Promising Effects of Digital Chest Tube Drainage System for Pulmonary Resection: A Systematic Review and Network Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Bias Assessment
2.4. Outcome Measures
2.5. Data Synthesis and Statistical Analysis
2.6. Quality Assessment
3. Results
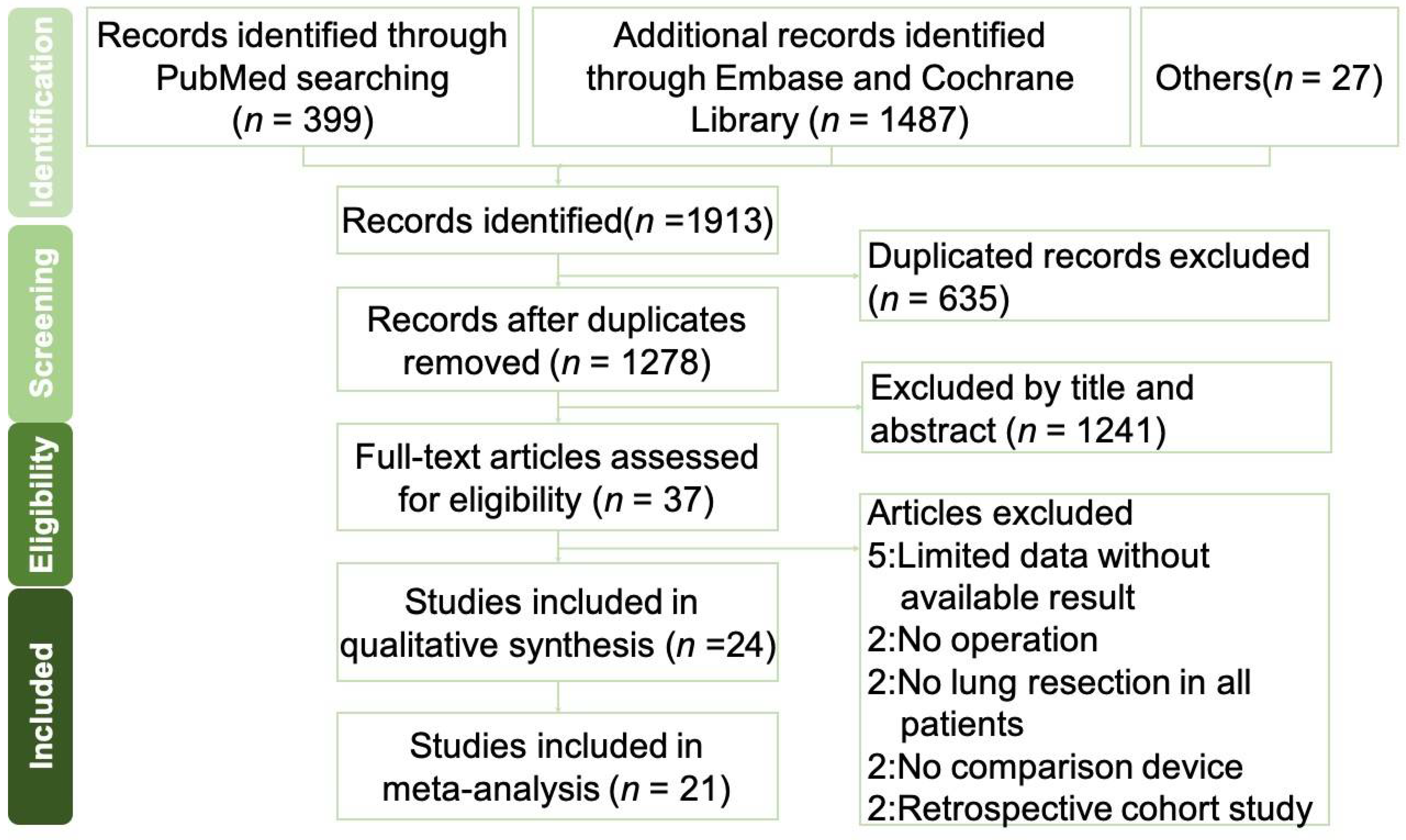
3.1. Systematic Literature Review
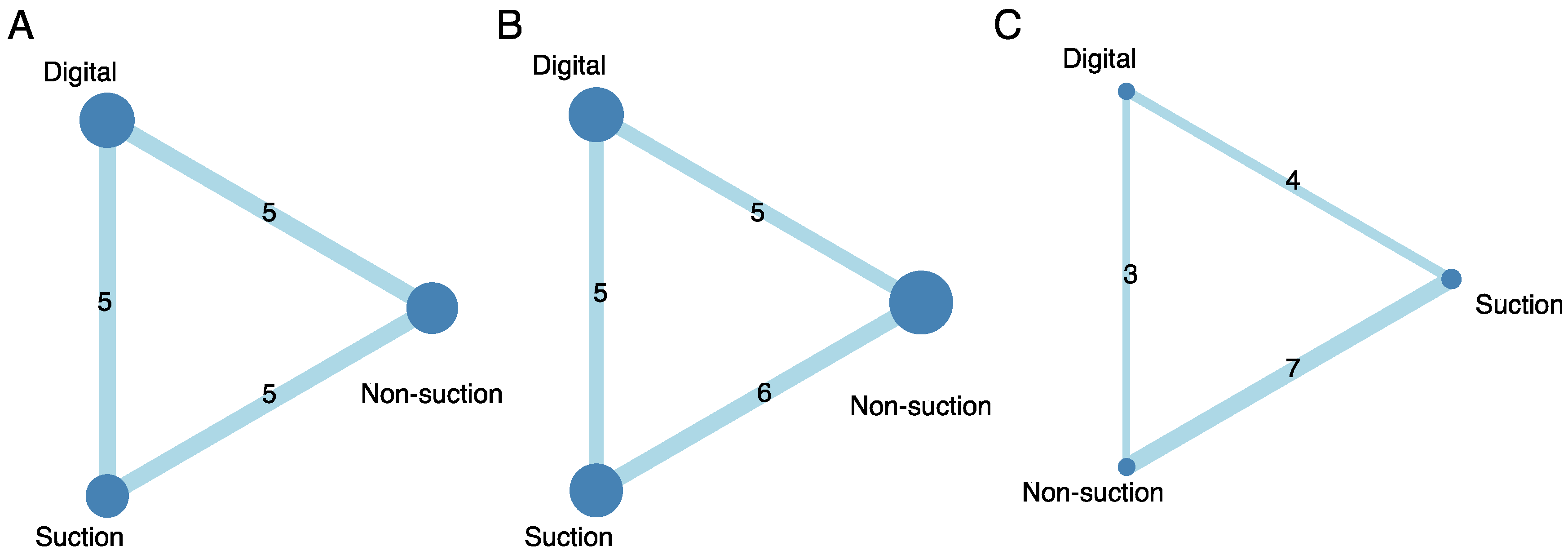
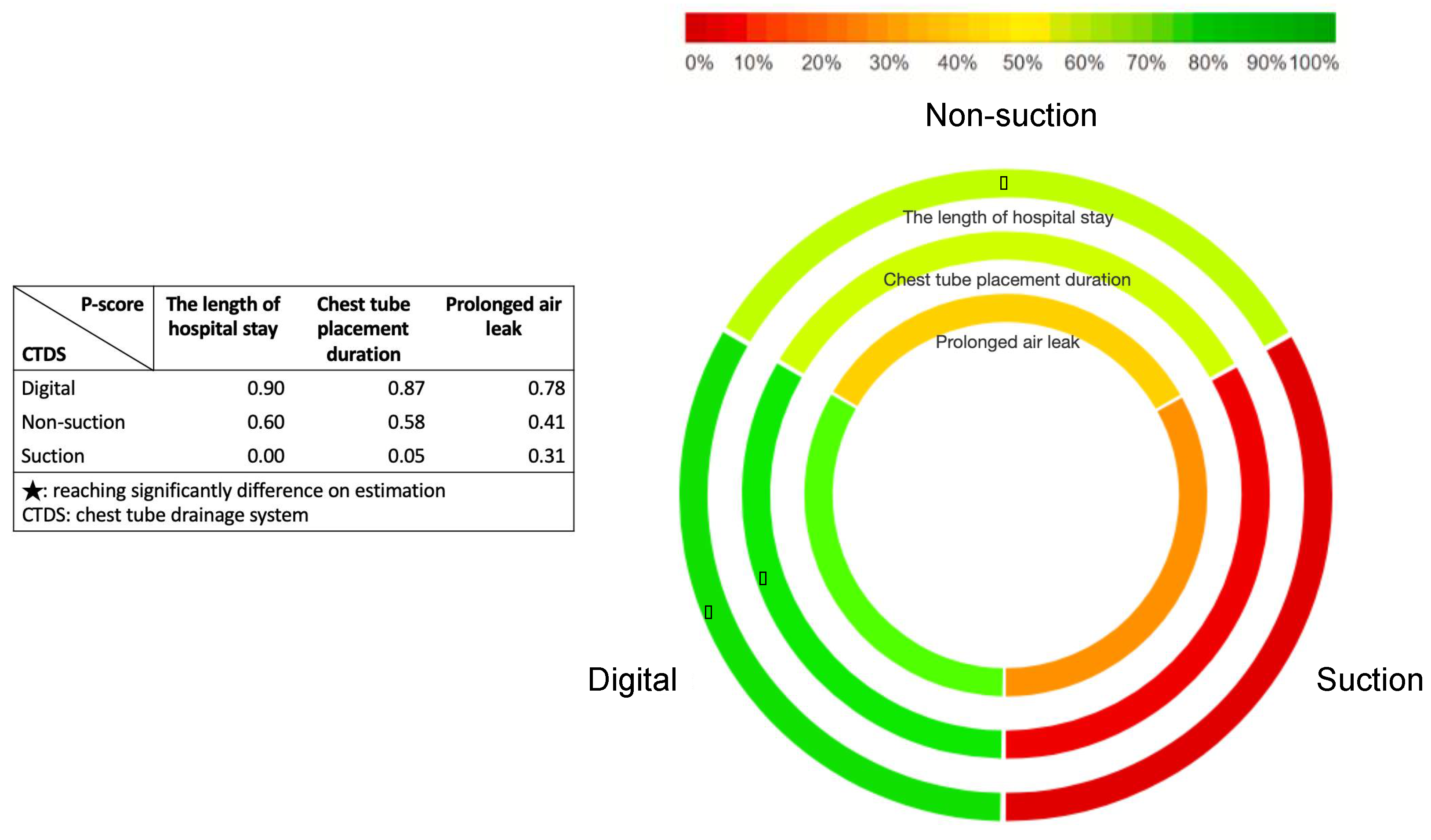
3.2. Results of Hospital Stay Length
3.3. Results of Chest Tube Placement Duration
3.4. Results of Prolonged Air Leak
3.5. Inconsistency, Risk of Bias, and Publication Bias
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lilienthal, H. Resection of the Lung for Suppurative Infections with a Report Based on 31 Operative Cases in which Resection Was Done or Intended. Ann. Surg. 1922, 75, 257–320. [Google Scholar] [CrossRef]
- Toth, J.W.; Reed, M.F.; Ventola, L.K. Chest Tube Drainage Devices. Semin. Respir. Crit. Care Med. 2019, 40, 386–393. [Google Scholar] [CrossRef]
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.; Gonzalez, M.; Ljungqvist, O.; Petersen, R.H.; Popescu, W.M.; Slinger, P.D.; et al. Guidelines for enhanced recovery after lung surgery: Recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardio-Thorac. Surg. 2018, 55, 91–115. [Google Scholar] [CrossRef]
- Attaar, A.; Tam, V.; Nason, K.S. Risk Factors for Prolonged Air Leak after Pulmonary Resection: A Systematic Review and Meta-analysis. Ann. Surg. 2020, 271, 834–844. [Google Scholar] [CrossRef]
- Varela, G.; Jiménez, M.F.; Novoa, N.M.; Aranda, J.L. Postoperative chest tube management: Measuring air leak using an electronic device decreases variability in the clinical practice. Eur. J. Cardio-Thorac. Surg. 2009, 35, 28–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baringer, K.; Talbert, S. Chest drainage systems and management of air leaks after a pulmonary resection. J. Thorac. Dis. 2017, 9, 5399–5403. [Google Scholar] [CrossRef] [Green Version]
- Cerfolio, R.J.; Bryant, A. The benefits of continuous and digital air leak assessment after elective pulmonary resection: A prospective study. Ann. Thorac. Surg. 2008, 86, 396–401. [Google Scholar] [CrossRef]
- Brunelli, A.; Salati, M.; Refai, M.; Di Nunzio, L.; Xiumé, F.; Sabbatini, A. Evaluation of a new chest tube removal protocol using digital air leak monitoring after lobectomy: A prospective randomised trial. Eur. J. Cardio-Thorac. Surg. 2010, 37, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Filosso, P.L.; Ruffini, E.; Solidoro, P.; Molinatti, M.; Bruna, M.C.; Oliaro, A. Digital air leak monitoring after lobectomy for primary lung cancer in patients with moderate COPD: Can a fast-tracking algorithm reduce postoperative costs and complications? J. Cardiovasc. Surg. 2010, 51, 429–433. [Google Scholar]
- Pompili, C.; Detterbeck, F.; Papagiannopoulos, K.; Sihoe, A.; Vachlas, K.; Maxfield, M.W.; Lim, H.C.; Brunelli, A. Multicenter International Randomized Comparison of Objective and Subjective Outcomes between Electronic and Traditional Chest Drainage Systems. Ann. Thorac. Surg. 2014, 98, 490–497, discussion 496–497. [Google Scholar] [CrossRef]
- Marshall, M.B.; Deeb, M.E.; Bleier, J.I.; Kucharczuk, J.C.; Friedberg, J.S.; Kaiser, L.R.; Shrager, J.B. Suction vs. water seal after pulmonary resection: A randomized prospective study. Chest 2002, 121, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Ayed, A.K. Suction versus water seal after thoracoscopy for primary spontaneous pneumothorax: Prospective randomized study. Ann. Thorac. Surg. 2003, 75, 1593–1596. [Google Scholar] [CrossRef]
- Brunelli, A.; Monteverde, M.; Borri, A.; Salati, M.; Marasco, R.D.; Al Refai, M.; Fianchini, A. Comparison of water seal and suction after pulmonary lobectomy: A prospective, randomized trial. Ann. Thorac. Surg. 2004, 77, 1932–1937, discussion 1937. [Google Scholar] [CrossRef]
- Alphonso, N.; Utley, M.; Cameron, R.; Dussek, J.; Lang-Lazdunski, L.; Tan, C.; Treasure, T. A prospective randomized controlled trial of suction versus non-suction to the under-water seal drains following lung resection. Eur. J. Cardio-Thorac. Surg. 2005, 27, 391–394. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, A.; Sabbatini, A.; Al Refai, M.; Salati, M.; Marasco, R. Alternate suction reduces prolonged air leak after pulmonary lobectomy: A randomized comparison versus water seal. Ann. Thorac. Surg. 2005, 80, 1052–1055. [Google Scholar] [CrossRef]
- Kakhki, A.; Pooya, M.; Pejhan, S.; Javaherzadeh, M.; Arab, M.; Shadmehr, M.B.; Abbasi, A. Effect of chest tube suction on air-leak following lung resection. Tanaffos 2006, 5, 37–43. [Google Scholar]
- Prokakis, C.; Koletsis, E.N.; Apostolakis, E.; Panagopoulos, N.; Kouki, H.S.; Sakellaropoulos, G.C.; Filos, K.; Dougenis, D.V. Routine Suction of Intercostal Drains Is Not Necessary After Lobectomy: A Prospective Randomized Trial. World J. Surg. 2008, 32, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Bertolaccini, L.; Rizzardi, G.; Filice, M.J.; Terzi, A. ‘Six Sigma approach’—An objective strategy in digital assessment of postoperative air leaks: A prospective randomised study. Eur. J. Cardio-Thoracic Surg. 2011, 39, e128–e132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunelli, A.; Salati, M.; Pompili, C.; Refai, M.; Sabbatini, A. Regulated tailored suction vs. regulated seal: A prospective randomized trial on air leak duration†. Eur. J. Cardio-Thorac. Surg. 2013, 43, 899–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leo, F.; Duranti, L.; Girelli, L.; Furia, S.; Billè, A.; Garofalo, G.; Scanagatta, P.; Giovannetti, R.; Pastorino, U. Does External Pleural Suction Reduce Prolonged Air Leak after Lung Resection? Results from the AirINTrial after 500 Randomized Cases. Ann. Thorac. Surg. 2013, 96, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Marjanski, T.; Sternau, A.; Rzyman, W. THORACIC SURGERY The implementation of a digital chest drainage system significantly reduces complication rates after lobectomy—A randomized clinical trial. Pol. J. Cardio-Thorac. Surg. 2013, 2, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, S.; McGuire, A.L.; Maghera, S.; Sundaresan, S.R.; Seely, A.J.; Maziak, D.E.; Shamji, F.M.; Villeneuve, P.J. Randomized trial of digital versus analog pleural drainage in patients with or without a pulmonary air leak after lung resection. J. Thorac. Cardiovasc. Surg. 2015, 150, 1243–1251. [Google Scholar] [CrossRef] [Green Version]
- Lijkendijk, M.; Licht, P.B.; Neckelmann, K. Electronic versus traditional chest tube drainage following lobectomy: A randomized trial. Eur. J. Cardio-Thorac. Surg. 2015, 48, 893–898, discussion 898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gocyk, W.; Kużdżał, J.; Włodarczyk, J.; Grochowski, Z.; Gil, T.; Warmus, J.; Kocoń, P.; Talar, P.; Obarski, P.; Trybalski, Ł. Comparison of Suction Versus Nonsuction Drainage After Lung Resections: A Prospective Randomized Trial. Ann. Thorac. Surg. 2016, 102, 1119–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lococo, F.; Nachira, D.; Ciavarella, L.P.; Congedo, M.T.; Porziella, V.; Meacci, E.; Margaritora, S.; Chiappetta, M. Digital Devices Improve Chest Tube Management: Results from a Prospective Randomized Trial. Thorac. Cardiovasc. Surg. 2017, 66, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Plourde, M.; Jad, A.; Dorn, P.; Harris, K.; Mujoomdar, A.; Henteleff, H.; French, D.; Bethune, E. Digital Air Leak Monitoring for Lung Resection Patients: A Randomized Controlled Clinical Trial. Ann. Thorac. Surg. 2018, 106, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Takamochi, K.; Nojiri, S.; Oh, S.; Matsunaga, T.; Imashimizu, K.; Fukui, M.; Suzuki, K. Comparison of digital and traditional thoracic drainage systems for postoperative chest tube management after pulmonary resection: A prospective randomized trial. J. Thorac. Cardiovasc. Surg. 2018, 155, 1834–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlin, S.; Emmerton-Coughlin, H.; Malthaner, R. Management of chest tubes after pulmonary resection: A systematic review and meta-analysis. Can. J. Surg. 2012, 55, 264–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, B.; Qian, K.; Zhou, J.-H.; Tan, Q.-Y.; Wang, R.-W. Optimization of Chest Tube Management to Expedite Rehabilitation of Lung Cancer Patients after Video-Assisted Thoracic Surgery: A Meta-Analysis and Systematic Review. World J. Surg. 2017, 41, 2039–2045. [Google Scholar] [CrossRef]
- Zhou, J.; Lyu, M.; Chen, N.; Wang, Z.; Hai, Y.; Hao, J.; Liu, L. Digital chest drainage is better than traditional chest drainage following pulmonary surgery: A meta-analysis. Eur. J. Cardio-Thorac. Surg. 2018, 54, 635–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Hu, W.; Ma, L.; Zhang, Y. Digital chest drainage system versus traditional chest drainage system after pulmonary resection: A systematic review and meta-analysis. J. Cardiothorac. Surg. 2019, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, N.; Hai, Y.; Lyu, M.; Wang, Z.; Gao, Y.; Pang, L.; Liao, H.; Liu, L. External suction versus simple water-seal on chest drainage following pulmonary surgery: An updated meta-analysis. Interact. Cardiovasc. Thorac. Surg. 2018, 28, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]; The Cochrane Collaboration: London, UK, 2011; Available online: www.handbook.cochrane.org (accessed on 1 February 2021).
- Stéphan, F.; Boucheseiche, S.; Hollande, J.; Flahault, A.; Cheffi, A.; Bazelly, B.; Bonnet, F. Pulmonary complications following lung resection: A comprehensive analysis of incidence and possible risk factors. Chest 2000, 118, 1263–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salanti, G.; Higgins, J.P.; Ades, A.; Ioannidis, J.P. Evaluation of networks of randomized trials. Stat. Methods Med. Res. 2008, 17, 279–301. [Google Scholar] [CrossRef] [PubMed]
- van Valkenhoef, G.; Lu, G.; de Brock, B.; Hillege, H.; Ades, A.E.; Welton, N.J. Automating network meta-analysis. Res. Synth. Methods 2012, 3, 285–299. [Google Scholar] [CrossRef]
- Rücker, G. Network meta-analysis, electrical networks and graph theory. Res. Synth. Methods 2012, 3, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Krahn, U.; Binder, H.; König, J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med. Res. Methodol. 2013, 13, 35. [Google Scholar] [CrossRef] [Green Version]
- Chaimani, A.; Higgins, J.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef]
- Jansen, J.P.; Trikalinos, T.; Cappelleri, J.C.; Daw, J.; Andes, S.; Eldessouki, R.; Salanti, G. Indirect Treatment Comparison/Network Meta-Analysis Study Questionnaire to Assess Relevance and Credibility to Inform Health Care Decision Making: An ISPOR-AMCP-NPC Good Practice Task Force Report. Value Health 2014, 17, 157–173. [Google Scholar] [CrossRef] [Green Version]
- Salanti, G.; Del Giovane, C.; Chaimani, A.; Caldwell, D.M.; Higgins, J.P.T. Evaluating the Quality of Evidence from a Network Meta-Analysis. PLoS ONE 2014, 9, e99682. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Jackson, D.; White, I.; Riley, R.D. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat. Med. 2012, 31, 3805–3820. [Google Scholar] [CrossRef] [Green Version]
- Veroniki, A.A.; Bender, R.; Glasziou, P.; Straus, S.E.; Tricco, A.C. The number needed to treat in pairwise and network meta-analysis and its graphical representation. J. Clin. Epidemiol. 2019, 111, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Dias, S.; Welton, N.J.; Sutton, A.J.; Caldwell, D.M.; Lu, G.; Ades, A.E. Evidence synthesis for decision making 4: Inconsistency in networks of evidence based on randomized controlled trials. Med. Decis. Mak. 2013, 33, 641–656. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Jackson, D.; Barrett, J.K.; Lu, G.; Ades, A.E.; White, I.R. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods 2012, 3, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Dias, S.; Welton, N.J.; Caldwell, D.M.; Ades, A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010, 29, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-K. Node-Splitting Generalized Linear Mixed Models for Evaluation of Inconsistency in Network Meta-Analysis. Value Health 2016, 19, 957–963. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puhan, M.A.; Schünemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, A.; Rodrigues, J.; Kavanagh, J.; Batchelor, T.; Lyen, S. Postoperative complications of pulmonary resection. Clin. Radiol. 2020, 75, 876.e1–876.e15. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-Y.; Hsu, P.-K.; Huang, C.-S.; Wu, Y.-C.; Hsu, H.-S. Complications after Chest Tube Removal and Reinterventions in Patients with Digital Drainage Systems. J. Clin. Med. 2019, 8, 2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruigrok, D.; Kunst, P.W.A.; Blacha, M.M.J.; Tomlow, B.; Herbrink, J.W.; Japenga, E.J.; Boersma, W.; Bresser, P.; van der Lee, I.; Mooren, K. Digital versus analogue chest drainage system in patients with primary spontaneous pneumothorax: A randomized controlled trial. BMC Pulm. Med. 2020, 20, 136. [Google Scholar] [CrossRef] [PubMed]
- Ades, A.E.; Sculpher, M.; Sutton, A.; Abrams, K.; Cooper, N.; Welton, N.; Lu, G. Bayesian Methods for Evidence Synthesis in Cost-Effectiveness Analysis. Pharmacoeconomics 2006, 24, 1–19. [Google Scholar] [CrossRef]
- Song, F.; Altman, D.G.; Glenny, A.-M.; Deeks, J. Validity of indirect comparison for estimating efficacy of competing interventions: Empirical evidence from published meta-analyses. BMJ 2003, 326, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, A.; Ades, A.E.; Cooper, N.; Abrams, K. Use of Indirect and Mixed Treatment Comparisons for Technology Assessment. Pharmacoeconomics 2008, 26, 753–767. [Google Scholar] [CrossRef]
- Mansournia, M.A.; Higgins, J.P.; Sterne, J.A.; Hernán, M.A. Biases in Randomized Trials: A Conversation between Trialists and Epidemiologists. Epidemiology 2017, 28, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.N.; Cosgrove, A.P.; Gibbons, J.R.; A McGuigan, J. Randomised clinical trial of chest drainage systems. Thorax 1992, 47, 461–462. [Google Scholar] [CrossRef] [Green Version]
- Waldhausen, J.H.; A Cusick, R.; Graham, D.; Pittinger, T.P.; Sawin, R.S. Removal of chest tubes in children without water seal after elective thoracic procedures: A randomized prospective study. J. Am. Coll. Surg. 2002, 194, 411–415. [Google Scholar] [CrossRef]
- Vuorisalo, S.; Aarnio, P.; Hannukainen, J. Comparison between Flutter Valve Drainage Bag and Underwater Seal Device for Pleural Drainage after Lung Surgery. Scand. J. Surg. 2005, 94, 56–58. [Google Scholar] [CrossRef] [Green Version]
- Lijkendijk, M.; Neckelmann, K.; Licht, P. Digital versus analogue chest tube drainage following lobectomy: A randomised trial. Interact. Cardiovasc. Thorac. Surg. 2014, 19. [Google Scholar] [CrossRef] [Green Version]
- De Waele, M.; Agzarian, J.; Hanna, W.C.; Schieman, C.; Finley, C.J.; Macri, J.; Shargall, Y. Does the usage of digital chest drainage systems reduce pleural inflammation and volume of pleural effusion following oncologic pulmonary resection?—A prospective randomized trial. J. Thorac. Dis. 2017, 9, 1598–1606. [Google Scholar] [CrossRef] [Green Version]
- Mier, J.M.; Cortés-Julián, G.; Berrios-Mejía, J.; Víctor-Valdivia, Z. The benefits of digital chest drainage in pleural decortication in thoracic empyema. Prospective, randomized, control trial. Cir. Cir. 2017, 85, 522–525. [Google Scholar]
- Barozzi, L.; Biagio, L.S.; Meneguzzi, M.; Courvoisier, D.; Cregan, I.; Walpoth, B.; Faggian, G. Do We Still Need Wall Suction for Chest Drainage? Heart Lung Circulation 2018, 27, S502. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, Y.; Xu, C.; Ding, C.; Chen, J.; Li, C.; Zhao, J. Comparison of the results of two chest tube managements during an enhanced recovery program after video-assisted thoracoscopic lobectomy: A randomized trial. Thorac. Cancer 2019, 10, 1993–1999. [Google Scholar] [CrossRef]
- Marulli, G.; Comacchio, G.M.; Nosotti, M.; Rosso, L.; Mendogni, P.; Natale, G.; Andriolo, L.; Imbriglio, G.; LaRocca, V.; Brascia, D.; et al. Multicenter randomized study on the comparison between electronic and traditional chest drainage systems. Trials 2019, 20, 1–8. [Google Scholar] [CrossRef]

| Author, Year | Patient Number | Gender (Male/ Female%) | Age (Mean ±SD) | Comorbidities (Number) | Surgical Indication | Surgical Approach | Size of Drain | Resection Type | Reported Incidence of Adverse Events (%) and Associated Items |
|---|---|---|---|---|---|---|---|---|---|
| Marshall 2002 [11] | 68 | M: 49% F: 51% | 63.4 ± 2.8 | NR | Benign and malignant lung tumors | NR | NR | NR | NR |
| Ayed 2003 [12] | 100 | M: 94% F: 6% | 23.0 ± 3.7 | Patients with underlying lung disease were excluded. | Primary spontaneous pneumothorax | VATS: 100% Thoracotomy: 0% | 28 Fr. | Wedge resection: 100% | NR |
| Brunelli 2004 [13] | 145 | M: 80.69% F: 19.31% | 68.4 ± 9.2 | NR | Nonsmall cell carcinoma. | VATS: 0% Thoracotomy: 100% | 28 Fr. | Lobectomy or bilobectomy: 100% | 24.83% (Atelectasis requiring bronchoscopy, pneumonia, pulmonary edema, adult respiratory distress syndrome, pulmonary embolism, pleural empyema, cardiac failure, arrhythmia requiring medical treatment, myocardial infarction, acute renal failure, and stroke.) |
| Alphonso 2005 [14] | 254 | M: 61.51% F: 38.49% | 57.5 ± NR | Previous pneumothorax(71) | NR | VATS: 42.26% Thoracotomy: 57.74% | NR | Lobectomy: 46.44% Wedge resection: 44.77% Lung biopsy: 8.79% | NR |
| Brunelli 2005 [15] | 94 | M: 76.60% F: 23.40% | 66.7 ± 10.1 | NR | Nonsmall cell carcinoma. | VATS: 0% Thoracotomy: 100% | 28 Fr. | Bilobectomy: 9.57% Lobectomy: 90.43% | 24.47% (Atelectasis requiring bronchoscopy, pneumonia, pulmonary edema, adult respiratory distress syndrome, pulmonary embolism, pleural empyema, cardiac failure, arrhythmia requiring medical treatment, myocardial infarction, acute renal failure, and stroke) |
| Kakhki 2006 [16] | 31 | M: 70.97% F: 29.03% | 36.8 ± 16.4 | NR | NR | VATS: 0% Thoracotomy: 100% | NR | NR (excluding pneumonectomy or bronchoplasty) | NR |
| Cerfolio 2008 [7] | 100 | M: 51% F: 49% | 62.0 ± NR | NR | Nonsmall cell carcinoma. | VATS: 0% Thoracotomy: 100% | NR | Lobectomy: 55% Segmentectomy: 16% Wedge resection: 29% | NR |
| Prokakis 2008 [17] | 91 | M: 63.74% F: 36.26% | 59.5 ± 8.4 | NR | Lung malignancies. | VATS: 0% Thoracotomy: 100% | 32 Fr. | Bilobectomy: 14.29% Lobectomy: 85.71% | 61.54% (Significant bleeding, sputum retention, atelectasis, pneumonia, cardiac arrhythmias, ventilatory support, empyema) |
| Brunelli 2010 [8] | 166 | M: 72.96% F: 27.04% | 66.7 ± 10.9 | Co-morbidity index(mean, (SD)): 1.69(1.65) | Lung cancer. | VATS: 0% Thoracotomy: 100% | 28 Fr. | Lobectomy: 100% | 15.06% (Only cardiopulmonary complications mentioned) |
| Filosso 2010 [9] | 31 | M: 67.74% F: 32.26% | 69.6 ± 3.4 | NR | Lung cancer. | VATS: 0% Thoracotomy: 100% | 24 and 28 Fr. | Lobectomy: 100% | NR |
| Bertolaccini 2011 [18] | 100 | M: 59% F: 41% | 65.5 ± 13.6 | NR | NR | NR | 24 and 28 Fr. | Lobectomy: 48% Segmentectomy: 6% Wedge resection: 46% | 2% (Reoperation for bleeding, and one for exploratory thoracotomy) |
| Marjański 2013 [21] | 64 | M: 59.38% F: 40.62% | 63.0 ± 21.5 | Htpertension (25) Diabetes mellitus (7) Cardiovascular disease (6) | Lung cancer. | VATS: 51.56% Thoracotomy: 48.44% | 28 Fr. | Lobectomy: 100% | 37.50% (Atrial fibrillation, atelectasis requiring bronchial aspiration, prolonged air leak, redrainage, bronchial stump fistula, or pneumonia) |
| Brunelli 2013 [19] | 100 | M: 70% F: 30% | 67.3 ± 10.6 | Diabetes mellitus (13) Cardiovascular disease (14) | Lung cancer. | VATS: 0% Thoracotomy: 100% | 28 Fr. | Lobectomy: 100% | 13% (Only mentioning other cardiopulmonary complications) |
| Leo 2013 [20] | 500 | M: 64.40% F: 35.60% | 63.5 ± NR | Chronic obstructive lung disease (114) Diabetes mellitus (77) | NR | NR | 28 Fr. | NR | 45.8% (Pneumothorax, subcutaneous emphysema, empyema without fistula, pulmonary pneumonia, atelectasis Requiring bronchoscopy, respiratory failure, atrial arrhythmia, pulmonary edema, cardiac ischemia, bronchial fistula, bleeding, reoperation for other reasons, laryngeal nerve palsy, and others) |
| Pompili 2014 [10] | 390 | M: 52.30% F: 47.70% | 66.2 ± NR | NR | NR | VATS: 80.84% Thoracotomy: 19.16% | 24 Fr. | Lobectomy: 85.30%% Segmentectomy: 14.70% | NR |
| Gilbert 2015 [22] | 176 | M: 36.36% F: 63.64% | 68.0 ± NR | Co-morbidity index(mean):1 | Benign or neoplastic lung disease | VATS: 72.09% Thoracotomy: 27.91% | NR | Lobectomy: 76.74% Segmentectomy: 23.26% | 13.64% (New or worsening pneumothorax and/or increasing subcutaneous emphysema requiring chest tube reinsertion) |
| Lijkendijk 2015 [23] | 105 | M: 37.14% F: 62.86% | 68.3 ± NR | NR | Lung cancer. | VATS: 39.04% Thoracotomy: 60.96% | 24 Fr. | Lobectomy: 100% | NR |
| Gocyk 2016 [24] | 254 | M: 62.20% F: 37.80% | 60.3 ± NR | NR | Malignant, benign and metastatic lung tumors. | NR | NR | Lobectomy: 55.51% Wedge resection: 44.49% | 5.91% (Empyema, residual pneumothorax, peritonitis due to colon necrosis) |
| Chiappetta 2017 [25] | 95 | M: 51.58% F: 48.42% | 63.6 ± 13.0 | Htpertension (45) Diabetes mellitus (9) Cardiovascular disease (7) Chronic obstructive lung disease (26) | Benign or malignant lung disease | NR | 28 Fr. | Lobectomy: 52.63% Wedge resection: 47.37% | 2.11% (Reopening after clamping test, complication after chest tube removal) |
| Plourde 2018 [26] | 215 | M: 43.26% F: 56.74% | 67.5 ± 9.3 | NR | Benign or malignant lung tumors | VATS: 83.72% Thoracotomy: 16.28% | 28 Fr. | Lobectomy: 93.49% Segmentectomy: 4.19% Wedge resection: 2.32% | 5.12% (Pneumothorax, hemothorax, and empyema after tube removal) |
| Takamochi 2018 [27] | 320 | M: 50.31% F: 49.69% | 67.3 ± 11.7 | Diabetes mellitus (36) Cardiovascular disease (14) Cerebrovascular disease (7) Chronic obstructive lung disease (82) Interstitial pneumonia (28) | Malignant, benign and metastatic lung tumors. | VATS: 0% Thoracotomy: 100% | NR | Lobectomy: 79.26% Segmentectomy: 20.74% | 21.25% (Pneumonia, atelectasis, bleeding, arrhythmia, chylothorax, and others) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, P.-C.; Chen, K.-H.; Jhou, H.-J.; Lee, C.-H.; Chou, S.-H.; Chen, P.-H.; Chang, T.-W. Promising Effects of Digital Chest Tube Drainage System for Pulmonary Resection: A Systematic Review and Network Meta-Analysis. J. Pers. Med. 2022, 12, 512. https://doi.org/10.3390/jpm12040512
Chang P-C, Chen K-H, Jhou H-J, Lee C-H, Chou S-H, Chen P-H, Chang T-W. Promising Effects of Digital Chest Tube Drainage System for Pulmonary Resection: A Systematic Review and Network Meta-Analysis. Journal of Personalized Medicine. 2022; 12(4):512. https://doi.org/10.3390/jpm12040512
Chicago/Turabian StyleChang, Po-Chih, Kai-Hua Chen, Hong-Jie Jhou, Cho-Hao Lee, Shah-Hwa Chou, Po-Huang Chen, and Ting-Wei Chang. 2022. "Promising Effects of Digital Chest Tube Drainage System for Pulmonary Resection: A Systematic Review and Network Meta-Analysis" Journal of Personalized Medicine 12, no. 4: 512. https://doi.org/10.3390/jpm12040512
APA StyleChang, P.-C., Chen, K.-H., Jhou, H.-J., Lee, C.-H., Chou, S.-H., Chen, P.-H., & Chang, T.-W. (2022). Promising Effects of Digital Chest Tube Drainage System for Pulmonary Resection: A Systematic Review and Network Meta-Analysis. Journal of Personalized Medicine, 12(4), 512. https://doi.org/10.3390/jpm12040512








