Exploring Rare Disease Patient Attitudes and Beliefs regarding Genetic Testing: Implications for Person-Centered Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Focus Group Discussions
2.3. Analysis
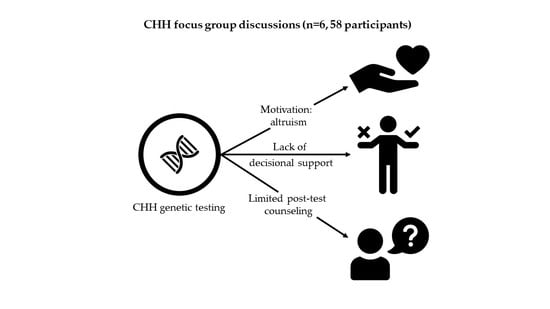
3. Results
3.1. Pre-Test Experiences
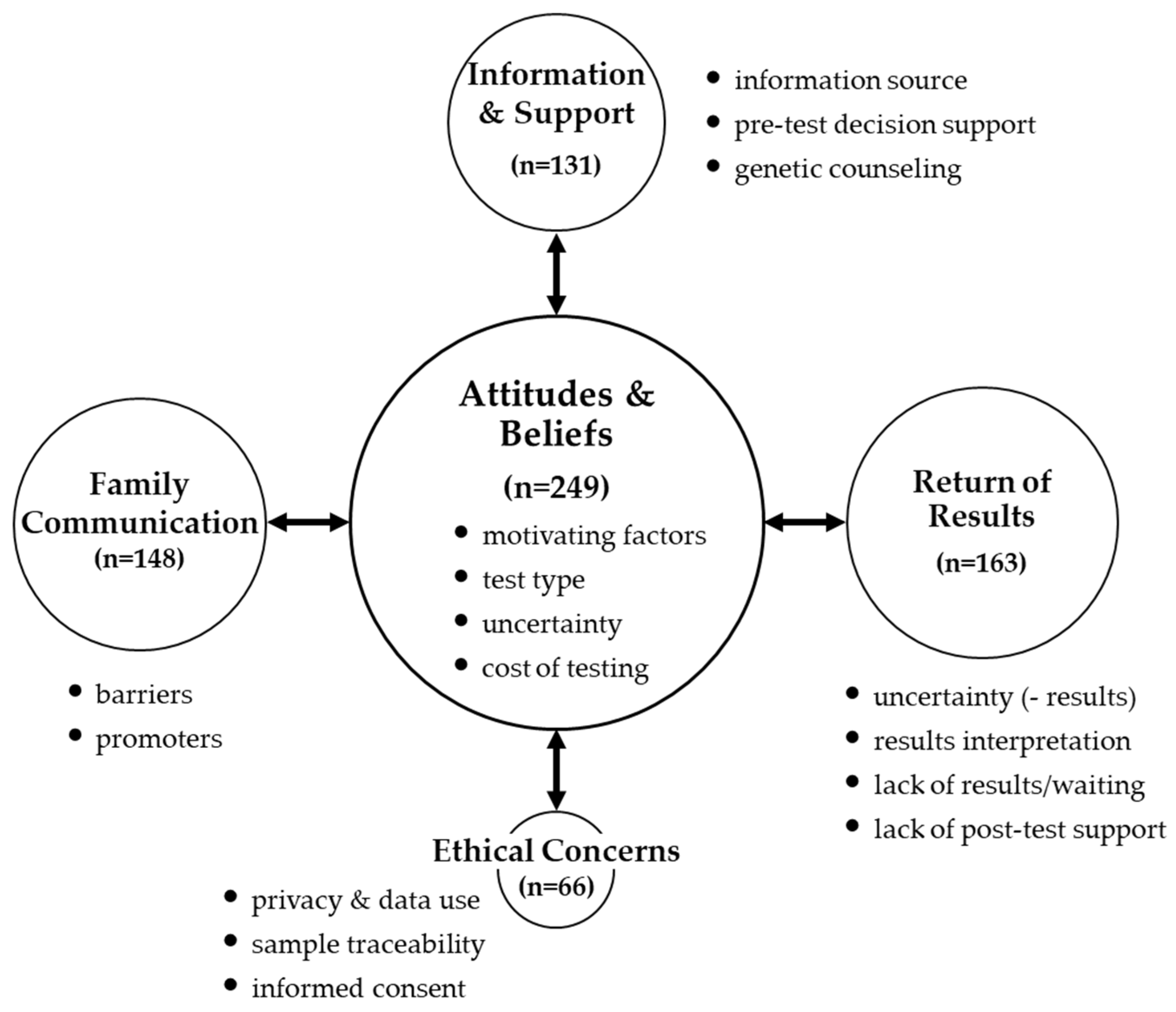
3.1.1. Theme: ‘Attitudes & Beliefs’
3.1.2. Theme: ‘Information and Support’
3.2. Post-Test Experiences
3.2.1. Theme: ‘Return of Results’
3.2.2. Theme: ‘Family Communication’
3.3. Theme: ‘Ethical Concerns’
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Focus Group Questions/Prompts
References
- Wu, A.C.; McMahon, P.; Lu, C. Ending the Diagnostic Odyssey—Is Whole-Genome Sequencing the Answer? JAMA Pediatr. 2020, 174, 821. [Google Scholar] [CrossRef] [PubMed]
- Eurordis. The Voice of 12,000 Patients: Experiences and Expectations of Rare Disease Patients on Diagnosis and Care in Europe; Impression Design: Boulogne-Billancourt, France, 2009. [Google Scholar]
- Smedley, D.; Smith, K.R.; Martin, A.; Thomas, E.A.; McDonagh, E.M.; Cipriani, V.; Ellingford, J.M.; Arno, G.; Tucci, A.; Vandrovcova, J.; et al. 100,000 Genomes Pilot on Rare-Disease Diagnosis in Health Care—Preliminary Report. N. Engl. J. Med. 2021, 385, 1868–1880. [Google Scholar] [CrossRef] [PubMed]
- Eggermann, T.; Elbracht, M.; Kurth, I.; Juul, A.; Johannsen, T.H.; Netchine, I.; Mastorakos, G.; Johannsson, G.; Musholt, T.J.; Zenker, M.; et al. Genetic testing in inherited endocrine disorders: Joint position paper of the European reference network on rare endocrine conditions (Endo-ERN). Orphanet J. Rare Dis. 2020, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Xu, C.; Papadakis, G.E.; Acierno, J.S.; Maione, L.; Hietamäki, J.; Raivio, T.; Pitteloud, N. Clinical Management of Congenital Hypogonadotropic Hypogonadism. Endocr. Rev. 2019, 40, 669–710. [Google Scholar] [CrossRef] [PubMed]
- Boehm, U.; Bouloux, P.-M.; Dattani, M.T.; de Roux, N.; Dodé, C.; Dunkel, L.; Dwyer, A.; Giacobini, P.; Hardelin, J.-P.; Juul, A.; et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism—Pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 2015, 11, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, E.-M.; Vaaralahti, K.; Tommiska, J.; Eklund, E.; Tervaniemi, M.; Valanne, L.; Raivio, T. Incidence, Phenotypic Features and Molecular Genetics of Kallmann Syndrome in Finland. Orphanet J. Rare Dis. 2011, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Dzemaili, S.; Tiemensma, J.; Quinton, R.; Pitteloud, N.; Morin, D.; Dwyer, A.A. Beyond hormone replacement: Quality of life in women with congenital hypogonadotropic hypogonadism. Endocr. Connect. 2017, 6, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, G.; Corona, G.; Mannucci, E.; Maggi, M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: A meta-analytic study. Andrology 2014, 2, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.A.; Smith, N.; Quinton, R. Psychological Aspects of Congenital Hypogonadotropic Hypogonadism. Front. Endocrinol. 2019, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.A. Psychosexual effects resulting from delayed, incomplete, or absent puberty. Curr. Opin. Endocr. Metab. Res. 2020, 14, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.; Palmert, M.R. Distinguishing Self-limited Delayed Puberty from Permanent Hypogonadotropic Hypogonadism: How and Why? J. Clin. Endocrinol. Metab. 2021, 106, e5264–e5266. [Google Scholar] [CrossRef]
- Swee, D.S.; Quinton, R. Congenital Hypogonadotrophic Hypogonadism: Minipuberty and the Case for Neonatal Diagnosis. Front. Endocrinol. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.A.; Quinton, R.; Pitteloud, N.; Morin, D. Psychosexual Development in Men with Congenital Hypogonadotropic Hypogonadism on Long-Term Treatment: A Mixed Methods Study. Sex. Med. 2015, 3, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, B.; Swee, D.S.; Quinton, R.; Bonomi, M. Genetics of congenital hypogonadotropic hypogonadism: Peculiarities and phenotype of an oligogenic disease. Qual. Life Res. 2020, 140, 77–111. [Google Scholar] [CrossRef]
- Hennink, M.M.; Kaiser, B.; Weber, M.B. What Influences Saturation? Estimating Sample Sizes in Focus Group Research. Qual. Health Res. 2019, 29, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V. One size fits all? What counts as quality practice in (reflexive) thematic analysis? Qual. Res. Psychol. 2021, 18, 328–352. [Google Scholar] [CrossRef]
- Dwyer, A.A.; Tiemensma, J.; Quinton, R.; Pitteloud, N.; Morin, D. Adherence to treatment in men with hypogonadotrophic hypogonadism. Clin. Endocrinol. 2017, 86, 377–383. [Google Scholar] [CrossRef]
- Biesecker, B. Genetic Counseling and the Central Tenets of Practice. Cold Spring Harb. Perspect. Med. 2019, 10, a038968. [Google Scholar] [CrossRef]
- George, R.; Kovak, K.; Cox, S.L. Aligning Policy to Promote Cascade Genetic Screening for Prevention and Early Diagnosis of Heritable Diseases. J. Genet. Couns. 2015, 24, 388–399. [Google Scholar] [CrossRef]
- Au, M.G.; Crowley, W.F.; Buck, C.L. Genetic counseling for isolated GnRH deficiency. Mol. Cell. Endocrinol. 2011, 346, 102–109. [Google Scholar] [CrossRef][Green Version]
- Maione, L.; Dwyer, A.; Francou, B.; Guiochon-Mantel, A.; Binart, N.; Bouligand, J.; Young, J. GENETICS IN ENDOCRINOLOGY: Genetic counseling for congenital hypogonadotropic hypogonadism and Kallmann syndrome: New challenges in the era of oligogenism and next-generation sequencing. Eur. J. Endocrinol. 2018, 178, R55–R80. [Google Scholar] [CrossRef]
- Maiese, D.R.; Keehn, A.; Lyon, M.; Flannery, D.; Watson, M. Current conditions in medical genetics practice. Genet. Med. 2019, 21, 1874–1877. [Google Scholar] [CrossRef]
- Abacan, M.; Alsubaie, L.; Barlow-Stewart, K.; Caanen, B.; Cordier, C.; Courtney, E.; Davoine, E.; Edwards, J.; Elackatt, N.J.; Gardiner, K.; et al. The Global State of the Genetic Counseling Profession. Eur. J. Hum. Genet. 2018, 27, 183–197. [Google Scholar] [CrossRef]
- Gorrie, A.; Gold, J.; Cameron, C.; Krause, M.; Kincaid, H. Benefits and limitations of telegenetics: A literature review. J. Genet. Couns. 2021, 30, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Schmidlen, T.; Schwartz, M.; DiLoreto, K.; Kirchner, H.L.; Sturm, A.C. Patient assessment of chatbots for the scalable delivery of genetic counseling. J. Genet. Couns. 2019, 28, 1166–1177. [Google Scholar] [CrossRef]
- Fox, S. Peer-to-Peer Healthcare: Many People—Especially Those Living with Chronic or Rare Diseases—Use Online Connections to Supplement Professional Medical Advice; Pew Internet: Washington, DC, USA, 2011. [Google Scholar]
- Katapodi, M.C.; Jung, M.; Schafenacker, A.M.; Milliron, K.J.; E Mendelsohn-Victor, K.; Merajver, S.D.; Northouse, L.L.; Underhill, M.; Son, Y.A. Development of a Web-based Family Intervention for BRCA Carriers and Their Biological Relatives: Acceptability, Feasibility, and Usability Study. JMIR Cancer 2018, 4, e7. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.A.; Quinton, R.; Morin, D.; Pitteloud, N. Identifying the unmet health needs of patients with congenital hypogonadotropic hypogonadism using a web-based needs assessment: Implications for online interventions and peer-to-peer support. Orphanet J. Rare Dis. 2014, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.; Hesse-Biber, S.; Flynn, B.; Remick, S. Parent of Origin Effects on Family Communication of Risk in BRCA+ Women: A Qualitative Investigation of Human Factors in Cascade Screening. Cancers 2020, 12, 2316. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.A.; Hesse-Biber, S.; Shea, H.; Zeng, Z.; Yi, S. Coping response and family communication of cancer risk in men harboring a BRCA mutation: A mixed methods study. Psycho-Oncology 2021, 31, 486–495. [Google Scholar] [CrossRef]
- James, C.A.; Hadley, D.W.; Holtzman, N.A.; Winkelstein, J.A. How does the mode of inheritance of a genetic condition influence families? A study of guilt, blame, stigma, and understanding of inheritance and reproductive risks in families with X-linked and autosomal recessive diseases. Genet. Med. 2006, 8, 234–242. [Google Scholar] [CrossRef]
- Katapodi, M.C.; Northouse, L.L.; Milliron, K.J.; Liu, G.; Merajver, S.D. Individual and family characteristics associated with BRCA1/2 genetic testing in high-risk families. Psycho-Oncology 2012, 22, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Morris, Z.; Wooding, S.; Grant, J. The answer is 17 years, what is the question: Understanding time lags in translational research. J. R. Soc. Med. 2011, 104, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Slomp, C.; Morris, E.; Gen, C.S.; Price, M.; Elliott, A.M.; Austin, J. The stepwise process of integrating a genetic counsellor into primary care. European journal of human genetics. Eur. J. Hum. Genet. 2022. [Google Scholar] [CrossRef]
- Burnett-Hartman, A.N.; Blum-Barnett, E.; Carroll, N.M.; Madrid, S.D.; Jonas, C.; Janes, K.; Alvarado, M.; Bedoy, R.; Paolino, V.; Aziz, N.; et al. Return of Research-Related Genetic Test Results and Genetic Discrimination Concerns: Facilitators and Barriers of Genetic Research Participation in Diverse Groups. Public Health Genom. 2020, 23, 59–68. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Returning Individual Research Results to Participants: Guidance for a New Research Paradigm; The National Academies Press: Washington, DC, USA, 2018. [Google Scholar]
- Kasperbauer, T.; Halverson, C.; Garcia, A.; Schmidt, K.K.; Schwartz, P.H. Biobank Participants’ Attitudes toward Requiring Understanding for Biobank Consent. Ethic-Hum. Res. 2021, 44, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.A.; Au, M.G.; Smith, N.; Plummer, L.; Lippincott, M.F.; Balasubramanian, R.; Seminara, S.B. Evaluating co-created patient-facing materials to increase understanding of genetic test results. J. Genet. Couns. 2020, 30, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, A.A.; Zeng, Z.; Lee, C.S. Validating online approaches for rare disease research using latent class mixture modeling. Orphanet J. Rare Dis. 2021, 16, 209. [Google Scholar] [CrossRef] [PubMed]
- Zhong, A.; Darren, B.; Loiseau, B.; He, L.Q.B.; Chang, T.; Hill, J.; Dimaras, H. Ethical, social, and cultural issues related to clinical genetic testing and counseling in low- and middle-income countries: A systematic review. Genet. Med. 2021, 23, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Fraiman, Y.S.; Wojcik, M.H. The influence of social determinants of health on the genetic diagnostic odyssey: Who remains undiagnosed, why, and to what effect? Pediatr. Res. 2020, 89, 295–300. [Google Scholar] [CrossRef] [PubMed]
| In-Person (n = 33) | Virtual (n = 25) | Total (n = 58) | |
|---|---|---|---|
| male patients (n) | 19 | 11 | 30 |
| age range (yrs.) | 20–72 | 22–75 | 20–75 |
| median age (yrs.) | 37.0 | 60.0 | 40.5 |
| mean ± SD | 39.7 ± 14.4 | 53.4 ± 18.2 * | 44.7 ± 17.0 |
| female patients (n)† | 6 | 12 * | 18 |
| age range (yrs.) | 24–68 | 33–52 | 24–68 |
| median age (yrs.) | 33.0 | 43.0 | 40.0 |
| mean ± SD | 37.5 ± 16.2 | 42.8 ± 7.5 | 40.7 ± 11.5 |
| parents/guardians (n) | 8 | 2 | 10 |
| age range (yrs.) | 35–62 | 30–32 | 30–62 |
| median age (yrs.) | 47.5 | 31 * | 45.0 |
| mean ± SD | 49.0 ± 9.9 | - | 45.4 ± 11.6 |
| Pre-Test Theme: ‘Attitudes & Beliefs’ |
|---|
| Sub-theme: ‘motivating factors’ Dimension: reasons for not testing
|
| Sub-theme: ‘uncertainty’ Dimension: uncertainty about results
|
| Pre-Test Theme: ‘Information & Support’ |
|---|
| Sub-theme: ‘information source’ Dimension: specialist (endocrinologist)
|
| Sub-themes: ‘decision support’/‘genetic counseling’ Dimension: lack of decisional support/genetic counseling
|
| Post-Test Theme: ‘Return of Results’ |
|---|
| Sub-theme: ‘uncertainty’ Dimension: not telling the “full story”
|
| Sub-theme: ‘results interpretation’ Dimension: complexity of genetic information
|
| Sub-theme: ‘lack of results/waiting’ Dimension: extended waiting
|
| Sub-theme: ‘lack of post-test support’ Dimension: lack of understanding about the impact of diagnosis
|
| Post-Test Theme: ‘Family Communication’ |
|---|
| Sub-theme: ‘barriers’ Dimension: unaccepting family members (e.g., denial, parental guilt)
|
| Sub-theme: ‘facilitators’ (promoters) Dimension: family dynamics—open communication pattern
|
| Theme: ‘Ethical Concerns’ |
|---|
| Sub-theme: ‘privacy & data use’ Dimension: privacy threats—research vs. for-profit
|
| Sub-theme: ‘sample traceability & use’ Dimension: sample traceability
|
| Sub-theme: ‘informed consent’ Dimension: receiving results
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dwyer, A.A.; Uveges, M.K.; Dockray, S.; Smith, N. Exploring Rare Disease Patient Attitudes and Beliefs regarding Genetic Testing: Implications for Person-Centered Care. J. Pers. Med. 2022, 12, 477. https://doi.org/10.3390/jpm12030477
Dwyer AA, Uveges MK, Dockray S, Smith N. Exploring Rare Disease Patient Attitudes and Beliefs regarding Genetic Testing: Implications for Person-Centered Care. Journal of Personalized Medicine. 2022; 12(3):477. https://doi.org/10.3390/jpm12030477
Chicago/Turabian StyleDwyer, Andrew A., Melissa K. Uveges, Samantha Dockray, and Neil Smith. 2022. "Exploring Rare Disease Patient Attitudes and Beliefs regarding Genetic Testing: Implications for Person-Centered Care" Journal of Personalized Medicine 12, no. 3: 477. https://doi.org/10.3390/jpm12030477
APA StyleDwyer, A. A., Uveges, M. K., Dockray, S., & Smith, N. (2022). Exploring Rare Disease Patient Attitudes and Beliefs regarding Genetic Testing: Implications for Person-Centered Care. Journal of Personalized Medicine, 12(3), 477. https://doi.org/10.3390/jpm12030477