Dental and Skeletal Side Effects of Oral Appliances Used for the Treatment of Obstructive Sleep Apnea and Snoring in Adult Patients—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
- Study design: randomized clinical trials (RCTs), quasi-randomized clinical trials, and non-randomized prospective and retrospective trials (non-RCTs), without any restriction in language and time of publication, were considered eligible for inclusion in this review;
- Participants: adult patients with obstructive sleep apnea syndrome or snoring;
- Interventions: studies that treated obstructive sleep apnea and/or snoring patients with an oral appliance that protruded the mandible forward;
- Comparisons: comparisons were made between baseline and follow-up patient characteristics;
- Outcomes measures: any objective dental and skeletal change, in the treated patients.
2.3. Information Sources, Search Strategy and Study Selection
2.4. Data Items and Collection Extraction and Management
2.5. Risk of Bias/Quality Assessment in Individual Studies
- Low risk of bias if all key domains of the study were at low risk of bias;
- Unclear risk of bias if one or more key domains of the study were unclear;
- High risk of bias if one or more key domains were at high risk of bias.
2.6. Additional Analyses
3. Results
3.1. Study Selection and Characteristics
3.2. Risk of Bias within Studies
3.3. Results of Meta-Analysis
3.4. Dental Changes
3.5. Skeletal Changes
4. Discussion
Summary of Evidence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Koutsourelakis, I.; Perraki, E.; Zakynthinos, G.; Minaritzoglou, A.; Vagiakis, E.; Zakynthinos, S. Clinical and polysomnographic determinants of snoring. J. Sleep Res. 2012, 21, 693–699. [Google Scholar] [CrossRef]
- Tsolakis, I.A.; Venkat, D.; Hans, M.G.; Alonso, A.; Palomo, J.M. When static meets dynamic: Comparing cone-beam computed tomography and acoustic reflection for upper airway analysis. Am. J. Orthod Dentofac. Orthop. 2016, 150, 643–650. [Google Scholar] [CrossRef]
- Rohra, A.K., Jr.; Demko, C.A.; Hans, M.G.; Rosen, C.; Palomo, J.M. Sleep disordered breathing in children seeking orthodontic care. Am. J. Orthod Dentofac. Orthop. 2018, 154, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Caples, S.M.; Gami, A.S.; Somers, V.K. Obstructive sleep apnea. Ann. Intern. Med. 2005, 142, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Behrents, R.G.; Shelgikar, A.V.; Conley, R.S.; Flores-Mir, C.; Hans, M.; Levine, M.; McNamara, J.A.; Palomo, J.M.; Pliska, B.; Stockstill, J.W.; et al. Obstructive sleep apnea and orthodontics: An American Association of Orthodontists White Paper. Am. J. Orthod Dentofac. Orthop. 2019, 156, 13–28.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punjabi, N.M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.K.; Ravenell, J.; Jean-Louis, G.; Zizi, F.; Underberg, J.A.; McFarlane, S.I.; Ogedegbe, G. Resistant hypertension and sleep apnea: Pathophysiologic insights and strategic management. Curr. Diab. Rep. 2011, 11, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Z.A.; Chai-Coetzer, C.L.; Antic, N.A.; McEvoy, R.D. Obstructive sleep apnoea in adults. Postgrad. Med. J. 2013, 89, 148–156. [Google Scholar] [CrossRef]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J. Clin. Sleep Med. 2015, 11, 773–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marklund, M.; Braem, M.J.A.; Verbraecken, J. Update on oral appliance therapy. Eur. Respir. Rev. 2019, 28, 190083. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Burger, M.S.; Rietdijk-Smulders, M.A.W.J.; Smeenk, F.W.J.M. Mandibular advancement device: Effectiveness and dental side effects. A real-life study. Cranio 2020, 40, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Hoekema, A.; Stegenga, B.; De Bont, L.G. Efficacy and co-morbidity of oral appliances in the treatment of obstructive sleep apnea-hypopnea: A systematic review. Crit Rev. Oral Biol. Med. 2004, 15, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Araie, T.; Okuno, K.; Ono Minagi, H.; Sakai, T. Dental and skeletal changes associated with long-term oral appliance use for obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 41, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rinchuse, D.; Zullo, T.; Wadhwa, R. Long-term dental and skeletal effects of mandibular advancement devices in adults with obstructive sleep apnoea: A systematic review. Int. Orthod. 2019, 17, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, M.L.; Bortolotti, F.; Martina, S.; Corazza, G.; Michelotti, A.; Alessandri-Bonetti, G. Dental and skeletal long-term side effects of mandibular advancement devices in obstructive sleep apnea patients: A systematic review with meta-regression analysis. Eur. J. Orthod. 2019, 41, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Martins, O.F.M.; Chaves Junior, C.M.; Rossi, R.R.P.; Cunali, P.A.; Dal-Fabbro, C.; Bittencourt, L. Side effects of mandibular advancement splints for the treatment of snoring and obstructive sleep apnea: A systematic review. Dent. Press J. Orthod. 2018, 23, 45–54. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Bondemark, L. Does 2 years’ nocturnal treatment with a mandibular advancement splint in adult patients with snoring and OSAS cause a change in the posture of the mandible? Am. J. Orthod. Dentofac. Orthop. 1999, 116, 621–628. [Google Scholar] [CrossRef]
- Robertson, C.J. Dental and skeletal changes associated with long-term mandibular advancement. Sleep 2001, 24, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Fransson, A.M.; Tegelberg, A.; Svenson, B.A.; Lennartsson, B.; Isacsson, G. Influence of mandibular protruding device on airway passages and dentofacial characteristics in obstructive sleep apnea and snoring. Am. J. Orthod. Dentofac. Orthop. 2002, 122, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.C.; Staats, R.; Virchow, C., Jr.; Jonas, I.E. Occlusal and skeletal effects of an oral appliance in the treatment of obstructive sleep apnea. Chest 2002, 122, 871–877. [Google Scholar] [CrossRef]
- Robertson, C.; Herbison, P.; Harkness, M. Dental and occlusal changes during mandibular advancement splint therapy in sleep disordered patients. Eur. J. Orthod. 2003, 25, 371–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringqvist, M.; Walker-Engström, M.L.; Tegelberg, A.; Ringqvist, I. Dental and skeletal changes after 4 years of obstructive sleep apnea treatment with a mandibular advancement device: A prospective, randomized study. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 53–60. [Google Scholar] [CrossRef]
- Hou, H.M.; Sam, K.; Hägg, U.; Rabie, A.B.M.; Bendeus, M.; Yam, L.Y.C.; Ip, M.S. Long-term dentofacial changes in Chinese obstructive sleep apnea patients after treatment with a mandibular advancement device. Angle Orthod. 2006, 76, 432–440. [Google Scholar] [PubMed]
- Almeida, F.R.; Lowe, A.A.; Sung, J.O.; Tsuiki, S.; Otsuka, R. Long-term sequellae of oral appliance therapy in obstructive sleep apnea patients: Part 1. Cephalometric analysis. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.J.; Gotsopoulos, H.; Shen, G.; Petocz, P.; Cistulli, P.A.; Darendeliler, M.A. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 806–814. [Google Scholar] [CrossRef]
- Doff, M.H.; Hoekema, A.; Pruim, G.J.; Huddleston Slater, J.J.; Stegenga, B. Long-term oral-appliance therapy in obstructive sleep apnea: A cephalometric study of craniofacial changes. J. Dent. 2010, 38, 1010–1018. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gong, X.; Yu, Z.; Gao, X.; Zhao, Y. Follow-up study of dental and skeletal changes in patients with obstructive sleep apnea and hypopnea syndrome with long-term treatment with the Silensor appliance. Am. J. Orthod. Dentofac. Orthop. 2015, 147, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Minagi, H.O.; Okuno, K.; Nohara, K.; Sakai, T. Predictors of Side Effects with Long-Term Oral Appliance Therapy for Obstructive Sleep Apnea. J. Clin. Sleep Med. 2018, 14, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hamoda, M.M.; Almeida, F.R.; Pliska, B.T. Long-term side effects of sleep apnea treatment with oral appliances: Nature, magnitude and predictors of long-term changes. Sleep Med. 2019, 56, 184–191. [Google Scholar] [CrossRef]
- Fransson, A.M.C.; Benavente-Lundahl, C.; Isacsson, G. A prospective 10-year cephalometric follow-up study of patients with obstructive sleep apnea and snoring who used a mandibular protruding device. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Furuhashi, A.; Komori, E.; Ishiyama, H.; Hasebe, D.; Sato, K.; Yuasa, H. The Most Effective Amount of forwarding Movement for Oral Appliances for Obstructive Sleep Apnea: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 3248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurzo, A.; Urbanová, W.; Novák, B.; Waczulíková, I.; Varga, I. Utilization of a 3D Printed Orthodontic Distalizer for Tooth-Borne Hybrid Treatment in Class II Unilateral Malocclusions. Materials 2022, 15, 1740. [Google Scholar] [CrossRef] [PubMed]






| ((“Sleep Apnea Syndromes”(Mesh)) AND “adverse effects” (Subheading)) | 2163 results |
| (((“Sleep Apnea Syndromes” (Mesh)) AND “adverse effects” (Subheading)) AND “Jaw” (Mesh)) | 94 results |
| ((“Sleep Apnea Syndromes” (Mesh)) AND “Jaw” (Mesh) AND “Tooth” (Mesh)) | 33 results |
| ((((“Sleep Apnea Syndromes” (Mesh)) AND “adverse effects” (Subheading)) AND “Jaw” (Mesh)) AND “Tooth” (Mesh)) | 7 results |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies that refer to oral appliance use for the treatment of OSA/snoring and its side effects in occlusion and skeletal tissues. | Studies that refer to non-specific side effects of oral appliance use or treatment of OSA/snoring, such as tooth discomfort and increased salivation. |
| RCTs, non-randomized trials (prospective or retrospective). | Studies that refer to side effects of oral appliance use for other reasons, than to treat OSA/snoring. |
| Studies in humans. | Case reports, case series, reviews, guidelines, and authors’ opinion. |
| Studies in adults with sufficient number of teeth to retain the oral appliance. |
| Authors/Publication Year | Study Design | Participants (Number-Age-Gender-AHI) | Intervention/Appliance | Treatment Duration/Observational Period | Outcomes | Method of Outcome Assessment | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Bondemark [19] (1999) | Prospective | 30 obstructive sleep apnea (OSA)/snoring patients (21 males (M), 9 females (F), mean age 55.3 ± 8.61 months) | Monobloc acrylic mandibular advancement splint, with 8 posterior stainless steel caps and full tooth coverage | 2 years (y) |
| Baseline and follow-up cephalometric radiographs |
| Forward and downward change in mandibular position, due to increase in mandibular length |
| Robertson [20] (2001) | Prospective | 100 OSA/snoring patients (87M, 13F, mean age 49 ± 8.5 years) | Non-adjustable mandibular advancement splint with full tooth coverage | 6–30 months (6 months intervals) |
| Baseline and follow-up cephalometric radiographs |
| Mainly minor skeletal and dental changes |
| Fransson et al. [21]. (2002) | Prospective | 65 patients (52M, 13F, mean age 54.8 ± 9.0 years, 44 OSA, 21 snoring) | Monobloc heat-cured methyl methacrylate mandibular protruding device with 4 metal caps for molars and full tooth coverage | 2 years |
| Baseline and follow-up cephalometric radiographs |
| Posterior rotation of the mandible and proclination of mandibular incisors |
| Rose et al. [22] (2002) | Retrospective | 34 mild–moderate OSA patients (mean age 52.9 ± 9.6 years, mean body mass index (BMI) 28.6 ± 4.2 kg/m2) | Mandibular advancement device (MAD) consisted of 2 hard acrylic plates joined by U-shaped clasps (Karwwetzky U-clasp activator) | 29.6 ± 5.1 months |
| Baseline and follow-up dental casts and cephalometric radiographs |
| Incisor inclination and mesial shift of the occlusion |
| Robertson et al. [23] (2003) | Longitudinal, observational study | 100 OSA/snoring patients (87M, 13F, mean age 49 ± 8.5 years) | Non-adjustable mandibular advancement splint ith full tooth coverage | 6–30 months (6 months intervals) |
| Baseline and follow-up cephalometric radiographs | Combined group: increase in SNA, ANB, anterior facial height, posterior and mainly in lower facial height, maxillary length, vertical mandibular position. Mandibular first molars and maxillary first premolars overeruption, retroclination of upper incisors and proclination of lower incisors, reduction in OJ, OB and maxillary arch length. Positive correlation between device advancement and ANB angle. 6 months: facial height increase, downward mandibular position, OJ and OB decrease 12 months: vertical mandibular position increase 18 months: total and lower facial height increase, vertical mandibular position, OJ and OB decrease 24 months: increase in facial height, SNA, vertical mandibular position. Over-eruption of mandibular first molars and maxillary premolars and proclination of mandibular incisors 30 months: proclination of mandibular incisors, OJ and OB decrease. Reduced lower facial height compared with 18 and 24 months. Positive correlation of MAD anterior opening and OB change. | Changes in facial height, overjet, overbite, and position of the mandible even before 6 months of device use. Over-eruption of upper first premolars and lower first molars and proclination of lower incisors occurred after 2 years of device use. Overbite changes might be decreased by keeping a minimum bite opening |
| Ringqvist [24] (2003) | Randomized clinical trial | 45 OSA patients treated with MAD (mean age 48.9 years, mean weight 87.8 kg, mean BMI 27.0 kg/m2) 43 OSA patients treated with uvulopalatopharungoplasty (UPPP): mean age 51 years, mean weight 87.8 kg, mean BMI 27.1 kg/m2 | MAD was a mono-bloc device consisted of heat-cured methyl methacrylate. | MAD patients (4.1 years, 30 patients completed the follow-up and 27 were only treated with MAD) UPPP patients (4.3 years, 37 completed the follow-up and 27 were only treated with UPPP) | O1: MAD group dental and skeletal measurements O2: UPPP group dental and skeletal measurements | Lateral cephalometric radiographs with the patient in supine position. | O1:
| Minor dental and skeletal changes after 4 years of MAD use. No clinically important differences between MAD and UPPP groups |
| Hou et al. [25] (2006) | Prospective | 67 Chinese OSA patients (50M, 17F, mean age 46.9 ± 8.9 years) | modified Harvold monobloc type of functional appliance | 1–3 years 1 year: n = 63 2 years: n = 43 3 years: n = 30 |
| Baseline and follow-up cephalometric radiographs |
| Small dentofacial changes and main OJ and OB reduction during early treatment |
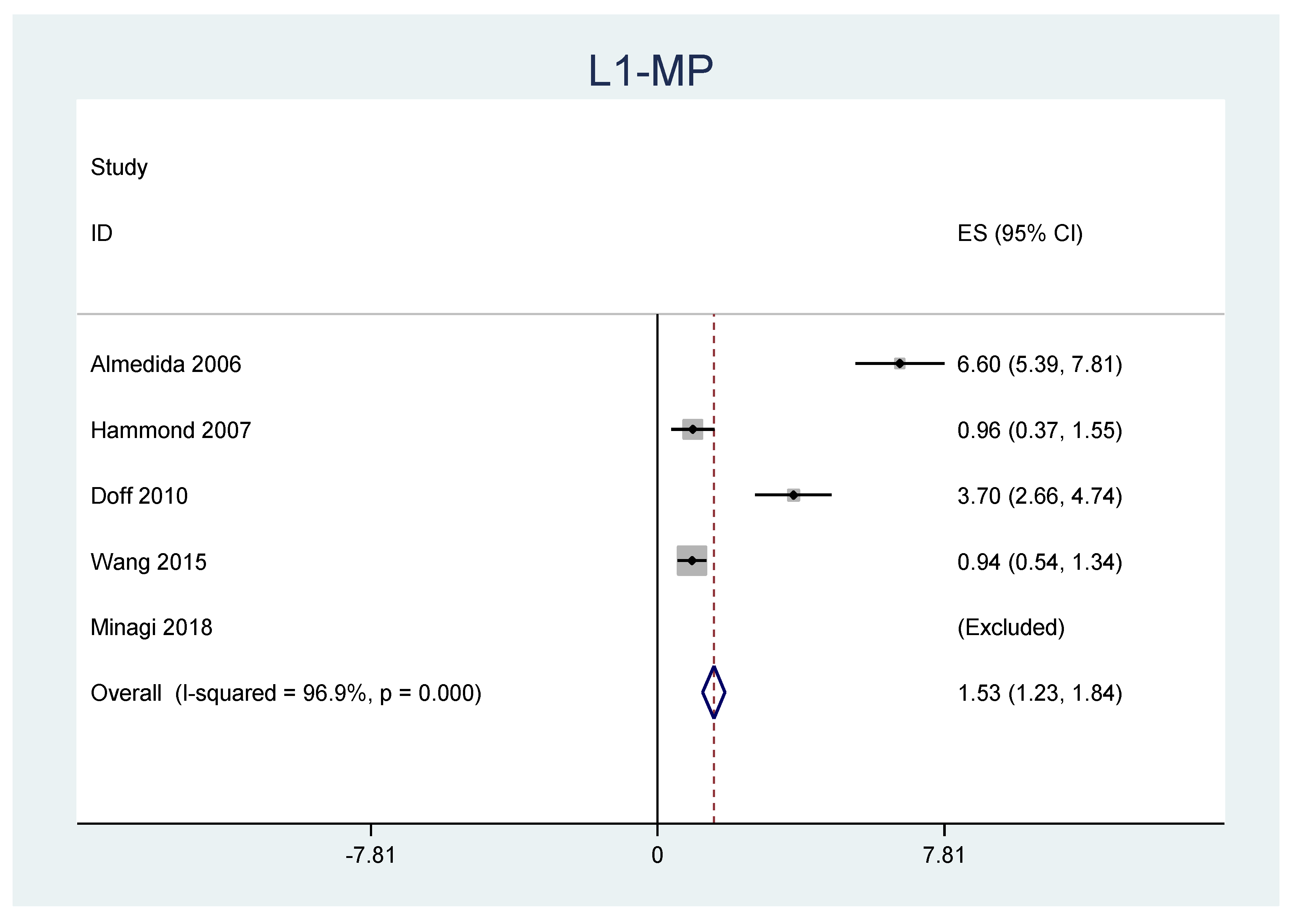
| Almeida et al. [26] (2006) | Retrospective | 71 OSA patients (63M, 8F, mean age 49.7 ± 9.7 years, respiratory disturbance index 28.9 ± 17.0/h, BMI 29.3 ± 5.9 kg/m2) | Klearway oral appliance | 7.3 ± 2.1 years on average |
| Baseline and follow-up cephalometric radiographs |
| Craniofacial and dental changes occur after long-term OA use |
| Hammond et al. [27] (2007) | Retrospective | 64 OSA patients (50M, 14F) | 2-piece acrylic appliance with full occlusal coverage and a screw that titrates the device (Mehta et al.) | 25.1 ± 11.8 months on average |
| Baseline and follow-up cephalometric radiographs, study model analysis and anthropometric measurements Questionnaire | Cephalometric analysis on 46 patients (34M, 12F):
| Minor dental and skeletal side effects |
| Doff et al. [28] (2010) | Randomized clinical trial | 103 OSA patients (51 with MAD) | Thorton Adjustable positioner | 2.3 ± 0.2 years on average |
| Baseline and follow-up cephalometric radiographs |
| Mainly dental changes |
| Wang et al. [29] (2015) | Prospective | 42 patients OSA patients (31M, 11F, mean age 47 ± 10 years, mean AHI 27 ± 19) | Silensor appliance | 4 ± 3 years on average |
| Questionnaire and baseline and follow-up cephalometric radiographs |
| Minor dental and skeletal side effects (1–3 years of treatment mainly skeletal changes, after 3 years of treatment dental and skeletal changes) |
| Minagi et al. [30] (2018) | Retrospective | 64 OSA patients (44M, 20F, mean age 57.7 ± 14.2 years, mean BMI 23.9 ± 3.6 kg/m2, mean apnea-hypopnea index (AHI) 24.9 ± 14.7 | Mad consisted of two separate acrylic monoblock modified plates (ERKODRNT) | 4.3 ± 2.1 years on average |
| Baseline and follow-up cephalometric radiographs |
| Dental side effects (low number of maxillary teeth and MAD treatment duration, use frequency and mandibular advancement correlated with OJ reduction) |
| Hamoda et al. [31] (2018) | Retrospective | 62 patients with primary snoring or mild to severe OSA (52M, 10F, mean age 49 ± 8.6 years, mean BMI 29.1 ± 6.9 kg/m2, mean AHI 30.0 ± 14.6 for 56 patients, Angle Class I/Class II/Class III 31/26/4) | Klearway® or SomnoDent® | 12.6 ± 3.9 years on average |
| Baseline and follow-up cephalometric radiographs (up to 9 cephalometric radiographs for some patients) |
| Dental changes happen progressively and duration of mandibular advancement device treatment is the greatest factor of their magnitude Minor skeletal changes that occur are not clinically significant |
| Fransson et al. [32] (2020) | Prospective | 65 patients (52M, 13F, mean age 54.8 ± 9.0 years, 44 OSA, 21 snoring) | Monobloc heat-cured methyl methacrylate mandibular protruding device with 4 metal caps for molars and full tooth coverage | 10 years |
| Baseline and follow-up cephalometric radiographs |
| Posterior rotation of the mandible and proclination of mandibular incisors |
| Author (Year) | Outcomes | Random Sequence Generation | Allocation Concealment | Performance Bias | Detection Bias | Attrition Bias | Selective Reporting | Other | Overall |
|---|---|---|---|---|---|---|---|---|---|
| Ringqvist et al. [24] (2003) | O1: dental and skeletal measurements on MAD patients O2: dental and skeletal measurements on UPPP patients | Unclear for all outcomes (‘…45 were randomly assigned to treatment with the mandibular advancement device (MAD) group and 43 to treatment with UPPP’) not possible to conclude if randomization was successful | Unclear for all outcomes (not mentioned concealment of allocation, probably not performed) | Low for all outcomes (not mentioned blinding of participants/personnel but the outcome is not likely to be affected) | Unclear for all outcomes (blinding of outcome assessor is not mentioned) | High for all outcomes (patients that did not attend the 4-year follow-up were 15 in the MAD group and 6 in the UPPP group) | Low for all outcomes (all pre-specified variables were measured) | High for all outcomes (patients received both treatments, 3 patients in the MAD group and 10 in the UPPP group) | High for all outcomes (patients not attending the follow-up and patients receiving both treatments can affect the outcomes) |
| Doff et al. [28] (2010) | Craniofacial changes | Unclear for all outcomes (‘…patients were randomized’) not possible to conclude if randomization was successful | Unclear for all outcomes (not mentioned concealment of allocation, probably not performed) | Low for all outcomes (not mentioned blinding of participants/personnel but the outcome is not likely to be affected) | Low for all outcomes (‘…one blinded observer (MD) performed all tracings’) | Low for all outcomes (number of missing outcome data balanced among groups-reasons not related to outcome) | Low for all outcomes (all pre-specified variables were measured) | Low for all outcomes (patients that randomized in oral appliance treatment, and after treated for 3 months, changed to CPAP treatment were excluded) | Low for all outcomes (no concealed allocation but baseline characteristics that can affect the outcome -AHI, BMI, number of teeth, appliance usage, were similar among groups) |
| Author (Year) | Outcomes | Bias Due to Confounding | Bias in Selection of Participants into the Study | Bias in Measurement of Interventions | Bias Due to Departures from Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Overall Bias |
|---|---|---|---|---|---|---|---|---|---|
| Bondemark [19] (1999) | Mandibular and dentofacial changes | Low for all outcomes | Low for all outcomes (all eligible participants were included and start of intervention and follow-up coincide) | Low for all outcomes (well-defined intervention status) | Low for all outcomes (no bias due to departure from intervention is expected) | Low for all outcomes (data were reasonably complete) | Low for all outcomes (objective method of outcome assessment, any error is unrelated to intervention status) | Low for all outcomes (all reported results correspond to intended outcome) | Low for all outcomes |
| Robertson [20] (2001) | Dentoalveolar and skeletal changes | Low for all outcomes | Low for all outcomes | Low for all outcomes | Low for all outcomes | Low for all outcomes (no missing outcome data) | Low for all outcomes (all pre-specified variables were measured) | Low for all outcomes (no possible risk of bias from other source) | Low for all outcomes |
| Robertson et al. [23] (2003) | Dentoalveolar and skeletal changes | Low for all outcomes | Low for all outcomes | Low for all outcomes | Low for all outcomes | Low for all outcomes (no missing outcome data) | Low for all outcomes (all pre-specified variables were measured) | Low for all outcomes (no possible risk of bias from other source) | Low for all outcomes |
| Rose et al. [22] (2002) | Dentofacial cephalometric and dental casts measurements | Low for all outcomes | Low for all outcomes | Low for all outcomes | Low for all outcomes | Low for all outcomes | Low for all outcomes | Low for all outcomes | Low for all outcomes |
| Fransson et al. [21] (2002) | O1: airway changes O2: skeletal, dental, soft tissue changes | Low for all outcomes | Low for all outcomes (all eligible participants were included and start of intervention and follow-up coincide) | Serious for all outcomes (intervention status regarding usage frequency not well-defined) | Low for all outcomes (no bias due to departure from intervention is expected) | Low for all outcomes (data were reasonably complete) | Low for all outcomes (objective method of outcome assessment, any error is unrelated to intervention status, outcome assessor was blinded during cephalometric analysis.) | Low for all outcomes (all reported results correspond to intended outcome) | Low for all outcomes |
| Hou et al. [25] (2006) | Long-term dentofacial changes | Serious for all outcomes (at least one critically important domain not appropriately measured or not adjusted for) | Low for all outcomes (all eligible participants were included and start of intervention and follow-up coincide) | Serious for all outcomes (intervention status not well-defined) | Serious for all outcomes (switches in treatment is apparent and are not adjusted in for the analysis) | Low for all outcomes (data were reasonably complete) | Low for all outcomes (objective method of outcome assessment, any error is unrelated to intervention status) | Low for all outcomes (all reported results correspond to intended outcome) | Serious for all outcomes (the study is judged to be in serious risk of bias in at least one domain) |
| Almeida et al. [26] (2006) | Skeletal, dental, and occlusal changes | Serious for all outcomes (at least one critically important domain not appropriately measured or not adjusted for) | Serious for all outcomes (retrospective study (start follow-up did not coincide) selection into the study was related to intervention and possibly to outcome) | Serious for all outcomes (intervention status not well-defined) | Low for all outcomes (no bias due to departure from intervention is expected) | Low for all outcomes (data were reasonably complete) | Serious for all outcomes (outcome assessor was aware of the intervention received by the participants) | Low for all outcomes (all reported results correspond to intended outcome) | Serious for all outcomes (the study is judged to be in serious risk of bias in at least one domain) |
| Hammond et al. [27] (2007) | O1: long-term subjective side-effects O2: long-term dental and skeletal effects side-effects | Serious for all outcomes (at least one critically important domain not appropriately measured or not adjusted for) | Serious for all outcomes (inception bias) | Serious for all outcomes (intervention status not well-defined) | Low for O1 outcomes Serious for O2 outcomes (switches in treatment) | Serious for all outcomes (missing data-baseline characteristics; the risk of bias cannot be removed trough appropriate analysis) | Serious for O1 (subjective method of outcome assessment) Serious for O2 (outcome assessor was aware of the intervention received by the participants) | Low for all outcomes (all reported results correspond to intended outcome) | Serious for all outcomes (the study is judged to be in serious risk of bias in at least one domain) |
| Wang et al. [29] (2015) | O1: long-term subjective side-effects O2: long-term dental and skeletal effects side-effects | Serious for all outcomes (at least one critically important domain not appropriately measured or not adjusted for) | Low for all outcomes (all eligible participants were included and start of intervention and follow-up coincide) | Low for all outcomes (well-defined intervention status) | Low for all outcomes (no bias due to departure from intervention is expected) | Low for all outcomes (data were reasonably complete) | Serious for O1 outcome (subjective method of outcome assessment) Low for O2 outcomes (objective method of outcome assessment, any error is unrelated to intervention status, outcome assessor was blinded during cephalometric analysis.) | Low for all outcomes (all reported results correspond to intended outcome) | Serious for all outcomes (the study is judged to be in serious risk of bias in at least one domain) |
| Minagi et al. [30] (2018) | causing factors and predictors of orthodontic changes after long-term use | Low for all outcomes | Low for all outcomes (all eligible participants were included and start of intervention and follow-up coincide) | Low for all outcomes (well-defined intervention status) | Low for all outcomes (no bias due to departure from intervention is expected) | Low for all outcomes (data were reasonably complete) | Low for all outcomes (objective method of outcome assessment, any error is unrelated to intervention status, outcome assessor was blinded during cephalometric analysis.) | Low for all outcomes (all reported results correspond to intended outcome) | Low for all outcomes |
| Hamoda et al. [31] (2018) | O1: dental and skeletal changes O2: Rate and predictors of changes | Serious for all outcomes (at least one critically important domain not appropriately measured or not adjusted for) | Serious for all outcomes (retrospective study) | Serious for all outcomes (intervention status regarding usage frequency not well-defined) | Low for all outcomes (no bias due to departure from intervention is expected) | Low for all outcomes (data were reasonably complete) | Low for all outcomes (objective method of outcome assessment) | Low for all outcomes (all reported results correspond to intended outcome) | Serious for all outcomes (the study is judged to be in serious risk of bias in at least one domain) |
| Fransson et al. [32] (2020) | O1: airway changes O2: skeletal, dental, soft tissue changes | Serious for all outcomes (at least one critically important domain not appropriately measured or not adjusted for) | Low for all outcomes (all eligible participants were included and start of intervention and follow-up coincide) | Low for all outcomes (well-defined intervention status) | Low for all outcomes (no bias due to departure from intervention is expected) | Low for all outcomes (data were reasonably complete) | Low for all outcomes (objective method of outcome assessment, any error is unrelated to intervention status, outcome assessor was blinded during cephalometric analysis.) | Low for all outcomes (all reported results correspond to intended outcome) | Low for all outcomes |
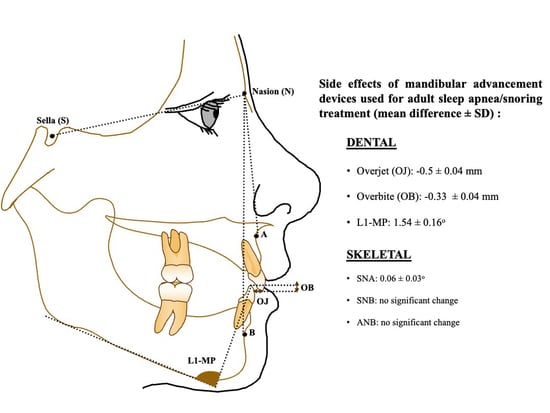
| Parameters | ES (Mean Diff.) | Upper Limit | SD |
|---|---|---|---|
| SNA | 0.061 | 0.116 | 0.028 |
| SNB | 0.019 | 0.088 | 0.035 |
| ANB | 0.067 | 0.143 | 0.039 |
| Overjet | −0.506 | −0.420 | 0.044 |
| Overbite | −0.326 | −0.255 | 0.036 |
| L1-MP | 1.535 | 1.838 | 0.155 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsolakis, I.A.; Palomo, J.M.; Matthaios, S.; Tsolakis, A.I. Dental and Skeletal Side Effects of Oral Appliances Used for the Treatment of Obstructive Sleep Apnea and Snoring in Adult Patients—A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 483. https://doi.org/10.3390/jpm12030483
Tsolakis IA, Palomo JM, Matthaios S, Tsolakis AI. Dental and Skeletal Side Effects of Oral Appliances Used for the Treatment of Obstructive Sleep Apnea and Snoring in Adult Patients—A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2022; 12(3):483. https://doi.org/10.3390/jpm12030483
Chicago/Turabian StyleTsolakis, Ioannis A., Juan Martin Palomo, Stefanos Matthaios, and Apostolos I. Tsolakis. 2022. "Dental and Skeletal Side Effects of Oral Appliances Used for the Treatment of Obstructive Sleep Apnea and Snoring in Adult Patients—A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 12, no. 3: 483. https://doi.org/10.3390/jpm12030483
APA StyleTsolakis, I. A., Palomo, J. M., Matthaios, S., & Tsolakis, A. I. (2022). Dental and Skeletal Side Effects of Oral Appliances Used for the Treatment of Obstructive Sleep Apnea and Snoring in Adult Patients—A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 12(3), 483. https://doi.org/10.3390/jpm12030483