Abstract
Systemic amyloidosis arises from monoclonal CD38+ plasma cells that produce misfolded immunoglobulin light chains, which form amyloid fibrils that are deposited into different tissues, leading to organ damage. Daratumumab is a human IgG/k monoclonal antibody that targets CD38, a glycoprotein uniformly expressed on human plasma cells. Daratumumab has been utilized in recent years with unprecedented responses in multiple myeloma. In patients with relapsed or refractory AL amyloidosis, daratumumab has shown promising efficacy in terms of hematologic responses and improvement in organ function. Here, we report real-life treatment with Daratumumab in 33 AL amyloidosis patients treated within the Regional Tuscan Myeloma network at 5 centers with associated MGUS or SMM (n = 15) or symptomatic MM (n = 18). Patients were treated at relapsed/refractory disease stages (n = 29) with a median of one previous line of therapy or at diagnosis (n = 4). Daratumumab showed good efficacy, representing 60% of good hematological responses and 50% of organ responses in a real-life population of patients with an acceptable toxicity profile.
1. Introduction
Systemic immunoglobulin light-chain amyloidosis (AL) is a multisystem disease caused by the deposition of amyloid fibrils in organ tissues, arising from misfolded light chains produced most commonly by clonal expansion of monoclonal plasma cells (PC) [1]. The resulting organ damage most frequently involves the heart, kidney, liver, and peripheral nervous system [2]. The estimated incidence is 8–12 cases per million people [3]. In systemic AL amyloidosis, the associated PC disorder usually has a low tumor burden (median marrow infiltration, 10%), i.e., monoclonal gammopathy of undetermined significance (MGUS). Less frequently, smoldering multiple myeloma (SMM) or symptomatic MM can be seen. Recurrent cytogenetic abnormalities in AL-amyloidosis-related clones are: t(11;14) and gain 1(q21), which are found in 50% and 20% of the clones, respectively [4]. Tissue biopsy stained with Congo red demonstrating amyloid deposits with apple-green birefringence and verification that amyloid is composed of immunoglobulin light chains are required for diagnosis [5]. The major determinant of the outcome in amyloidosis is the extent of cardiac involvement, and the N-terminal pro-brain natriuretic peptide (NT-proBNP), serum troponin T, and the difference between involved and uninvolved immunoglobulin free-light-chain (FLC) values are used to assess prognosis in the revised Mayo Clinic staging system [6]. Treatment of AL amyloidosis is primarily based on multiple myeloma (MM) therapies, and a combination of bortezomib, cyclophosphamide, and melphalan (CyBordex; VMdex) is the most commonly used regimen in the frontline setting [7]. Autologous stem cell transplant (ASCT) is still the preferred treatment option for AL amyloidosis, but only 20% of patients are eligible [8]. Immunomodulatory agents (IMiDs) lenalidomide and pomalidomide are often used in relapsed/refractory patients [9,10]. Despite therapeutic advances, outcomes remain poor and alternative therapies are needed.
CD38 and Daratumumab
The human CD38 antigen is a 46-kilodalton (kDa) type II transmembrane glycoprotein with a short N-terminal cytoplasmic tail and a long extracellular domain. It is present in hematopoietic cells and can also be expressed on regulatory T cells, regulatory B cells, and myeloid-derived suppressor cells with a high surface expression associated with compromised immune surveillance for malignancies. CD38 is present in most of the circulating T- and B-cells; it is also present on monocytes, natural killer cells, dendritic cells, and plasma cells [11]. CD38 is a great target for Monoclonal Antibodies (MoA) therapy in MM because MM plasma cells express higher levels of CD38 compared with normal cells. Daratumumab (Dara), a human IgG-k monoclonal antibody that targets CD38, is a highly effective antiplasma cell therapy, and it was recently added to the therapeutic MM armamentarium with an unprecedented depth of responses [12,13,14,15].
Dara is an ideal agent for the treatment of AL amyloidosis. In the phase 3 ANDROMEDA trial, subcutaneous daratumumab plus bortezomib, cyclophosphamide, and dexamethasone showed efficacy and safety in newly diagnosed AL amyloidosis [16,17]. Dara-containing regimens also appeared to be highly active in patients with relapsed/refractory AL amyloidosis, providing rapid hematological responses with a good safety profile [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Although different studies have been reported in the literature, most are case reports, and real-life clinical studies are needed to confirm the efficacy and toxicities of Dara therapeutic regimens. Herein, we report the results of a retrospective real-life analysis of daratumumab-based therapies in patients with AL amyloidosis treated within the Regional Tuscan Myeloma Network (RTM).
2. Materials and Methods
Patients consecutively presented to the RTM centers with biopsy-proven systemic AL, measurable disease, and with both newly diagnosed and relapsed/refractory AL amyloidosis were included in this study. There were no exclusion criteria regarding the presence of concomitant symptomatic MM or other comorbidities of any degree. This study received approval from ethics committees and regulatory authorities. It was conducted according to the Declaration of Helsinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice. All patients provided written informed consent prior to entering the study. This multicenter retrospective study was conducted at 5 Italian hematological centers (Siena, Firenze, Pisa, and Arezzo, Empoli) belonging to the RTM, and subjects were enrolled between July of 2018 and August of 2021. Daratumumab was administered IV (at that time SC formulation was not available yet) as monotherapy or in combination with lenalidomide and dexamethasone (DaraRd) or bortezomib and dexamethasone (DaraVd), according to the preferred choice of each center. Premedication with paracetamol, antihistamines, and corticosteroids (IV methylprednisolone, 100 mg, or dexamethasone, 20 mg) was administered to all patients to minimize the risk of daratumumab-associated infusion reactions. The primary end point was progression-free survival (PFS) and overall survival (OS). Secondary end points included best hematological response, time to hematological response, organ response, and safety and tolerability. PFS was defined as time to progression or death—whichever occurred first. Initial diagnostic evaluation and response to previous therapy were documented for all patients at enrollment. Fluorescence in situ hybridization (FISH) was reported when available. In particular, probes for t(11;14); del 17p; t(14;16); and amp 1q. were utilized [39]. Definitions of hematological response and organ response were based on the standard and updated international response criteria for amyloidosis; VGPR was defined as dFLC < 40 mg/L, complete response (CR) was defined as negative serum and urine immunofixation plus normalized FLC ratio (with no bone marrow evaluation requested), and partial response (PR) was defined as a reduction > 50% of the dFLC. Overall response rate (ORR) was defined as PR or better [6]. Recently, the 2012 response criteria were updated by the International Society of Amyloidosis (ISA) to define complete hematological response as the absence of amyloidogenic light chains (either free or part of a complete immunoglobulin) described as negative serum and urine immunofixation and either a FLC ratio within the reference range or an abnormal FLC ratio as long as the uninvolved FLC concentration is greater than the involved FLC concentration. The meaning of this clarification is that an abnormal FLC ratio does not preclude the achievement of CR when the concentration of uninvolved, nonamyloidogenic FLC is greater than that of the involved, amyloidogenic FLC. This is particularly relevant today when highly effective antiplasma cell therapies are available that can cause profound reductions in both involved FLC and uninvolved-FLC, possibly resulting in an inverted FLC ratio favoring nonamyloidogenic FLC [40].
Relapse definition was based on the start of a new line of therapy (for insufficient hematological or clinical response or organ progression), reappearance (on immunofixation) of the original monoclonal protein in the serum or urine, or an increase in the serum involved FLC (iFLC) of at least double that of the normal range for CR, or an increase in the serum iFLC concentration of 50%, and this must increase to a value greater than (100 mg/L) for PR [41]. All patients underwent cardiac ultrasound, while MRI was performed only to confirm cardiac involvement in cases with positive cardiac ultrasound. Cardiac response was defined by a reduction in NT-proBNP of 30% and >300 ng/L over the starting value (baseline NT-proBNP had to be ≥650 ng/L to be measurable). Renal response was defined by a 30% reduction in 24 h urine protein excretion or a drop of proteinuria below 0.5 g per 24 h in the absence of progressive renal insufficiency, defined as a decrease in eGFR to 25% over baseline. Toxicities were graded according to National Cancer Institute Common Toxicity Criteria of Adverse Events (version 4.0). Survival analysis used the Kaplan–Meier method to estimate PFS and OS. Quantitative data were reported using median, interquartile range (IQR), and range; qualitative data were reported with frequency and percentages. Response rates were reported as point estimates with exact 95% confidence interval (CI). Statistical analyses were performed using MedCalc for Windows, version 19.4 (MedCalc Software, Ostend, Belgium).
3. Results
3.1. Patients’ Characteristics
By February of 2022, 33 patients were included in the study. Table 1 summarizes baseline patient and disease characteristics. The median age was 64 years (range, 44–82). A total of 18 (54.5%) patients presented symptomatic multiple myeloma, 11 (33.3%) had smoldering myeloma, and only 4 (12.1%) presented MGUS. FISH was performed in 23 (69.7%) patients: 6 (26.1%) of them presented t(11;14), 4 (17.4%) had amp1q, and 9 were negative. Overall, 27 (81.8%) patients had cardiac involvement, 13 (39.3%) had renal involvement, and 16 (48.5%) had ≥2 organs involved. The median dFLC level at baseline was 478.5 mg/L (IQR, 52–6405). Median NT-proBNP at baseline was 1307 ng/L (IQR, 140–31,947). A total of 11 (33.3%) patients had creatinine clearance < 60 mL/min. According to the revised Mayo clinic staging system, 2 (6.1%) patients were stage I, 11 (33.3%) stage II, 10 (30.3%) stage III, and 10 (30.3%) stage IV. A total of 4 (12.2%) patients received daratumumab as a first-line therapy, while 29 (87.8%) patients were relapsed/refractory to previous treatments, and 22 (66.6%) of them received daratumumab as a second-line therapy. Of note, five (15.2%) patients had previously received autologous stem cell transplantation (ASCT).

Table 1.
Patients’ characteristics.
3.2. Treatment
Six (18.1%) patients received daratumumab as a monotherapy, while twenty-two (66.6%) and five (15.2%) received daratumumab in combination with the DaraRd and DaraVd scheme, respectively. Treatments were chosen based on center-based criteria. MGUS-associated AL amyloidosis patients tended to receive Dara monotherapy, while MM patients tended to receive combined therapy with lenalidomide or bortezomib (if renal failure was absent or present, respectively). The median treatment duration was eight cycles (IQR, 1–44), and 18 (54.5%) patients are still on treatment with daratumumab at the time of writing. MGUS/SMM-associated AL amyloidosis patients received treatment for a limited time, while symptomatic MM patients received treatment until progression. Seven patients discontinued treatment because of disease progression and/or absence of hematological response. Of note, two patients received ABMT after achieving CR or VGPR with daratumumab. No one stopped treatment or died as a result of drug toxicity.
3.3. Response Evaluation
Hematological and Organ Response
Hematological and organ responses are summarized in Table 2. At the last evaluation, 22 (66.6%) of 33 patients achieved VGPR or better, 11 patients (33.3%) achieved CR (4 MGUS/SMM and 7 MM), and 11 patients (33.3%) achieved VGPR (3 MGUS/SMM and 8 MM). Eight (24.3%) additional patients had a PR for an ORR of 90%. A total of 20 patients (60.6%) achieved hematological response within 6 months of treatment, and 8 (24.2%) of them responded after 1 month of therapy. The median time to best hematological response was 4.5 months. The median dFLC after 1 month of daratumumab was 32.5 mg/L (IQR, 1–400). During follow-up, 15 of 27 (55.5%) patients with baseline cardiac involvement had a cardiac response. Concerning the 13 patients with renal involvement, 8 (61.5%) had a renal response. No differences were seen in survival rates according to FISH analysis.

Table 2.
Hematological response and organ response.
3.4. Safety
Overall, five serious adverse events (AEs) were reported in five patients, including three deaths (one related to acute renal injury, one to congestive heart failure, and one to meningitis), and two patients were reported to experience septicemia; none were considered treatment-related. A total of 11 patients had grade 3/4 AEs; 3 grade 3 AEs were considered treatment-related: cutaneous rash after the first infusion that did not recur in 1 patient and neutropenia and lymphopenia in 2 patients. Other grade 3/4 AEs included heart failure (three patients), pneumonia (one patient), cholecystitis (one patient), herpes zoster (one patient), urinary tract infection (one patient), and osteomyelitis (one patient). The most common AEs were grade 1/2 infusion reactions after the first dose, seen in 12 (36.4%) patients, as previously reported for other IV daratumumab trials. No grade 4 or 5 therapy-related AEs were recorded.
3.5. Survival Outcomes and Durability of Response
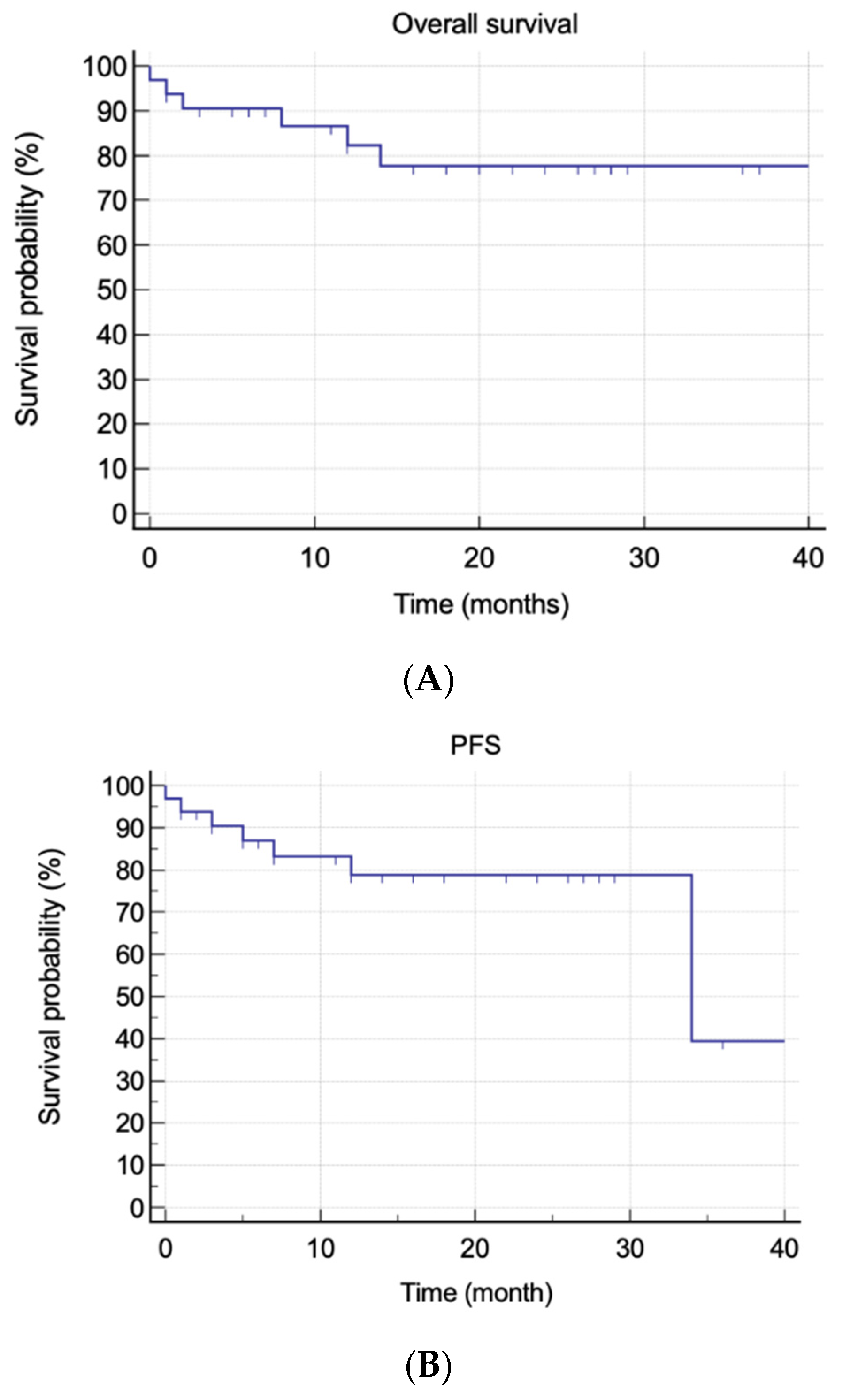
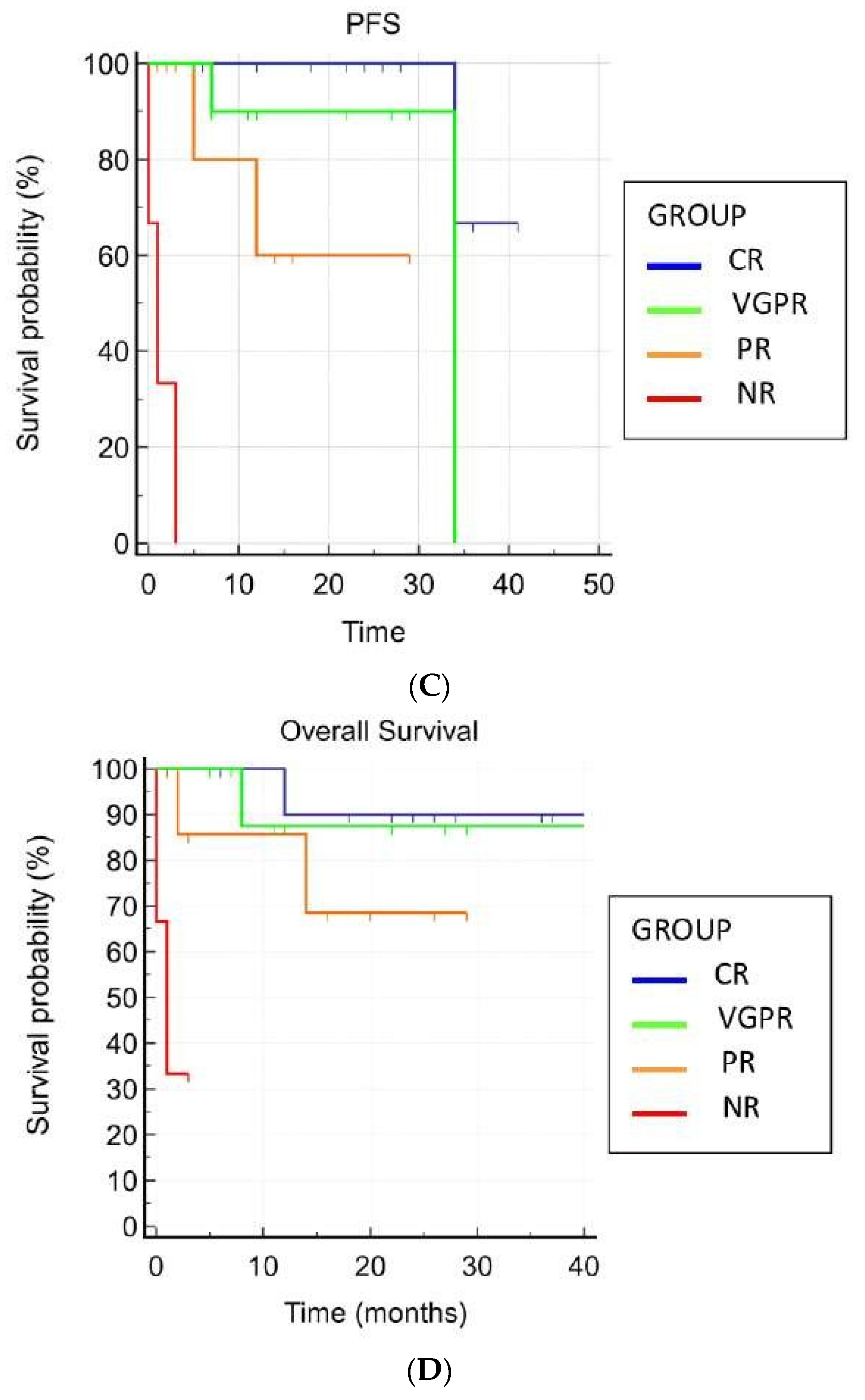
At the time of writing, no patient has been lost to follow-up. The median actual follow-up was 17 months (IQR, 1–34). Overall, six (18.1%) patients died, three of them due to disease progression and three due to SAEs. Five (15.2%) patients relapsed, and three (9.1%) patients were considered refractory to daratumumab. The mean OS was 34.9 months (95% CI, 29.25–40.69), whereas the mean PFS was 30.63 months (95% CI, 25.02–36.24). The median OS was not reached, whereas the median PFS estimate was not reached at 34 months (95% CI, 34–34). Achieving hematological response was the only statistically significant predictive factor for better OS and PFS (p = 0.0002 and p < 0.0001, respectively) (Figure 1).
Figure 1.
Overall and progression-free survivals. (A) Overall survival in all patients. (B) Progression-free survival in all patients. (C) Overall survival and hematological response. (D) Progression-free survival and hematological response.
4. Discussion
In this real-life multicenter study of the RTM, we analyzed the outcomes of 33 patients with AL amyloidosis treated with daratumumab as a monotherapy or in combination regimens. Efficacy was confirmed as in other previously reported trials (ORR rate of 90%), and we observed very good hematological responses in two-thirds of the patients, and more than 50% of them also obtained an organ response (55% cardiac, 61% renal). Response was also rapid with a median of 30 days, and the best response was reached at 4 months. Hematologic response is the first prerequisite for organ response [5]. In fact, the disappearance of the amyloidogenic clone should be the first goal of therapy in order to improve organ response and ultimately survival. In this view, the speed of response is also important. Although the number of patients was small, this study is important because we tested and confirmed the efficacy of the anti-CD38 monoclonal antibody daratumumab in AL Amyloidosis without significant toxicity related to the drug in a real-life population. In fact, clinical trial exclusion criteria can vary, thus encompassing a large population of patients. In the Andromeda study, for example, in which daratumumab was given with cyclophosphamide, bortezomib, and dexamethasone (CYBorDex) to patients with AL amyloidosis, exclusion criteria were: symptomatic MM, previous treatment, a poor performance status (ECOG > 2), an estimated glomerular filtration rate of less than 20 mL × 1.73 m2 of body surface area, and a IIIb or IV heart failure NYHA score. In clinical practice, those patients represent a considerable part of the AL amyloidosis patients, who require more attention and probably represent the real unmet need in this disease.
In a previously reported study [18], twenty-two patients were treated with Daratumumab monotherapy with a 96% ORR and a high rate of >VGPR. Roussel et al. [19] reported on 40 patients treated with daratumumab monotherapy, for which severe cardiac failure was an exclusion criterium, and patients were 75% ECOG 0–1. Responses were seen in 52% of the patients. The biggest studies were performed by Kimmich and Milani from Heidelberg and Pavia, respectively [22,24]. In the first, 168 patients were treated with Dara monotherapy (n = 106) or daratumumab–Velcade–dexamethasone (n = 62). Patients mostly presented MGUS or SMM, with symptomatic MM patients representing 9% of the study group. Severe renal failure was reported in 10% of the patients, and 30% had severe cardiac failure. The ORR was 66% with >VGPR in nearly 50% of the patients. Cardiac response was reported in 22% of patients, and nephrotic albuminuria was reported as a poor prognostic factor. Milani et al. reported on 72 patients treated with daratumumab monotherapy (n = 46) or with bortezomib or lenalidomide. Only 5% of the patients had severe renal failure, and none experienced symptomatic myeloma, although the median proportion of bone marrow plasma cells was 20%. The ORR was 83% with cardiac and renal responses in 29% and 60% of patients, respectively.
In the present study, about 15% of the patients had severe heart failure and advanced renal disease. Although the study used a small population of patients and was not statistically significant, half of these patients had a hematological response that translated to an organ response in 30%. This is particularly important for a group of poor-prognosis patients, who usually have less than 12 months of median overall survival.
Another interesting point is that almost half of the patients had symptomatic MM and underwent long-term therapy with daratumumab. Although the median follow-up was less than two years, efficacy was confirmed and seemed to increase over time for those patients responding to continuous therapy. It has been reported that symptomatic MM-associated AL amyloidosis has worst survival compared to the MGUS-related one [42]. This could be due to the higher tumor burden associated with MM, which can be accompanied by lytic lesions, hypercalcemia, and anemia. On the other hand, novel therapies led to higher remission rates and survival in MM as well. In our study, no particular differences were seen between the two populations of patients (MGUS vs. MM), probably because the higher tumor burden in MM responded well to the Dara combination therapy.
Toxicity was acceptable, with IRRs being more frequent than has been reported in other MM trials [43,44,45]. Certainly, the subcutaneous formulation will help to overcome this side effect. Deaths were related to organ failure after disease progression (one cardiac and one renal), and one was related to infection in an MM patient with advanced disease stage who had received two previous therapies, pointing out the importance of immune system frailty in myeloma. No data were collected in this study on minimal residual disease (MRD), although it seems to be an important tool in AL amyloidosis and has been shown in many MM studies to be an indicator of depth of response and, ultimately, survival [46,47,48,49,50].
5. Conclusions
Daratumumab is efficacious in MGUS-, SMM-, and MM- associated AL amyloidosis patients. Real-life studies are needed to confirm the efficacy and safety of the drug in pretreated patients with poor performance statuses as well.
Author Contributions
Conceptualization, A.G. and V.S.; methodology, E.A., G.B., M.L.D.G., I.A., S.C., M.T.P., F.B., V.C., L.P. and U.O.; validation, M.B.; writing—original draft preparation, A.G., V.S. and F.P.; writing—review and editing, A.G. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Area Vasta Sud-Est (protocol code N. 21372/2022 of 17/01/2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Palladini, G.; Milani, P.; Merlini, G. Management of AL amyloidosis in 2020. Blood 2020, 136, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Merlini, G.; Dispenzieri, A.; Sanchorawala, V.; Schönland, S.O.; Palladini, G.; Hawkins, P.N.; Gertz, M.A. Systemic immunoglobulin light chain amyloidosis. Nat. Rev. Dis. Prim. 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Pinney, J.H.; Smith, C.; Taube, J.B.; Lachmann, H.; Venner, C.P.; Gibbs, S.D.J.; Dungu, J.; Banypersad, S.M.; Wechalekar, A.D.; Whelan, C.J.; et al. Systemic Amyloidosis in England: An epidemiological study. Br. J. Haematol. 2013, 161, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, E.; Dispenzieri, A.; Kumar, S.K.; Ketterling, R.P.; Dingli, D.; Lacy, M.Q.; Buadi, F.K.; Hayman, S.R.; Kapoor, P.; Leung, N.; et al. Interphase fluorescence in situ hybridization in untreated AL amyloidosis has an independent prognostic impact by abnormality type and treatment category. Leukemia 2017, 31, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A. Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. Am. J. Hematol. 2020, 95, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Colby, C.; Laumann, K.; Zeldenrust, S.R.; Leung, N.; Dingli, D.; et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J. Clin. Oncol. 2012, 30, 989–995. [Google Scholar] [CrossRef] [Green Version]
- Palladini, G.; Hegenbart, U.; Milani, P.; Kimmich, C.; Foli, A.; Ho, A.D.; Rosin, M.V.; Albertini, R.; Moratti, R.; Merlini, G.; et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood 2014, 124, 2325–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palladini, G.; Sachchithanantham, S.; Milani, P.; Gillmore, J.; Foli, A.; Lachmann, H.; Basset, M.; Hawkins, P.; Merlini, G.; Wechalekar, A.D. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood 2015, 126, 612–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchorawala, V.; Sun, F.; Quillen, K.; Sloan, J.M.; Berk, J.L.; Seldin, D.C. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood 2015, 126, 2345–2347. [Google Scholar] [CrossRef] [Green Version]
- Sanchorawala, V.; Wright, D.G.; Rosenzweig, M.; Finn, K.T.; Fennessey, S.; Zeldis, J.B.; Skinner, M.; Seldin, D.C. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: Results of a phase 2 trial. Blood 2007, 109, 492–496. [Google Scholar] [CrossRef]
- Sanchorawala, V.; Shelton, A.C.; Lo, S.; Varga, C.; Sloan, J.M.; Seldin, D.C. Pomalidomide and dexamethasone in the treatment of AL amyloidosis: Results of a phase 1 and 2 trial. Blood 2016, 128, 1059–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malavasi, F.; Funaro, A.; Roggero, S.; Horenstein, A.; Calosso, L.; Mehta, K. Human CD38: A glycoprotein in search of a function. Immunol. Today 1994, 15, 95–97. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef] [Green Version]
- Gozzetti, A.; Candi, V.; Papini, G.; Bocchia, M. Therapeutic Advancements in Multiple Myeloma. Front. Oncol. 2014, 4, 241. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, S.R.; Foley, N.; Schroeder, M.A. Daratumumab for the treatment of multiple myeloma. Drugs Today 2021, 57, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Milani, P.; Malavasi, F.; Merlini, G. Daratumumab in the Treatment of Light-Chain (AL) Amyloidosis. Cells 2021, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Palladini, G.; Minnema, M.C.; Wechalekar, A.D.; Jaccard, A.; Lee, H.C.; Sanchorawala, V.; Gibbs, S.; Mollee, P.; Venner, C.P.; et al. Daratumumab-Based Treatment for Immunoglobulin Light-Chain Amyloidosis. N. Engl. J. Med. 2021, 385, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Sanchorawala, V.; Sarosiek, S.; Schulman, A.; Mistark, M.; Migre, M.E.; Cruz, R.; Sloan, J.M.; Brauneis, D.; Shelton, A.C. Safety, tolerability, and response rates of daratumumab in relapsed AL amyloidosis: Results of a phase 2 study. Blood 2020, 135, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.; Merlini, G.; Chevret, S.; Arnulf, B.; Stoppa, A.M.; Perrot, A.; Palladini, G.; Karlin, L.; Royer, B.; Huart, A.; et al. A prospective phase 2 trial of daratumumab in patients with previously treated systemic light-chain amyloidosis. Blood 2020, 135, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, G.P.; Schrier, S.L.; Lafayette, R.A.; Arai, S.; Witteles, R.M.; Liedtke, M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood 2017, 130, 900–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeykoon, J.P.; Zanwar, S.; Dispenzieri, A.; Gertz, M.A.; Leung, N.; Kourelis, T.; Gonsalves, W.; Muchtar, E.; Dingli, D.; Lacy, M.Q.; et al. Daratumumab-based therapy in patients with heavily-pretreated AL amyloidosis. Leukemia 2019, 33, 531–536. [Google Scholar] [CrossRef]
- Milani, P.; Fazio, F.; Basset, M.; Berno, T.; LaRocca, A.; Foli, A.; Riva, M.; Benigna, F.; Oliva, S.; Nuvolone, M.; et al. High rate of profound clonal and renal responses with daratumumab treatment in heavily pre-treated patients with light chain (AL) amyloidosis and high bone marrow plasma cell infiltrate. Am. J. Hematol. 2020, 95, 900–905. [Google Scholar] [CrossRef]
- Lecumberri, R.; Krsnik, I.; Askari, E.; Sirvent, M.; González-Pérez, M.S.; Escalante, F.; Pradillo, V.; Tamariz, L.E.; Cánovas, V.; Alegre, A.; et al. Treatment with daratumumab in patients with relapsed/refractory AL amyloidosis: A multicentric retrospective study and review of the literature. Amyloid 2020, 27, 163–167. [Google Scholar] [CrossRef]
- Kimmich, C.R.; Terzer, T.; Benner, A.; Dittrich, T.; Veelken, K.; Carpinteiro, A.; Hansen, T.; Goldschmidt, H.; Seckinger, A.; Hose, D.; et al. Daratumumab for systemic AL amyloidosis: Prognostic factors and adverse outcome with nephrotic-range albuminuria. Blood 2020, 135, 1517–1530. [Google Scholar] [CrossRef]
- Cohen, O.C.; Brodermann, M.H.; Blakeney, I.J.; Mahmood, S.; Sachchithanantham, S.; Ravichandran, S.; Law, S.; Lachmann, H.J.; Whelan, C.J.; Popat, R.; et al. Rapid response to single agent daratumumab is associated with improved progression-free survival in relapsed/refractory AL amyloidosis. Amyloid 2020, 27, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Khouri, J.; Kin, A.; Thapa, B.; Reu, F.; Bumma, N.; Samaras, C.J.; Liu, H.D.; Karam, M.A.; Reed, J.; Mathur, S.; et al. Daratumumab proves safe and highly effective in AL amyloidosis. Br. J. Haematol. 2019, 185, 342–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roccatello, D.; Fenoglio, R.; Naretto, C.; Baldovino, S.; Sciascia, S.; Ferro, M.; Rossi, D. Daratumumab Monotherapy in Severe Patients with AL Amyloidosis and Biopsy-Proven Renal Involvement: A Real Life Experience. J. Clin. Med. 2020, 9, 3232. [Google Scholar] [CrossRef] [PubMed]
- Shragai, T.; Gatt, M.; Lavie, N.; Vaxman, I.; Tadmor, T.; Rouvio, O.; Zektser, M.; Horowitz, N.; Magen, H.; Ballan, M.; et al. Daratumumab for relapsed AL amyloidosis—When cumulative real-world data precedes clinical trials: A multisite study and systematic literature review. Eur. J. Haematol. 2021, 106, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Sher, T.; Fenton, B.; Akhtar, A.; Gertz, M.A. First report of safety and efficacy of daratumumab in 2 cases of advanced immunoglobulin light chain amyloidosis. Blood 2016, 128, 1987–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gran, C.; Gahrton, G.; Alici, E.; Nahi, H. Case Report: Treatment of light-chain amyloidosis with daratumumab monotherapy in two patients. Eur. J. Haematol. 2018, 100, 386–388. [Google Scholar] [CrossRef]
- Arnall, J.R.; Usmani, S.Z.; Adamu, H.; Mishkin, J.; Bhutani, M. Daratumumab, pomalidomide, and dexamethasone as a bridging therapy to autologous stem cell transplantation in a case of systemic light-chain amyloidosis with advanced cardiac involvement. J. Oncol. Pharm. Pract. 2019, 25, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Canichella, M.; Serrao, A.; Annechini, G.; D’Elia, G.M.; De Luca, M.L.; Pulsoni, A. Long-term response to daratumumab in a patient with advanced immunoglobulin light-chain (AL) amyloidosis with organ damage. Ann. Hematol. 2019, 98, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.X.; Zhou, P.; Varga, C.; Fogaren, T.; Ho, K.; Ma, X.; Warner, M.; Toskic, D.; Kugelmass, A.; Comenzo, R.L. Daratumumab activity in relapsed or primary refractory systemic AL amyloidosis and Fcγ receptor 3A V158F polymorphisms. Amyloid 2019, 26, 101–102. [Google Scholar]
- Ghilardi, G.; Stussi, G.; Mazzucchelli, L.; Röcken, C.; Rossi, D.; Gerber, B. Venetoclax plus daratumumab induce hematological CR and organ response in an AL amyloidosis patient with t(11;14). Amyloid 2019, 26, 173–174. [Google Scholar] [CrossRef]
- Schwotzer, R.; Manz, M.G.; Pederiva, S.; Waibel, C.; Caspar, C.; Lerch, E.; Flammer, A.J.; Brouwers, S.; Seeger, H.; Heimgartner, R.; et al. Daratumumab for relapsed or refractory AL amyloidosis with high plasma cell burden. Hematol. Oncol. 2019, 37, 595–600. [Google Scholar] [CrossRef]
- Van de Wyngaert, Z.; Carpentier, B.; Pascal, L.; Lionne-Huyghe, P.; Leduc, I.; Srour, M.; Vasseur, M.; Demarquette, H.; Terriou, L.; Herbaux, C.; et al. Daratumumab is effective in the relapsed or refractory systemic light-chain amyloidosis but associated with high infection burden in a frail real-life population. Br. J. Haematol. 2020, 188, e24–e27. [Google Scholar] [CrossRef] [PubMed]
- Godara, A.; Siddiqui, N.S.; Lee, L.X.; Toskic, D.; Fogaren, T.; Varga, C.; Comenzo, R.L. Dual Monoclonal Antibody Therapy in Patients With Systemic AL Amyloidosis and Cardiac Involvement. Clin. Lymphoma Myeloma Leuk. 2020, 20, 184–189. [Google Scholar] [CrossRef]
- Chung, A.; Kaufman, G.P.; Sidana, S.; Eckhert, E.; Schrier, S.L.; Lafayette, R.A.; Arai, S.; Witteles, R.M.; Liedtke, M. Organ responses with daratumumab therapy in previously treated AL amyloidosis. Blood Adv. 2020, 4, 458–466. [Google Scholar] [CrossRef]
- Gozzetti, A.; Le Beau, M.M. Fluorescence in situ hybridization: Uses and limitations. Semin Hematol. 2000, 37, 320–333. [Google Scholar] [CrossRef]
- Palladini, G.; Schönland, S.O.; Sanchorawala, V.; Kumar, S.; Wechalekar, A.; Hegenbart, U.; Milani, P.; Ando, Y.; Westermark, P.; Dispenzieri, A.; et al. Clarification on the definition of complete haematologic response in light-chain (AL) amyloidosis. Amyloid 2021, 28, 1–2. [Google Scholar] [CrossRef]
- Palladini, G.; Merlini, G. When should treatment of AL amyloidosis start at relapse? Early, to prevent organ progression. Blood Adv. 2019, 3, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Muchtar, E.; Gertz, M.A.; Kourelis, T.V.; Sidana, S.; Go, R.S.; Lacy, M.Q.; Buadi, F.K.; Dingli, D.; Hayman, S.R.; Kapoor, P.; et al. Bone marrow plasma cells 20% or greater discriminate presentation, response, and survival in AL amyloidosis. Leukemia 2020, 34, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Mateos, M.V.; Nahi, H.; Legiec, W.; Grosicki, S.; Vorobyev, V.; Spicka, I.; Hungria, V.; Korenkova, S.; Bahlis, N.; Flogegard, M.; et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): A multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020, 7, e370–e380. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Nahi, H.; Mateos, M.-V.; Van De Donk, N.W.C.J.; Chari, A.; Kaufman, J.; Moreau, P.; Oriol, A.; Plesner, T.; Benboubker, L.; et al. Subcutaneous delivery of daratumumab in relapsed or refractory multiple myeloma. Blood 2019, 134, 668–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozzetti, A.; Bacchiarri, F.; Sammartano, V.; Defina, M.; Sicuranza, A.; Mecacci, B.; Zappone, E.; Cencini, E.; Fabbri, A.; Raspadori, D.; et al. Long-Term Safety of Rapid Daratumumab Infusions in Multiple Myeloma Patients. Front. Oncol. 2020, 10, 570187. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.A.; Guillen-Grima, F.; Moreno, C.; Panizo, C.; Pérez-Robles, C.; Mata-Molanes, J.J.; Moreno, L.; Arana, P.; Chocarro, S.; Merino, J. A simple flow-cytometry method to evaluate peripheral blood contamination of bone marrow aspirates. J. Immunol. Methods 2017, 442, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Loken, M.R.; Chu, S.-C.; Fritschle, W.; Kalnoski, M.; Wells, D.A. Normalization of bone marrow aspirates for hemodilution in flow cytometric analyses. Cytom. B Clin. Cytom. 2008, 76, 27–36. [Google Scholar] [CrossRef]
- Lionetti, M.; Neri, A. Utilizing next-generation sequencing in the management of multiple myeloma. Expert Rev. Mol. Diagn. 2017, 17, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Orfao, A.; Chim, C. Molecular detection of minimal residual disease in multiple myeloma. Br. J. Haematol. 2018, 181, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozzetti, A.; Raspadori, D.; Bacchiarri, F.; Sicuranza, A.; Pacelli, P.; Ferrigno, I.; Tocci, D.; Bocchia, M. Minimal Residual Disease in Multiple Myeloma: State of the Art and Applications in Clinical Practice. J. Pers. Med. 2020, 10, 120. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).