxiety and depression in patients with rheumatoid arthritis. Eur. J. Clin. Investig. 2020, 50, e13268. [Google Scholar] [CrossRef] [PubMed]
- Margaretten, M.; Barton, J.; Julian, L.; Katz, P.; Trupin, L.; Tonner, C.; Graf, J.; Imboden, J.; Yelin, E. Socioeconomic determinants of disability and depression in patients with rheumatoid arthritis. Arthritis Care Res. 2011, 63, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Matcham, F.; Galloway, J.; Hotopf, M.; Roberts, E.; Scott, I.C.; Steer, S.; Norton, S. The Impact of Targeted Rheumatoid Arthritis Pharmacologic Treatment on Mental Health: A Systematic Review and Network Meta-Analysis. Arthritis Rheumatol. 2018, 70, 1377–1391. [Google Scholar] [CrossRef]
- Rathbun, A.M.; Reed, G.W.; Harrold, L.R. The temporal relationship between depression and rheumatoid arthritis disease activity, treatment persistence and response: A systematic review. Rheumatology 2013, 52, 1785–1794. [Google Scholar] [CrossRef] [Green Version]
- Kekow, J.; Moots, R.; Khandker, R.; Melin, J.; Freundlich, B.; Singh, A. Improvements in patient-reported outcomes, symptoms of depression and anxiety, and their association with clinical remission among patients with moderate-to-severe active early rheumatoid arthritis. Rheumatology 2011, 50, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Arya, S.; Talapatra, P.; Lather, K.; Mathur, R.; Singhania, A.; Chaudhary, V. Assessment of fatigue in rheumatoid arthritis (by Functional Assessment of Chronic Illness Therapy-Fatigue score) and its relation to disease activity and anemia. J. Clin. Rheumatol. 2014, 20, 87–90. [Google Scholar] [CrossRef]
- Holten, K.; Paulshus Sundlisater, N.; Lillegraven, S.; Sexton, J.; Nordberg, L.B.; Moholt, E.; Hammer, H.B.; Uhlig, T.; Kvien, T.K.; Haavardsholm, E.A.; et al. Fatigue in patients with early rheumatoid arthritis undergoing treat-to-target therapy: Predictors and response to treatment. Ann. Rheum Dis. 2021, 81, 344–350. [Google Scholar] [CrossRef]
- Curtis, J.R.; Greenberg, J.D.; Harrold, L.R.; Kremer, J.M.; Palmer, J.L. Influence of obesity, age, and comorbidities on the multi-biomarker disease activity test in rheumatoid arthritis. Semin Arthritis Rheum 2018, 47, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Vallerand, I.A.; Patten, S.B.; Barnabe, C. Depression and the risk of rheumatoid arthritis. Curr. Opin. Rheumatol. 2019, 31, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Kubera, M.; Obuchowiczwa, E.; Goehler, L.; Brzeszcz, J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro. Endocrinol. Lett. 2011, 32, 7–24. [Google Scholar] [PubMed]
- Maes, M.; Twisk, F.N. Why myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) may kill you: Disorders in the inflammatory and oxidative and nitrosative stress (IO&NS) pathways may explain cardiovascular disorders in ME/CFS. Neuroendocrinol. Lett. 2009, 30, 677–693. [Google Scholar]
- Maes, M. An intriguing and hitherto unexplained co-occurrence: Depression and chronic fatigue syndrome are manifestations of shared inflammatory, oxidative and nitrosative (IO&NS) pathways. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 784–794. [Google Scholar]
- Stoyanov, D.; Maes, M.H. How to construct neuroscience-informed psychiatric classification? Towards nomothetic networks psychiatry. World J. Psychiatry 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Nerurkar, L.; Siebert, S.; McInnes, I.B.; Cavanagh, J. Rheumatoid arthritis and depression: An inflammatory perspective. Lancet Psychiatry 2019, 6, 164–173. [Google Scholar] [CrossRef]
- Nishuty, N.L.; Khandoker, M.M.H.; Karmoker, J.R.; Ferdous, S.; Shahriar, M.; Qusar, M.; Islam, M.S.; Kadir, M.F.; Islam, M.R. Evaluation of Serum Interleukin-6 and C-reactive Protein Levels in Drug-naïve Major Depressive Disorder Patients. Cureus 2019, 11, e3868. [Google Scholar] [CrossRef] [Green Version]
- de Lima, C.A.D.; Rushansky, E.; Adelino, J.E.; de Oliveira Souza, A.P.; d’Emery Alves Santos, P.; de Araújo Mariano, M.H.Q.; Crovella, S.; de Azevêdo Silva, J.; Sandrin-Garcia, P. Are key cytokines genetic and serum levels variations related to rheumatoid arthritis clinical severity? Gene 2020, 722, 144098. [Google Scholar] [CrossRef]
- Mousa, R.F.; Al-Hakeim, H.K.; Alhaideri, A.; Maes, M. Chronic fatigue syndrome and fibromyalgia-like symptoms are an integral component of the phenome of schizophrenia: Neuro-immune and opioid system correlates. Metab. Brain Dis. 2021, 36, 169–183. [Google Scholar] [CrossRef]
- Choy, E.H.S.; Calabrese, L.H. Neuroendocrine and neurophysiological effects of interleukin 6 in rheumatoid arthritis. Rheumatology 2018, 57, 1885–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.D.; Wang, Y.L.; Gao, W.F. Honokiol possesses potential anti-inflammatory effects on rheumatoid arthritis and GM-CSF can be a target for its treatment. Int. J. Clin. Exp. Pathol. 2015, 8, 7929–7936. [Google Scholar] [PubMed]
- Schmidt, F.M.; Lichtblau, N.; Minkwitz, J.; Chittka, T.; Thormann, J.; Kirkby, K.C.; Sander, C.; Mergl, R.; Faßhauer, M.; Stumvoll, M.; et al. Cytokine levels in depressed and non-depressed subjects, and masking effects of obesity. J. Psychiatr. Res. 2014, 55, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yan, S.; Wang, H.; Gu, B.; Sun, K.; Yang, X.; Sun, B.; Wang, X. IL-29 Enhances LPS/TLR4-Mediated Inflammation in Rheumatoid Arthritis. Cell Physiol. Biochem. 2015, 37, 27–34. [Google Scholar] [CrossRef]
- Wu, M.K.; Huang, T.L.; Huang, K.W.; Huang, Y.L.; Hung, Y.Y. Association between toll-like receptor 4 expression and symptoms of major depressive disorder. Neuropsychiatr. Dis. Treat. 2015, 11, 1853–1857. [Google Scholar]
- Maes, M.; Mihaylova, I.; Leunis, J.-C. Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): Indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut–intestinal permeability. J. Affect. Disord. 2007, 99, 237–240. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro. Endocrinol. Lett. 2008, 29, 117–124. [Google Scholar]
- Jahid, M.; Rehan Ul, H.; Avasthi, R.; Ahmed, R.S. Interleukin10-1082 A/G polymorphism: Allele frequency, correlation with disease markers, messenger RNA and serum levels in North Indian rheumatoid arthritis patients. Clin. Biochem. 2018, 55, 80–85. [Google Scholar] [CrossRef]
- Wiener, C.D.; Moreira, F.P.; Portela, L.V.; Strogulski, N.R.; Lara, D.R.; da Silva, R.A.; Souza, L.D.M.; Jansen, K.; Oses, J.P. Interleukin-6 and Interleukin-10 in mood disorders: A population-based study. Psychiatry Res. 2019, 273, 685–689. [Google Scholar] [CrossRef]
- Jinquan, T.; Larsen, C.G.; Gesser, B.; Matsushima, K.; Thestrup-Pedersen, K. Human IL-10 is a chemoattractant for CD8+ T lymphocytes and an inhibitor of IL-8-induced CD4+ T lymphocyte migration. J. Immunol. 1993, 151, 4545–4551. [Google Scholar]
- Rousset, F.; Garcia, E.; Defrance, T.; Péronne, C.; Vezzio, N.; Hsu, D.H.; Kastelein, R.; Moore, K.W.; Banchereau, J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 1890–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsikis, P.D.; Chu, C.Q.; Brennan, F.M.; Maini, R.N.; Feldmann, M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J. Exp. Med. 1994, 179, 1517–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, E.; Moñux, G.; Mas, A.; Serrano, F.J.; de la Concha, E.G.; Urcelay, E. Role of IL-10 promoter polymorphisms in the development of severe aorto-iliac occlusive disease. Hum. Immunol. 2008, 69, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Kufi, S.N.; Al-Dujaili, A.H.; Maes, M. Serum Interleukin Levels and Insulin Resistance in Major Depressive Disorder. CNS Neurol. Disord. Drug Targets 2018, 17, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Van’t Hof, R.; Hocking, L.; Wright, P.; Ralston, S. Nitric oxide is a mediator of apoptosis in the rheumatoid joint. Rheumatology 2000, 39, 1004–1008. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, L.J.S.d.; Nunes-Souza, V.; Goulart, M.O.F.; Rabelo, L.A. Oxidative stress in rheumatoid arthritis: What the future might hold regarding novel biomarkers and add-on therapies. Oxid. Med. Cell. Longev. 2019, 7536805. [Google Scholar] [CrossRef] [Green Version]
- Morelli, N.R.; Maes, M.; Bonifacio, K.L.; Vargas, H.O.; Nunes, S.O.V.; Barbosa, D.S. Increased nitro-oxidative toxicity in association with metabolic syndrome, atherogenicity and insulin resistance in patients with affective disorders. J. Affect. Disord. 2021, 294, 410–419. [Google Scholar] [CrossRef]
- Denko, C.W.; Aponte, J.; Gabriel, P.; Petricevic, M. beta-Endorphin, immunological and biochemical changes in synovial fluid in rheumatic disorders. Clin. Rheumatol. 1986, 5, 25–32. [Google Scholar] [CrossRef]
- Moustafa, S.R.; Al-Rawi, K.F.; Stoyanov, D.; Al-Dujaili, A.H.; Supasitthumrong, T.; Al-Hakeim, H.K.; Maes, M. The Endogenous Opioid System in Schizophrenia and Treatment Resistant Schizophrenia: Increased Plasma Endomorphin 2, and kappa and mu Opioid Receptors Are Associated with Interleukin-6. Diagnostics 2020, 10, 633. [Google Scholar] [CrossRef]
- Luan, Y.-H.; Wang, D.; Yu, Q.; Chai, X.-Q. Action of β-endorphin and nonsteroidal anti-inflammatory drugs, and the possible effects of nonsteroidal anti-inflammatory drugs on β-endorphin. J. Clin. Anesth. 2017, 37, 123–128. [Google Scholar] [CrossRef]
- Machelska, H. Targeting of opioid-producing leukocytes for pain control. Neuropeptides 2007, 41, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Machelska, H.; Stein, C. Leukocyte-derived opioid peptides and inhibition of pain. J. Neuroimmune Pharm. 2006, 1, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; Straub, R.H.; Schäfer, M.; Stein, C. β-Endorphin, Met-enkephalin and corresponding opioid receptors within synovium of patients with joint trauma, osteoarthritis and rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 871–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Ying, J.; Wang, X.; Liu, X.; Zhao, T.; Yoon, S.; Zheng, Q.; Fang, Y.; Yang, D.; Hua, F. The Involvement of Lactosylceramide in Central Nervous System Inflammation Related to Neurodegenerative Disease. Front. Aging Neurosci. 2021, 13, 691230. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Hoshino, Y.; Yasuo, S.; Watanabe, M.; Nakane, Y.; Murai, A.; Ebihara, S.; Korf, H.W.; Yoshimura, T. Involvement of thyrotrOpin. in photoperiodic signal transduction in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 18238–18242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulbins, E.; Palmada, M.; Reichel, M.; Lüth, A.; Böhmer, C.; Amato, D.; Müller, C.P.; Tischbirek, C.H.; Groemer, T.W.; Tabatabai, G.; et al. Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs. Nat. Med. 2013, 19, 934–938. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, H.; Moriyama, Y.; Watanabe, K.; Tomizawa, S.; Yamazaki, R.; Takahashi, H.; Murayama, T. Lactosylceramide-Induced Phosphorylation Signaling to Group IVA Phospholipase A(2) via Reactive Oxygen Species in Tumor Necrosis Factor-α-Treated Cells. J. Cell. Biochem. 2017, 118, 4370–4382. [Google Scholar] [CrossRef]
- Chatterjee, S.; Balram, A.; Li, W. Convergence: Lactosylceramide-Centric Signaling Pathways Induce Inflammation, Oxidative Stress, and Other Phenotypic Outcomes. Int. J. Mol. Sci. 2021, 22, 1816. [Google Scholar] [CrossRef]
- Chang, K.; Yang, S.M.; Kim, S.H.; Han, K.H.; Park, S.J.; Shin, J.I. Smoking and rheumatoid arthritis. Int. J. Mol. Sci. 2014, 15, 22279–22295. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, D.; Shepstone, L.; Moots, R.; Lear, J. Lynch, Heavy cigarette smoking is strongly associated with rheumatoid arthritis (RA), particularly in patients without a family history of RA. Ann. Rheum. Dis. 2001, 60, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Mehterov, N.; Minchev, D.; Gevezova, M.P.; Sarafian, V.; Maes, M. Interactions among brain-derived neurotrophic factor and neuroimmune pathways are key components of the major psychiatric disorders. Res. Gate 2022. [Google Scholar] [CrossRef]
- Lai, N.S.; Yu, H.C.; Huang Tseng, H.Y.; Hsu, C.W.; Huang, H.B.; Lu, M.C. Increased Serum Levels of Brain-Derived Neurotrophic Factor Contribute to Inflammatory Responses in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 1841. [Google Scholar] [CrossRef] [PubMed]
- Furmaga, H.; Carreno, F.R.; Frazer, A. Vagal nerve stimulation rapidly activates brain-derived neurotrophic factor receptor TrkB in rat brain. PLoS ONE 2012, 7, e34844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkitny, L.; Younger, J. Reduced Pro-Inflammatory Cytokines after Eight Weeks of Low-Dose Naltrexone for Fibromyalgia. Biomedicines 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Raknes, G.; Småbrekke, L. Low dose naltrexone: Effects on medication in rheumatoid and seropositive arthritis. A nationwide register-based controlled quasi-experimental before-after study. PLoS ONE 2019, 14, e0212460, Erratum in: PLoS ONE 2019, 14, e0223545. [Google Scholar] [CrossRef] [Green Version]
- Stouten, V.; Pazmino, S.; Verschueren, P.; Mamouris, P.; Westhovens, R.; de Vlam, K.; Bertrand, D.; Van der Elst, K.; Vaes, B.; De Cock, D. Comorbidity burden in the first three years after diagnosis in patients with rheumatoid arthritis, psoriatic arthritis or spondyloarthritis: A general practice registry-based study. RMD Open 2021, 7, e001671. [Google Scholar] [CrossRef]
- Van der Elst, K.; Verschueren, P.; De Cock, D.; De Groef, A.; Stouten, V.; Pazmino, S.; Vriezekolk, J.; Joly, J.; Moons, P.; Westhovens, R. One in five patients with rapidly and persistently controlled early rheumatoid arthritis report poor well-being after 1 year of treatment. RMD Open 2020, 6, e001146. [Google Scholar] [CrossRef]
- Brites, L.; Rovisco, J.; Costa, F.; Freitas, J.P.D.; Jesus, D.; Eugénio, G.; Serra, S.; Duarte, C.; Ferreira, R.J.O.; da Silva, J.A.P.D. High patient global assessment scores in patients with rheumatoid arthritis otherwise in remission do not reflect subclinical inflammation. Jt. Bone Spine 2021, 88, 105242. [Google Scholar] [CrossRef]
- Anderson, G.; Berk, M.; Maes, M. Biological phenotypes underpin the physio-somatic symptoms of somatization, depression, and chronic fatigue syndrome. Acta Psychiatr. Scand. 2014, 129, 83–97. [Google Scholar] [CrossRef]
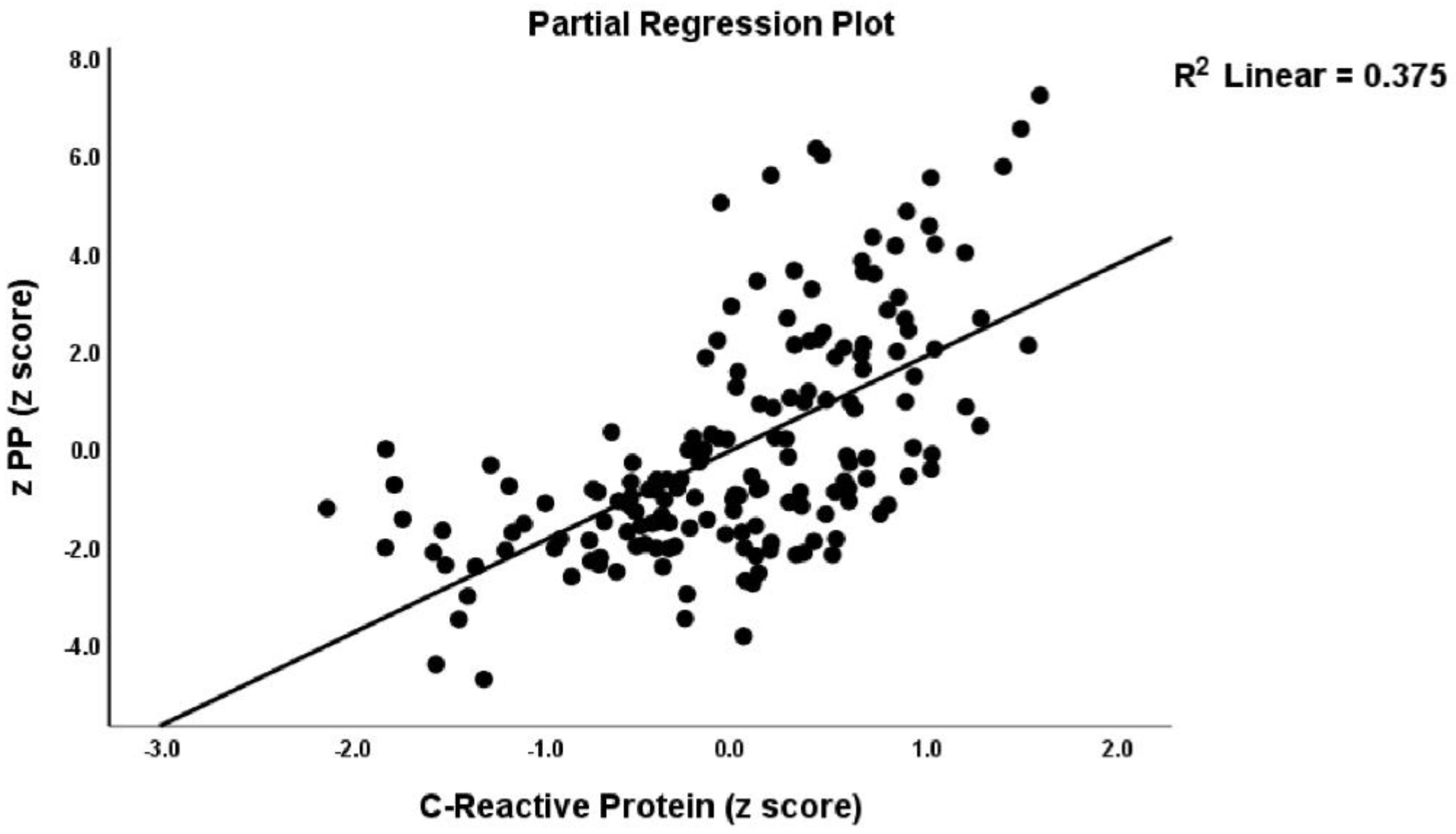




| Variables | HC A n = 50 | RA B n = 59 | RA + PP C n = 59 | F/χ2 | df | p |
|---|---|---|---|---|---|---|
| Age years | 48.7 ± 4.6 | 49.8 ± 7.3 | 50.3 ± 6.8 | 0.90 | 2/165 | 0.407 |
| Sex F/M | 27/23 | 36/23 | 33/26 | 0.60 | 2 | 0.471 |
| BMI kg/m2 | 27.44 ± 2.43 | 27.85 ± 2.65 | 27.60 ± 2.87 | 0.33 | 2/165 | 0.721 |
| TUD No/Yes | 44/6 C | 57/2 C | 45/14 A,B | 10.80 | 2 | 0.005 |
| RF No/Yes | 50/0 B,C | 14/45 A,C | 9/50 A,B | 93.50 | 2 | <0.001 |
| ACPA No/Yes | 50/0 B,C | 27/32 A,C | 24/35 A,B | 47.54 | 2 | <0.001 |
| DAS28-4 | 1.61 ± 0 B,C | 4.50 ± 0.65 A,C | 5.26 ± 0.67 A,B | 650.49 | 2/165 | <0.001 |
| CDAI | 0.0 | 16.7 ± 6.0 C | 22.9 ± 7.1 B | KWT | - | <0.001 |
| SDAI | 0.0 | 20.1 ± 6.7 C | 28.3 ± 7.7 B | KWT | - | <0.001 |
| PGA | 0.0 | 4.99 (1.97) C | 6.31 (2.23) B | KWT | - | <0.001 |
| EGA | 0.0 | 4.36 (1.89) C | 5.72 (2.15) B | KWT | - | <0.001 |
| Tender joint count | 0.0 | 4.9 ± 2.2 C | 7.2 ± 2.7 B | KWT | - | <0.001 |
| Swollen joint count | 0.0 | 2.4 ± 1.6 C | 3.7 ± 1.4 B | KWT | - | <0.001 |
| Total BDI-II | 8.9 ± 2.0 B,C | 11.3 ± 5.9 A,C | 27.5 ± 11.0 A,B | 103.62 | 2/165 | <0.001 |
| Total FF | 5.9 ± 1.7 B,C | 12.8 ± 5.3 A,C | 24.5 ± 6.2 A,B | 203.44 | 2/165 | <0.001 |
| Total HAMA | 6.0 ± 1.7 B,C | 13.2 ± 3.9 A,C | 23.1 ± 4.7 A,B | 288.14 | 2/165 | <0.001 |
| Type | Dependent Variables | Explanatory Variables | F | df | p | Partial η2 |
|---|---|---|---|---|---|---|
| Multivariate | CRP, GM-CSF, TLR4, FBXW7, CD17, IL-6, IL-10, B-EN, MOR, EP2, KOR | HC/RA/RA + PP | 11.50 | 22/290 | <0.001 | 0.466 |
| Sex | 0.68 | 11/144 | 0.753 | 0.050 | ||
| Age | 1.08 | 11/144 | 0.378 | 0.077 | ||
| BMI | 0.69 | 11/144 | 0.749 | 0.050 | ||
| TUD | 5.47 | 11/144 | <0.001 | 0.295 | ||
| Tests for between-subject effects | CRP | HC/RA/RA + PP | 234.54 | 2/154 | <0.001 | 0.753 |
| IL-10 | HC/RA/RA + PP | 55.45 | 2/154 | <0.001 | 0.419 | |
| GM-CSF | HC/RA/RA + PP | 33.49 | 2/154 | <0.001 | 0.303 | |
| IL-6 | HC/RA/RA + PP | 31.96 | 2/154 | <0.001 | 0.293 | |
| TLR4 | HC/RA/RA + PP | 21.19 | 2/154 | <0.001 | 0.216 | |
| CD17 | HC/RA/RA + PP | 7.71 | 2/154 | 0.001 | 0.091 | |
| KOR | HC/RA/RA + PP | 6.56 | 2/154 | 0.002 | 0.079 | |
| FBXW7 | HC/RA/RA + PP | 6.16 | 2/154 | 0.003 | 0.074 | |
| EP2 | HC/RA/RA + PP | 5.66 | 2/154 | 0.004 | 0.069 | |
| MOR | HC/RA/RA + PP | 1.07 | 2/154 | 0.346 | 0.014 | |
| B-EN | HC/RA/RA + PP | 0.67 | 2/154 | 0.515 | 0.009 |
| Biomarkers | HC A n = 50 | RA B n = 59 | RA + PP C n = 59 |
|---|---|---|---|
| CRP mg/L | 5.04 (2.06) B,C | 34.35 (1.92) A,C | 52.31 (1.93) A,B |
| GM-CSF pg/mL | 22.66 (6.98) B,C | 73.71 (6.51) A,C | 106.24 (6.53) A,B |
| TLR4 ng/mL | 4.01 (0.65) B,C | 9.60 (0.60) A | 9.33 (0.61) A |
| FBXW7 ng/mL | 16.08 (1.71) B,C | 25.09 (1.60) A | 22.38 (1.60) A |
| CD17 ng/mL | 1737.0 (207.6) B,C | 2777.4 (193.5) A | 2877.0 (194.0) A |
| IL-6 pg/mL | 6.8 (1.2) B,C | 11.5 (1.0) A | 13.0 (0.68) A |
| IL-10 pg/mL | 9.23 (0.98) B,C | 22.73 (0.91) A | 22.11 (0.91) A |
| B-EN pg/mL | 25.6 (2.6) | 25.2 (2.3) | 28.6 (2.4) |
| MOR pg/mL | 5.12 (0.43) | 5.32 (0.40) | 5.93 (0.40) |
| EP2 pg/mL | 383.0 (45.0) B,C | 522.8 (42.0) A | 588.8 (42.0) A |
| KOR ng/mL | 6.84 (0.74) B,C | 9.82 (0.69) A | 8.70 (0.70) A |
| Biomarkers | Total BDI | Total FF | Total HAMA | z PP |
|---|---|---|---|---|
| CRP | 0.562 *** | 0.834 *** | 0.736 *** | 0.812 *** |
| GM-CSF | 0.408 *** | 0.616 *** | 0.614 *** | 0.634 *** |
| TLR4 | 0.304 ***/ | 0.421 *** | 0.435 *** | 0.449 *** |
| FBXW7 | 0.113 | 0.163 * | 0.211 ** | 0.188 * |
| CD17 | 0.244 ** | 0.286 *** | 0.321 *** | 0.325 *** |
| IL-6 | 0.359 *** | 0.471 *** | 0.470 *** | 0.496 *** |
| IL-10 | 0.342 *** | 0.554 *** | 0.559 *** | 0.564 *** |
| B-EN | 0.165 * | 0.181 * | 0.140 | 0.185 * |
| MOR | 0.177 * | 0.182 * | 0.173 * | 0.188 * |
| EP2 | 0.293 *** | 0.339 *** | 0.243 ** | 0.324 *** |
| KOR | 0.188 * | 0.252 ** | 0.270 *** | 0.275 *** |
| Dependent Variables | Explanatory Variables | β | t | p | F model | df | p | R2 |
|---|---|---|---|---|---|---|---|---|
| #1. z PP | Model | 75.279 | 3/164 | <0.001 | 0.579 | |||
| CRP | 0.562 | 9.22 | <0.001 | |||||
| GM-CSF | 0.220 | 3.77 | <0.001 | |||||
| TLR4 | 0.133 | 2.38 | 0.019 | |||||
| #2. Total BDI | Model | 25.844 | 3/164 | <0.001 | 0.321 | |||
| CRP | 0.374 | 4.83 | <0.001 | |||||
| GM-CSF | 0.181 | 2.45 | 0.015 | |||||
| TLR4 | 0.147 | 2.07 | 0.040 | |||||
| #3. Total FF | Model | 83.02 | 3/164 | <0.001 | 0.603 | |||
| CRP | 0.624 | 10.96 | <0.001 | |||||
| GM-CSF | 0.179 | 3.11 | 0.002 | |||||
| EP2 | 0.121 | 2.29 | 0.023 | |||||
| #4. Total HAMA | Model | 46.19 | 5/162 | <0.001 | 0.588 | |||
| CRP | 0.416 | 5.75 | <0.001 | |||||
| GM-CSF | 0.179 | 3.00 | 0.003 | |||||
| RF | 0.172 | 2.64 | 0.009 | |||||
| Anti-CCP | 0.129 | 2.25 | 0.026 | |||||
| TLR4 | 0.116 | 2.06 | 0.041 | |||||
| #5. Key_BDI | Model | 32.31 | 2/165 | <0.001 | 0.281 | |||
| CRP | 0.427 | 5.543 | 0.000 | |||||
| GM-CSF | 0.183 | 2.422 | 0.016 | |||||
| #6. Key_HAMA | Model | 38.03 | 3/164 | <0.001 | 0.410 | |||
| CRP | 0.425 | 5.47 | <0.001 | |||||
| GM-CSF | 0.172 | 2.46 | 0.015 | |||||
| RF | 0.160 | 2.12 | 0.035 | |||||
| #7. Z PS | Model | 63.19 | 2/164 | <0.001 | 0.435 | |||
| CRP | 0.447 | 6.67 | <0.001 | |||||
| GM-CSF | 0.315 | 4.71 | <0.001 |
| Parameter | Total BDI | Total FF | Total HAMA | z PP |
|---|---|---|---|---|
| DAS28-4 | 0.637/0.609 | 0.782/0.545 | 0.779/0.497 | 0.838/0.632 |
| CDAI | 0.596/0.545 | 0.733/0.422 | 0.751/0.421 | 0.799/0.533 |
| SDAI | 0.619/0.592 | 0.777/0.537 | 0.771/0.484 | 0.829/0.617 |
| Tender joint count | 0.625/0.641 | 0.711/0.368 | 0.752/0.415 | 0.799/0.553 |
| Swollen joint count | 0.590/0.449 | 0.711/0.332 | 0.726/0.322 | 0.771/0.425 |
| PGA | 0.511/0.373 | 0.720/0.372 | 0.723/0.335 | 0.762/0.411 |
| EGA | 0.494/0.325 | 0.713/0.340 | 0.719/0.322 | 0.754/0.374 |
| Dependent Variables | Explanatory Variables | β | t | p | F model | df | p | R2 |
|---|---|---|---|---|---|---|---|---|
| #1. z PP | Model | 69.27 | 5/162 | <0.001 | 0.681 | |||
| Tender joint count | 0.445 | 5.61 | <0.001 | |||||
| CRP | 0.338 | 4.94 | <0.001 | |||||
| GM-CSF | 0.113 | 2.08 | 0.039 | |||||
| IL-10 | −0.165 | −2.64 | 0.009 | |||||
| Swollen joint count | 0.166 | 2.22 | 0.028 | |||||
| #2. Total BDI | Model | 43.84 | 4/156 | <0.001 | 0.529 | |||
| Tender joint count | 0.673 | 7.60 | <0.001 | |||||
| IL-10 | −0.267 | −3.52 | 0.001 | |||||
| Swollen joint count | 0.189 | 2.17 | 0.031 | |||||
| EP2 | 0.132 | 2.16 | 0.032 | |||||
| #3. Total FF | Model | 86.83 | 3/164 | <0.001 | 0.614 | |||
| CRP | 0.508 | 7.21 | <0.001 | |||||
| Tender joint count | 0.231 | 3.15 | 0.002 | |||||
| GM-CSF | 0.150 | 2.56 | 0.011 | |||||
| #4. Total HAMA | Model | 87.87 | 3/164 | <0.001 | 0.616 | |||
| Tender joint count | 0.404 | 5.52 | <0.001 | |||||
| CRP | 0.353 | 5.03 | <0.001 | |||||
| GM-CSF | 0.136 | 2.33 | 0.021 | |||||
| #5. Key_BDI | Model | 53.72 | 3/164 | <0.001 | 0.496 | |||
| Tender joint count | 0.674 | 7.34 | <0.001 | |||||
| IL-10 | −0.219 | −2.90 | 0.004 | |||||
| Swollen joint count | 0.193 | 2.14 | 0.034 | |||||
| #6. Key_HAMA | Model | 60.99 | 2/165 | <0.001 | 0.425 | |||
| CDAI | 0.389 | 4.25 | <0.001 | |||||
| CRP | 0.305 | 3.34 | 0.001 | |||||
| #7. Key_FF | Model | 22.63 | 5/161 | <0.001 | 0.413 | |||
| CRP | 0.327 | 3.45 | 0.001 | |||||
| GM-CSF | 0.240 | 3.27 | 0.001 | |||||
| PGA | 0.273 | 2.78 | 0.006 | |||||
| IL-10 | −0.229 | −2.67 | 0.008 | |||||
| EP2 | 0.143 | 2.11 | 0.036 | |||||
| #8. z PS | Model | 36.64 | 6/160 | <0.001 | 0.579 | |||
| Tender joint count | 0.449 | 4.82 | <0.001 | |||||
| GM-CSF | 0.181 | 2.83 | 0.005 | |||||
| IL-10 | −0.305 | −4.09 | <0.001 | |||||
| CRP | 0.215 | 2.72 | 0.007 | |||||
| Swollen joint count | 0.188 | 2.16 | 0.032 | |||||
| EP2 | 0.122 | 2.13 | 0.035 | |||||
| #.9 z Fatigue | Model | 29.88 | 5/162 | <0.001 | 0.480 | |||
| Tender joint count | 0.404 | 4.46 | <0.001 | |||||
| CRP | 0.299 | 3.49 | 0.001 | |||||
| EP2 | 0.204 | 3.21 | 0.002 | |||||
| IL-10 | −0.267 | −3.30 | 0.001 | |||||
| GM-CSF | 0.170 | 2.43 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smesam, H.N.; Qazmooz, H.A.; Khayoon, S.Q.; Almulla, A.F.; Al-Hakeim, H.K.; Maes, M. Pathway Phenotypes Underpinning Depression, Anxiety, and Chronic Fatigue Symptoms Due to Acute Rheumatoid Arthritis: A Precision Nomothetic Psychiatry Analysis. J. Pers. Med. 2022, 12, 476. https://doi.org/10.3390/jpm12030476
Smesam HN, Qazmooz HA, Khayoon SQ, Almulla AF, Al-Hakeim HK, Maes M. Pathway Phenotypes Underpinning Depression, Anxiety, and Chronic Fatigue Symptoms Due to Acute Rheumatoid Arthritis: A Precision Nomothetic Psychiatry Analysis. Journal of Personalized Medicine. 2022; 12(3):476. https://doi.org/10.3390/jpm12030476
Chicago/Turabian StyleSmesam, Hasan Najah, Hasan Abbas Qazmooz, Sinan Qayes Khayoon, Abbas F. Almulla, Hussein Kadhem Al-Hakeim, and Michael Maes. 2022. "Pathway Phenotypes Underpinning Depression, Anxiety, and Chronic Fatigue Symptoms Due to Acute Rheumatoid Arthritis: A Precision Nomothetic Psychiatry Analysis" Journal of Personalized Medicine 12, no. 3: 476. https://doi.org/10.3390/jpm12030476
APA StyleSmesam, H. N., Qazmooz, H. A., Khayoon, S. Q., Almulla, A. F., Al-Hakeim, H. K., & Maes, M. (2022). Pathway Phenotypes Underpinning Depression, Anxiety, and Chronic Fatigue Symptoms Due to Acute Rheumatoid Arthritis: A Precision Nomothetic Psychiatry Analysis. Journal of Personalized Medicine, 12(3), 476. https://doi.org/10.3390/jpm12030476








