Sensory Ion Channel Candidates Inform on the Clinical Course of Pancreatic Cancer and Present Potential Targets for Repurposing of FDA-Approved Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Obtain and Pre-Processing
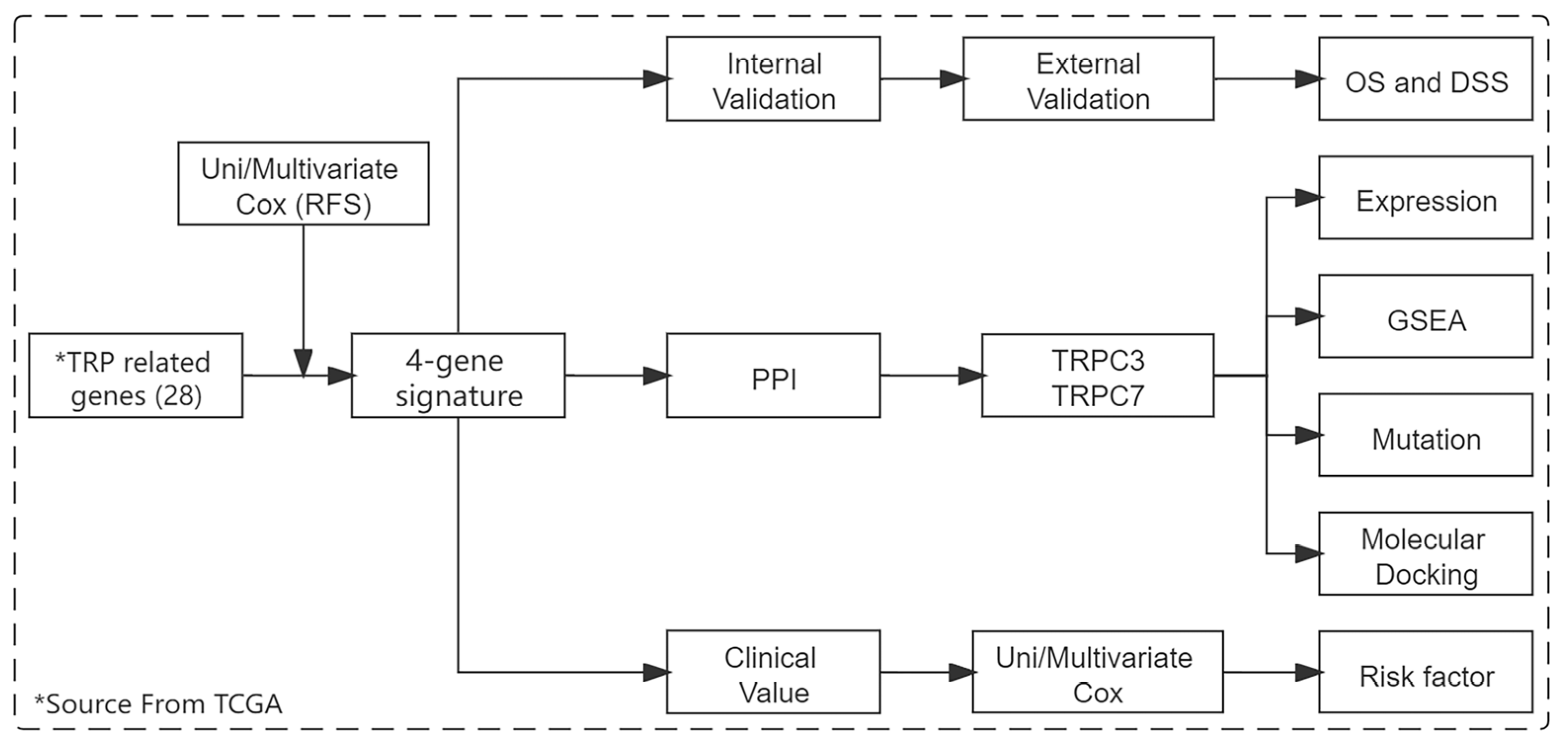
2.2. Signature Construction
2.3. Signature Validation
2.4. Prognostic Value Evaluation of Signature
2.5. Core Gene Screening and Expression Analysis
2.6. Core Gene Mutation Analysis
2.7. GSEA Enrichment Analysis
2.8. Virtual Screening
3. Results
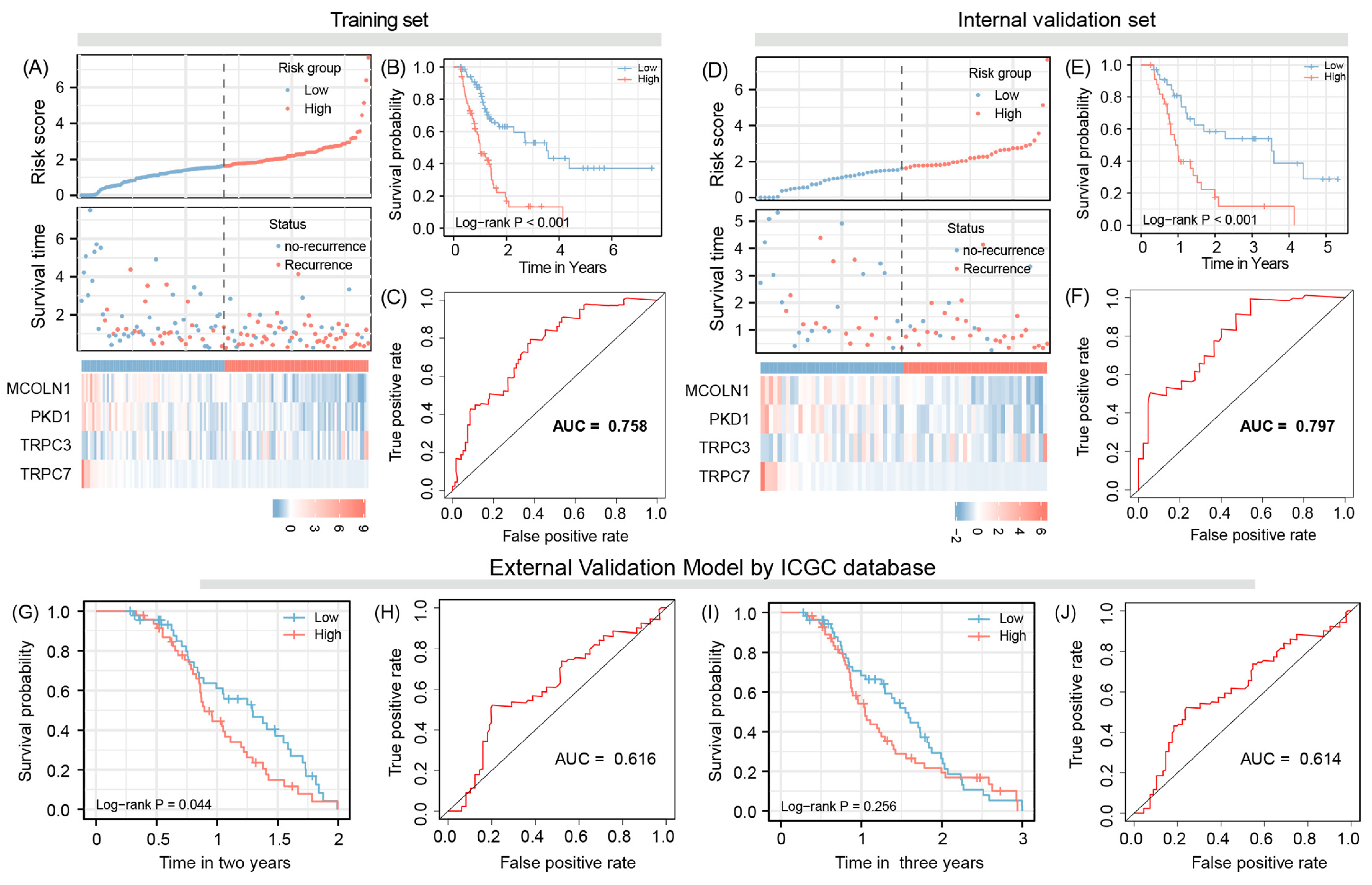
3.1. Risk Gene Identification and Model Construction
3.2. Evaluation Model Efficacy
3.3. Model Validation
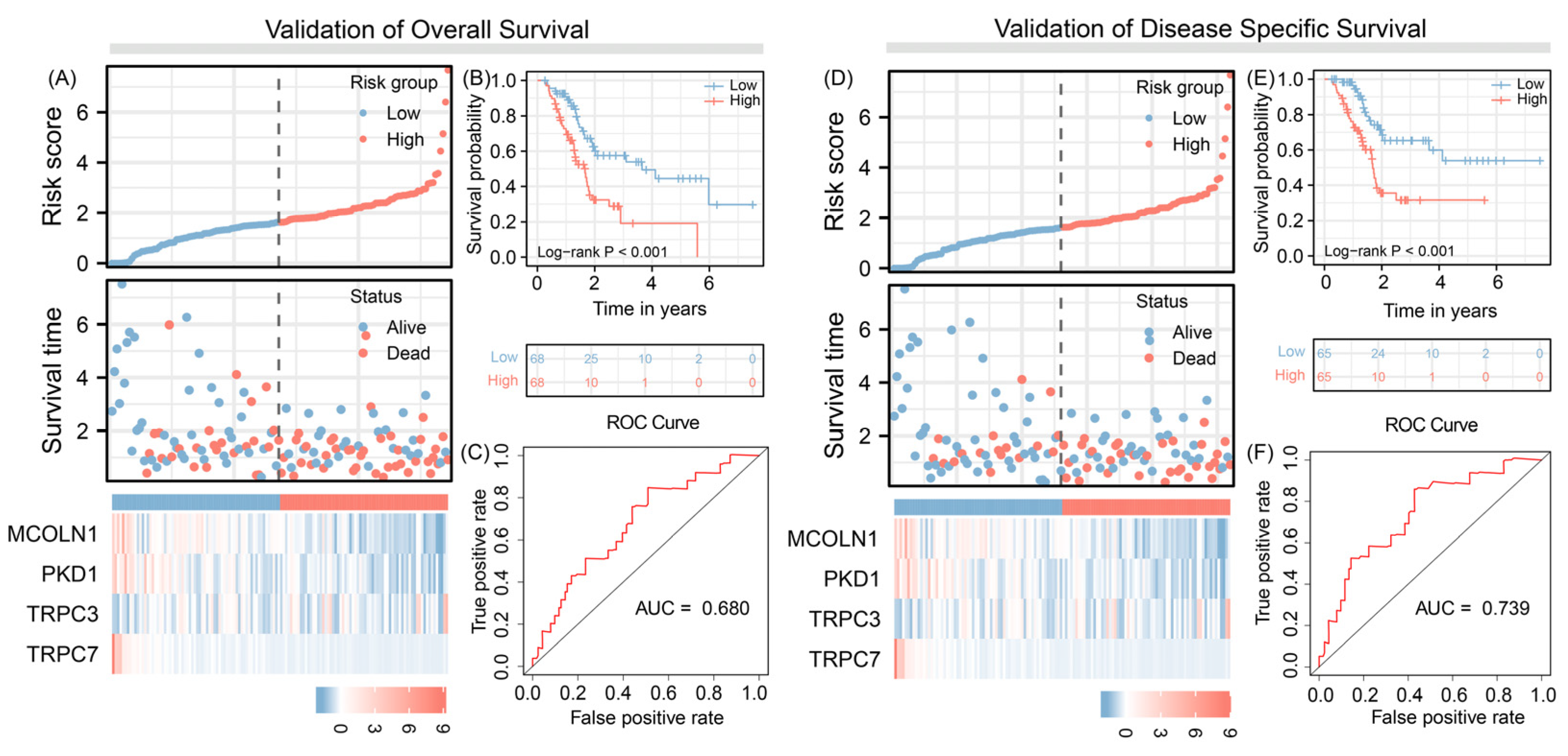
3.4. Predict Overall Survival and Disease-Specific Survival
3.5. Independent Prognosis Value Evaluation
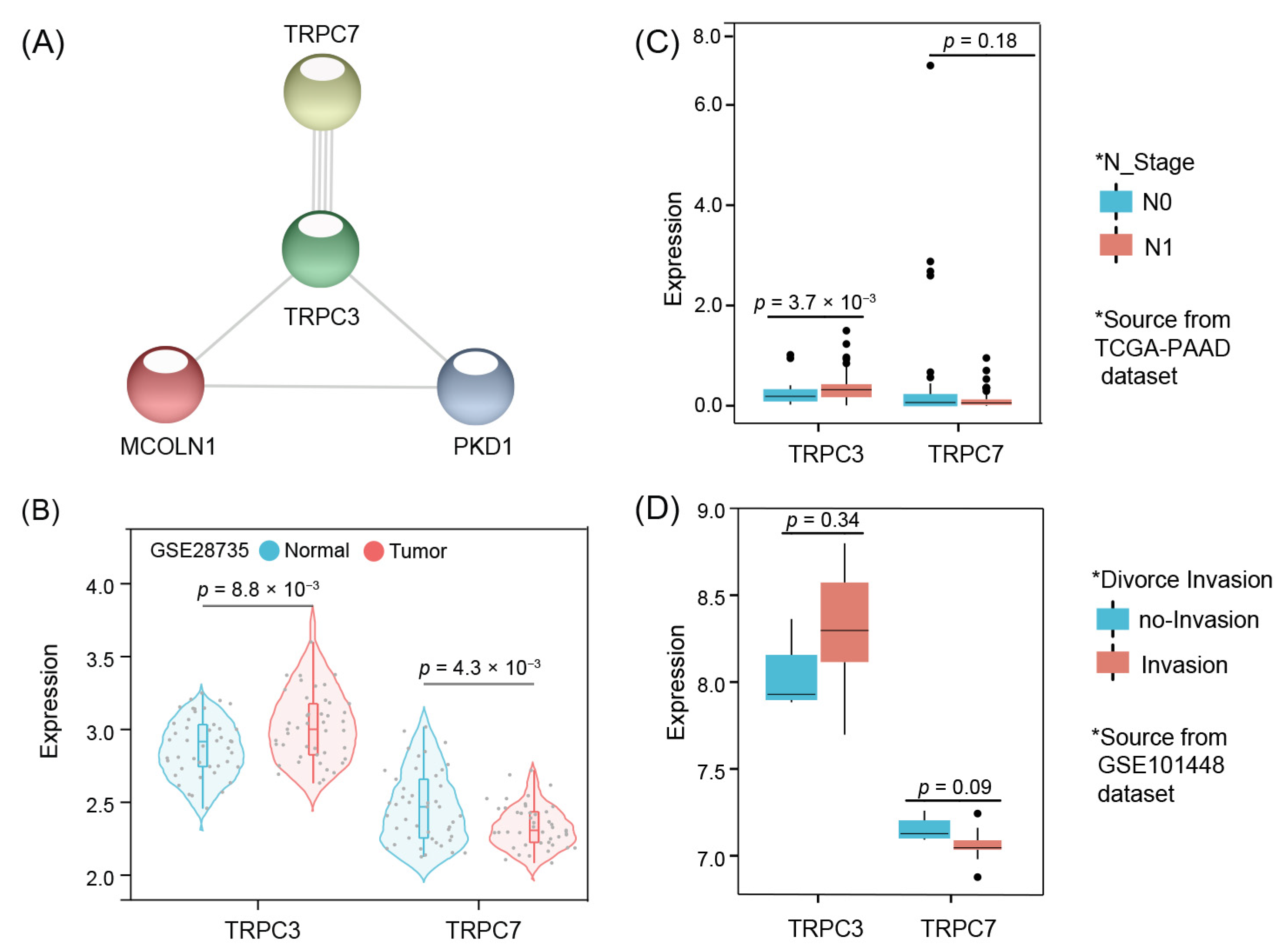
3.6. Model Core Genes and Expression Validation
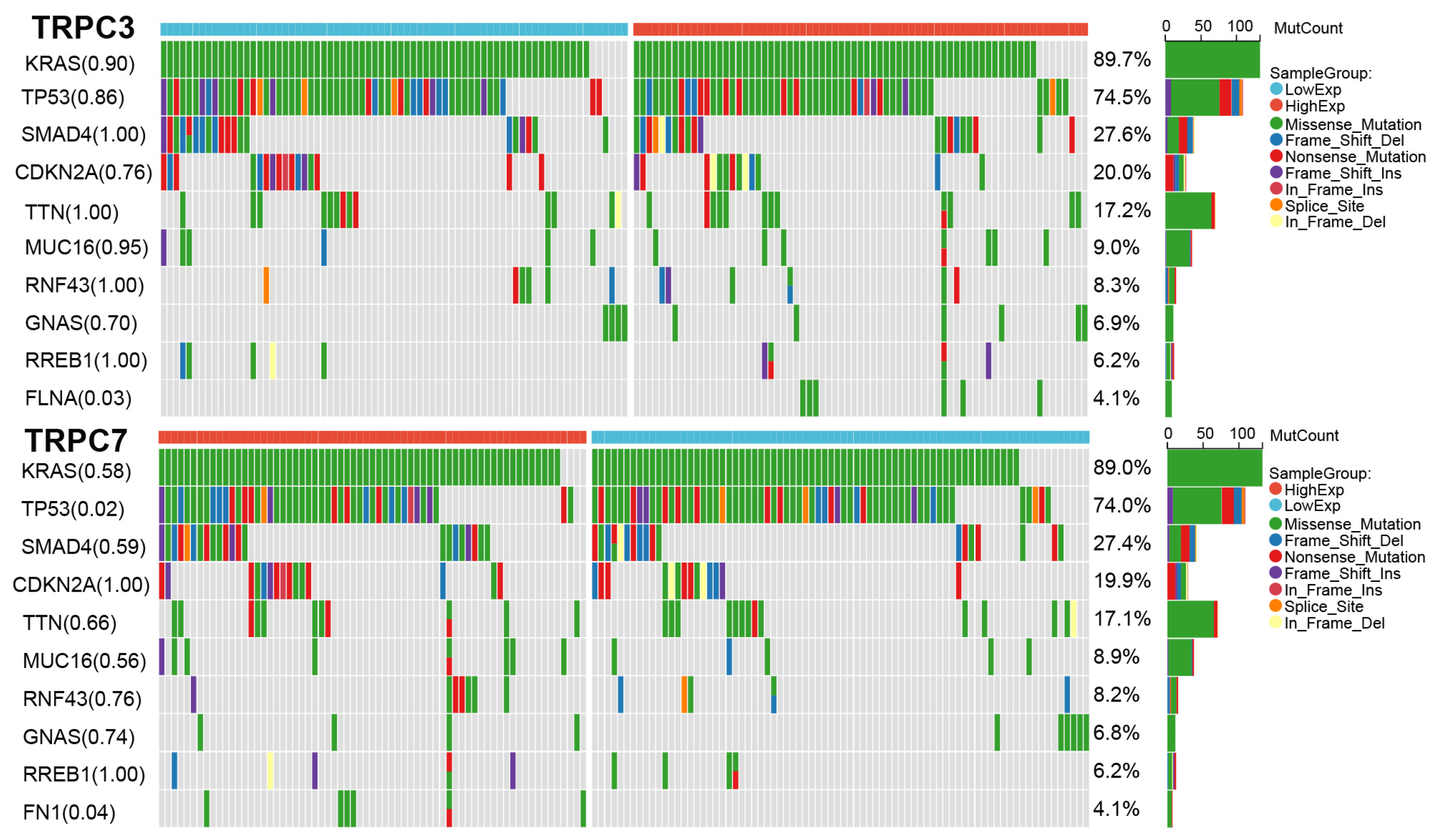
3.7. Mutation Results
3.8. Functional Annotation
3.9. Pathway Enrichment Analysis
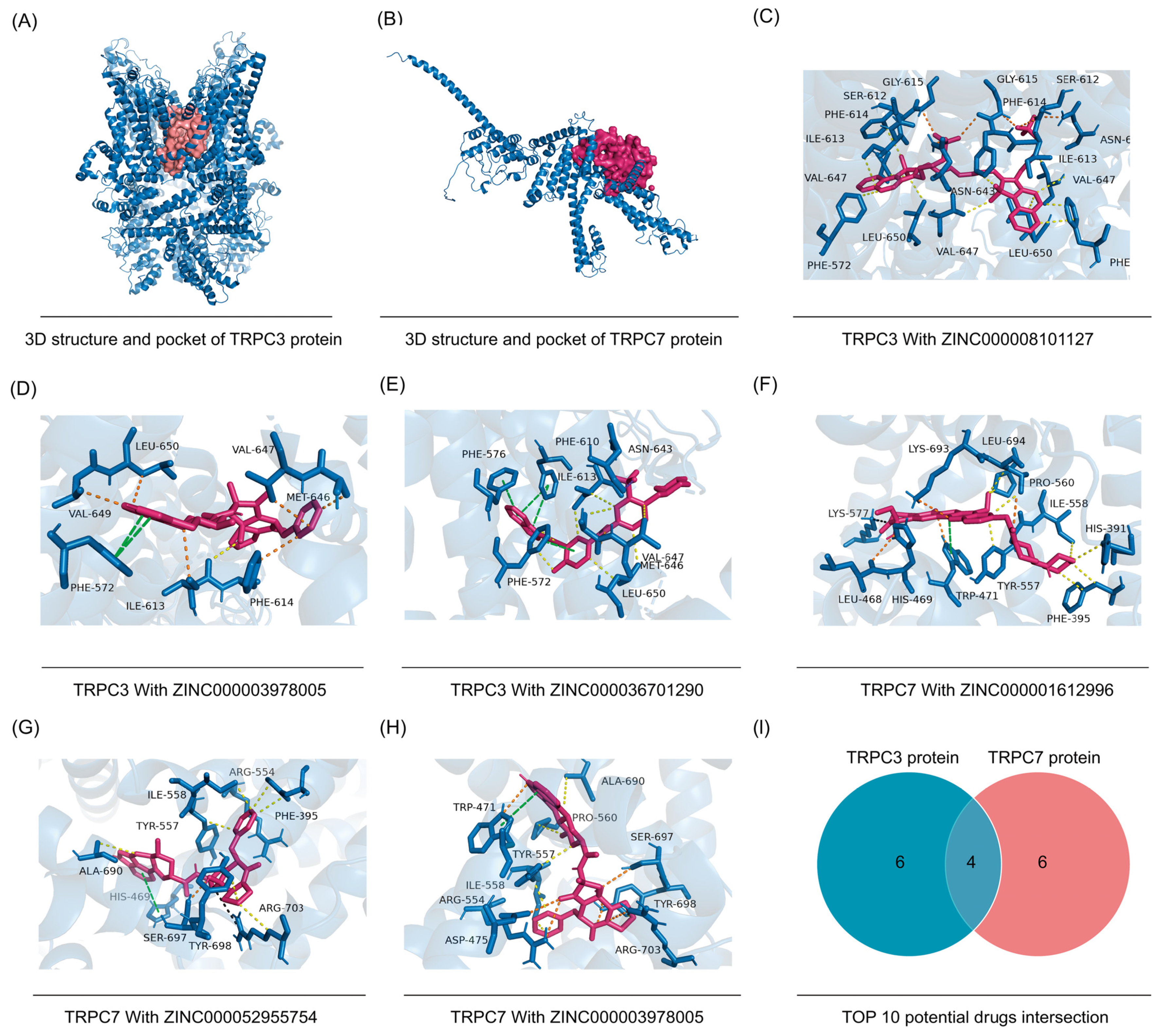
3.10. Candidate Compound Interventions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhan, W.; Shelton, C.A.; Greer, P.J.; Brand, R.E.; Whitcomb, D.C. Germline variants and risk for pancreatic cancer: A systematic review and emerging concepts. Pancreas 2018, 47, 924. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, Y.; Yang, F.; Zhu, L.; Zhu, X.-Q.; Wang, Z.-F.; Wu, X.-L.; Zhou, C.-H.; Yan, J.-Y.; Hu, B.-Y.; et al. The molecular biology of pancreatic adenocarcinoma: Translational challenges and clinical perspectives. Signal Transduct. Target. Ther. 2021, 6, 1–23. [Google Scholar] [CrossRef]
- Casolino, R.; Braconi, C.; Malleo, G.; Paiella, S.; Bassi, C.; Milella, M.; Dreyer, S.B.; Froeling, F.E.M.; Chang, D.K.; Biankin, A.V.; et al. Reshaping preoperative treatment of pancreatic cancer in the era of precision medicine. Ann. Oncol. 2021, 32, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef]
- Collisson, E.A.; Bailey, P.; Chang, D.K.; Biankin, A.V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 207–220. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, Q.; Wu, X.; Fan, Y.; Qian, J. Gene coexpression network approach to develop an immune prognostic model for pancreatic adenocarcinoma. World J. Surg. Oncol. 2021, 19, 1–9. [Google Scholar] [CrossRef]
- Meng, Z.; Yuan, Q.; Zhao, J.; Wang, B.; Li, S.; Offringa, R.; Jin, X.; Wu, H. The m6A-Related mRNA Signature Predicts the Prognosis of Pancreatic Cancer Patients. Mol. Ther.—Oncolytics 2020, 17, 460–470. [Google Scholar] [CrossRef]
- Feng, Z.; Li, K.; Lou, J.; Wu, Y.; Peng, C. An EMT-Related Gene Signature for Predicting Response to Adjuvant Chemotherapy in Pancreatic Ductal Adenocarcinoma. Front. Cell Dev. Biol. 2021, 9, 1153. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, H.; Reinach, P.S. Transient receptor potential (TRP) gene superfamily encoding cation channels. Hum. Genom. 2011, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, R.; Mori, Y. Transient receptor potential (TRP) channels: Biosensors for redox environmental stimuli and cellular status. Free Radic. Biol. Med. 2020, 146, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesquita, G.; Prevarskaya, N.; Schwab, A.; Lehen’Kyi, V. Role of the TRP Channels in Pancreatic Ductal Adenocarcinoma Development and Progression. Cells 2021, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Mayakonda, A.; Lin, D.-C.; Assenov, Y.; Plass, C.; Koeffler, H.P. Maftools: Efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018, 28, 1747–1756. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Westbrook, J.; Feng, Z.; Jain, S.; Bhat, T.N.; Thanki, N.; Ravichandran, V.; Gilliland, G.L.; Bluhm, W.F.; Weissig, H.; Greer, D.S.; et al. The Protein Data Bank: Unifying the archive. Nucleic Acids Res. 2002, 30, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Fährrolfes, R.; Bietz, S.; Flachsenberg, F.; Meyder, A.; Nittinger, E.; Otto, T.; Volkamer, A.; Rarey, M. ProteinsPlus: A web portal for structure analysis of macromolecules. Nucleic Acids Res. 2017, 45, W337–W343. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.F.; Owsianik, G.; Nilius, B. TRP channels: An overview. Cell Calcium 2005, 38, 233–252. [Google Scholar] [CrossRef]
- Barritt, G.; Rychkov, G. TRPs as mechanosensitive channels. Nat. Cell Biol. 2005, 7, 105–107. [Google Scholar] [CrossRef]
- Shekhar, S.; Liu, Y.; Wang, S.; Zhang, H.; Fang, X.; Zhang, J.; Fan, L.; Zheng, B.; Roman, R.J.; Wang, Z. Novel mechanistic insights and potential therapeutic impact of trpc6 in neurovascular coupling and ischemic stroke. Int. J. Mol. Sci. 2021, 22, 2074. [Google Scholar] [CrossRef]
- Vennekens, R.; Menigoz, A.; Nilius, B. TRPs in the brain. Rev. Physiol. Biochem. Pharmacol. 2012, 163, 27–64. [Google Scholar] [PubMed]
- Kwan, H.-Y.; Huang, Y.; Yao, X. TRP channels in endothelial function and dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 907–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damann, N.; Voets, T.; Nilius, B. TRPs in our senses. Curr. Biol. 2008, 18, R880–R889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, D.; Ohlsson, H.; Althini, C.; Bauden, M.; Zhou, Q.; Hu, D.; Andersson, R. The Hippo Signaling Pathway in Pancreatic Cancer. Anticancer Res. 2019, 39, 3317–3321. [Google Scholar] [CrossRef] [Green Version]
- Hao, H.B.; Webb, S.E.; Yue, J.; Moreau, M.; Leclerc, C.; Miller, A.L. TRPC3 is required for the survival, pluripotency and neural differentiation of mouse embryonic stem cells (mESCs). Sci. China Life Sci. 2018, 61, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wong, C.K.; Suen, C.H.; Wang, J.; Long, C.; Sauer, H.; Yao, X.; Tsang, S.Y. TRPC3 regulates the automaticity of embryonic stem cell-derived cardiomyocytes. Int. J. Cardiol. 2016, 203, 169–181. [Google Scholar] [CrossRef]
- Hsu, W.; Tsai, M.; Wu, C.; Liang, J.; Lu, J.; Kahle, J.S.; Yu, H.; Yen, C.; Yen, C.; Hsieh, Y.; et al. Nociceptive transient receptor potential canonical 7 (TRPC7) mediates aging-associated tumorigenesis induced by ultraviolet B. Aging Cell 2020, 19, e13075. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhao, R.; Ding, Q.; Yao, X.; Tsang, S.Y. TRPC7 regulates the electrophysiological functions of embryonic stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Yang, J.; Cai, W.; Lu, X.; Liu, S.; Zhao, S. RNA-Sequencing Analyses Demonstrate the Involvement of Canonical Transient Receptor Potential Channels in Rat Tooth Germ Development. Front. Physiol. 2017, 8, 455. [Google Scholar] [CrossRef] [Green Version]
- Ouwens, D.M.; Hewera, M.; Li, G.; Di, W.; Muhammad, S.; Hänggi, D.; Steiger, H.-J.; Dumitru, C.A.; Sandalcioglu, E.; Croner, R.S.; et al. Canonical WNT pathway inhibition reduces ATP synthesis rates in glioblastoma stem cells. Front. Biosci. 2022, 27, 35. [Google Scholar] [CrossRef]
- Tiapko, O.; Groschner, K. TRPC3 as a Target of Novel Therapeutic Interventions. Cells 2018, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, Z.; Ramos, A.; Boudreaux, J.P.; Thiagarajan, R.; Mattison, Y.B.; Dunham, M.E.; McWhorter, A.J.; Chen, Q.; Zhang, J.; et al. Detection of pancreatic cancer by indocyanine green-assisted fluorescence imaging in the first and second near-infrared windows. Cancer Commun. 2021, 41, 1431–1434. [Google Scholar] [CrossRef]
- Shirakawa, S.; Toyama, H.; Kido, M.; Fukumoto, T. A prospective single-center protocol for using near-infrared fluorescence imaging with indocyanine green during staging laparoscopy to detect small metastasis from pancreatic cancer. BMC Surg. 2019, 19, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-H.; Lee, A.Y.; Yu, K.-N.; Park, J.; Kim, K.P.; Cho, M.-H. Dihydroergotamine Tartrate Induces Lung Cancer Cell Death through Apoptosis and Mitophagy. Chemotherapy 2016, 61, 304–312. [Google Scholar] [CrossRef]
- Lambert, A.; Conroy, T.; Ducreux, M. Future directions in drug development in pancreatic cancer. Semin. Oncol. 2021, 48, 47–56. [Google Scholar] [CrossRef]
- Gao, G.; Li, C.; Fan, W.; Zhang, M.; Li, X.; Chen, W.; Li, W.; Liang, R.; Li, Z.; Zhu, X. Brilliant glycans and glycosylation: Seq and ye shall find. Int. J. Biol. Macromol. 2021, 189, 279–291. [Google Scholar] [CrossRef]
- Yi, S.Y.; Park, Y.S.; Kim, H.S.; Jun, H.J.; Kim, K.H.; Chang, M.H.; Park, M.J.; Uhm, J.E.; Lee, J.; Park, S.H.; et al. Irinotecan monotherapy as second-line treatment in advanced pancreatic cancer. Cancer Chemother. Pharmacol. 2009, 63, 1141–1145. [Google Scholar] [CrossRef]
- Rosti, G.; Palandri, F.; Castagnetti, F.; Breccia, M.; Levato, L.; Gugliotta, G.; Capucci, A.; Cedrone, M.; Fava, C.; Intermesoli, T.; et al. Nilotinib for the frontline treatment of Ph+ chronic myeloid leukemia. Blood 2009, 114, 4933–4938. [Google Scholar] [CrossRef] [Green Version]
- Kelley, R.K.; Ko, A.H. Erlotinib in the treatment of advanced pancreatic cancer. Biol. Targets Ther. 2008, 2, 83. [Google Scholar]
- Hurwitz, H.; Uppal, N.; Wagner, S.A.; Bendell, J.C.; Beck, J.T.; Wade, S.; Nemunaitis, J.J.; Stella, P.J.; Pipas, J.M.; Wainberg, Z.A.; et al. A randomized double-blind phase 2 study of ruxolitinib (RUX) or placebo (PBO) with capecitabine (CAPE) as second-line therapy in patients (pts) with metastatic pancreatic cancer (mPC). J. Clin. Oncol. 2014, 32, 4000. [Google Scholar] [CrossRef]
- Harder, J.; Ihorst, G.; Heinemann, V.; Hofheinz, R.; Moehler, M.; Buechler, P.; Kloeppel, G.; Röcken, C.; Bitzer, M.; Boeck, S.; et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br. J. Cancer 2012, 106, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Numaga, T.; Wakamori, M.; Mori, Y. Trpc7. In Transient Receptor Potential (TRP) Channels; Springer: Berlin/Heidelberg, Germany, 2007; pp. 143–151. [Google Scholar]

| Gene Symbol | Coef | Hazard Ratio | 95%CI (Low) | 95%CI (High) | p Value |
|---|---|---|---|---|---|
| MCOLN1 | −0.048 | 0.953 | 0.913 | 0.995 | 0.030 |
| PKD1 | −0.042 | 0.959 | 0.910 | 1.010 | 0.115 |
| TRPC3 | 1.072 | 2.922 | 1.230 | 6.940 | 0.015 |
| TRPC7 | −2.622 | 0.073 | 0.009 | 0.619 | 0.016 |
| Protein Name | Affinity | ZINC_ID | Drug Name |
|---|---|---|---|
| TRPC3 | −10.9 | ZINC000008101127 | Indocyanine Green |
| TRPC3 | −10.7 | ZINC000003978005 | Dihydroergotamine |
| TRPC3 | −10.6 | ZINC000036701290 | Ponatinib |
| TRPC3 | −10.4 | ZINC000000896717 | Accolate |
| TRPC3 | −10.2 | ZINC000164760756 | Olysio |
| TRPC3 | −10.2 | ZINC000052955754 | Ergotamine |
| TRPC3 | −10.2 | ZINC000006716957 | Nilotinib |
| TRPC3 | −10.1 | ZINC000068204830 | Daclatasvir |
| TRPC3 | −10.0 | ZINC000001612996 | Irinotecan |
| TRPC3 | −10.0 | ZINC000026664090 | Sqv |
| TRPC7 | −12.2 | ZINC000001612996 | Irinotecan |
| TRPC7 | −11.8 | ZINC000052955754 | Ergotamine |
| TRPC7 | −11.8 | ZINC000003978005 | Dihydroergotamine |
| TRPC7 | −11.7 | ZINC000006716957 | Nilotinib |
| TRPC7 | −11.6 | ZINC000066166864 | Alectinib |
| TRPC7 | −11.6 | ZINC000084668739 | Lifitegrast |
| TRPC7 | −11.4 | ZINC000064033452 | Lumacaftor |
| TRPC7 | −11.3 | ZINC000004214700 | Paliperidone |
| TRPC7 | −11.2 | ZINC000000538312 | Risperdal |
| TRPC7 | −11.2 | ZINC000003932831 | Avodart |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Li, C.; Wartmann, T.; Kahlert, C.; Du, R.; Perrakis, A.; Brunner, T.; Croner, R.S.; Kahlert, U.D. Sensory Ion Channel Candidates Inform on the Clinical Course of Pancreatic Cancer and Present Potential Targets for Repurposing of FDA-Approved Agents. J. Pers. Med. 2022, 12, 478. https://doi.org/10.3390/jpm12030478
Shi W, Li C, Wartmann T, Kahlert C, Du R, Perrakis A, Brunner T, Croner RS, Kahlert UD. Sensory Ion Channel Candidates Inform on the Clinical Course of Pancreatic Cancer and Present Potential Targets for Repurposing of FDA-Approved Agents. Journal of Personalized Medicine. 2022; 12(3):478. https://doi.org/10.3390/jpm12030478
Chicago/Turabian StyleShi, Wenjie, Chen Li, Thomas Wartmann, Christoph Kahlert, Renfei Du, Aristotelis Perrakis, Thomas Brunner, Roland S. Croner, and Ulf D. Kahlert. 2022. "Sensory Ion Channel Candidates Inform on the Clinical Course of Pancreatic Cancer and Present Potential Targets for Repurposing of FDA-Approved Agents" Journal of Personalized Medicine 12, no. 3: 478. https://doi.org/10.3390/jpm12030478
APA StyleShi, W., Li, C., Wartmann, T., Kahlert, C., Du, R., Perrakis, A., Brunner, T., Croner, R. S., & Kahlert, U. D. (2022). Sensory Ion Channel Candidates Inform on the Clinical Course of Pancreatic Cancer and Present Potential Targets for Repurposing of FDA-Approved Agents. Journal of Personalized Medicine, 12(3), 478. https://doi.org/10.3390/jpm12030478










