Lack of Association between Inhaled Corticosteroid Use and the Risk of Future Exacerbation in Patients with GOLD Group A Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement of Clinical Data
2.3. Outcome Measure
2.4. Ethics
2.5. Statistical Analysis
3. Results
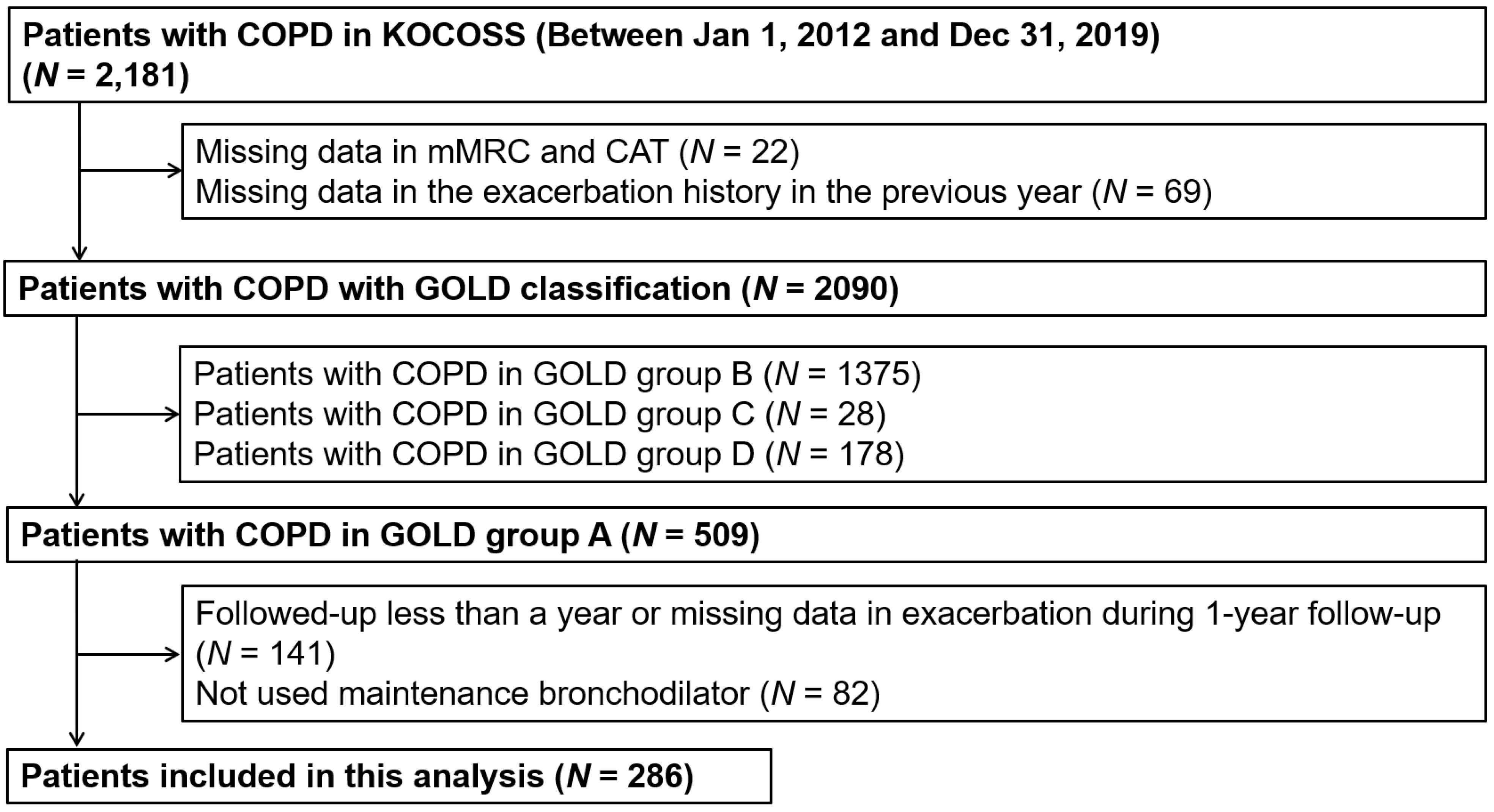
3.1. Description of Participants
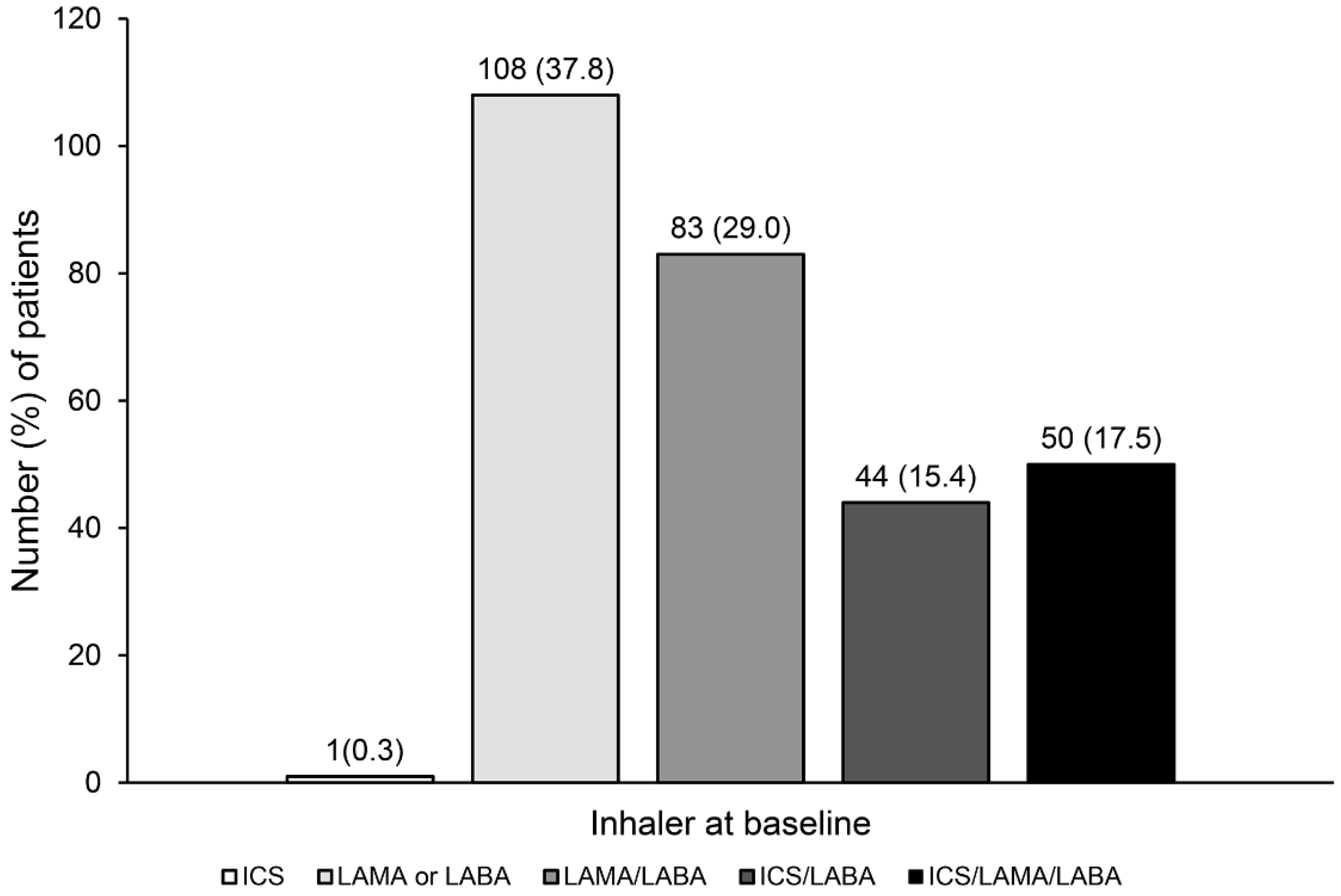
3.2. Inhaler Prescription Status
3.3. Clinical Characteristics According to ICS Use
3.4. Development of Exacerbation According ICS Use
3.5. Development of Exacerbation According the Type of Inhaler Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mannino, D.M.; Braman, S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2007, 4, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aymerich, J.; Serra Pons, I.; Mannino, D.M.; Maas, A.K.; Miller, D.P.; Davis, K.J. Lung function impairment, COPD hospitalisations and subsequent mortality. Thorax 2011, 66, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bourbeau, J. Outcomes and health-related quality of life following hospitalization for an acute exacerbation of COPD. Respirology 2005, 10, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax 2012, 67, 957–963. [Google Scholar] [CrossRef]
- Press, V.G.; Konetzka, R.T.; White, S.R. Insights about the economic impact of chronic obstructive pulmonary disease readmissions post implementation of the hospital readmission reduction program. Curr. Opin. Pulm. Med. 2018, 24, 138–146. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2021 Update. Available online: http://www.goldcopd.org (accessed on 30 August 2021).
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Celli, B.; Senn, S.; Burkhart, D.; Kesten, S.; Menjoge, S.; Decramer, M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 1543–1554. [Google Scholar] [CrossRef]
- Appleton, S.; Poole, P.; Smith, B.; Veale, A.; Lasserson, T.J.; Chan, M.M. Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2006, 3, Cd001104. [Google Scholar] [CrossRef]
- Barr, R.G.; Bourbeau, J.; Camargo, C.A.; Ram, F.S. Inhaled tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2005, 2005, Cd002876. [Google Scholar]
- Zhou, Y.; Zhong, N.S.; Li, X.; Chen, S.; Zheng, J.; Zhao, D.; Yao, W.; Zhi, R.; Wei, L.; He, B.; et al. Tiotropium in Early-Stage Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2017, 377, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Maltais, F.; Bjermer, L.; Kerwin, E.M.; Jones, P.W.; Watkins, M.L.; Tombs, L.; Naya, I.P.; Boucot, I.H.; Lipson, D.A.; Compton, C.; et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: The EMAX randomised trial. Respir. Res. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.R.; Deslée, G.; Jebrak, G.; Brinchault, G.; Caillaud, D.; Chanez, P.; Court-Fortune, I.; Escamilla, R.; Nesme-Meyer, P.; Paillasseur, J.L.; et al. Real-life use of inhaled corticosteroids in COPD patients versus the GOLD proposals: A paradigm shift in GOLD 2011? Eur. Respir. J. 2014, 43, 1201–1203. [Google Scholar] [CrossRef]
- Cabrera López, C.; Casanova Macario, C.; Marín Trigo, J.M.; de-Torres, J.P.; Sicilia Torres, R.; González, J.M.; Polverino, F.; Divo, M.; Pinto Plata, V.; Zulueta, J.J.; et al. Comparison of the 2017 and 2015 Global Initiative for Chronic Obstructive Lung Disease Reports. Impact on Grouping and Outcomes. Am. J. Respir. Crit. Care Med. 2018, 197, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Criner, R.N.; Labaki, W.W.; Regan, E.A.; Bon, J.M.; Soler, X.; Bhatt, S.P.; Murray, S.; Hokanson, J.E.; Silverman, E.K.; Crapo, J.D.; et al. Mortality and Exacerbations by Global Initiative for Chronic Obstructive Lung Disease Groups ABCD: 2011 Versus 2017 in the COPDGene® Cohort. Chronic Obstr. Pulm. Dis. 2019, 6, 64–73. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, C.H.; Um, S.J.; Park, Y.B.; Yoo, K.H.; Jung, K.S.; Lee, S.D.; Oh, Y.M.; Lee, J.H.; Kim, E.K.; et al. Clinical impacts of the classification by 2017 GOLD guideline comparing previous ones on outcomes of COPD in real-world cohorts. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3473–3484. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chon, G.R.; Rhee, C.K.; Kim, D.K.; Yoon, H.K.; Lee, J.H.; Yoo, K.H.; Lee, S.H.; Lee, S.Y.; Kim, T.E.; et al. Characteristics of Patients with Chronic Obstructive Pulmonary Disease at the First Visit to a Pulmonary Medical Center in Korea: The KOrea COpd Subgroup Study Team Cohort. J. Korean Med. Sci. 2016, 31, 553–560. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique--1995 update. Am. J. Respir. Crit. Care Med. 1995, 152, 2185–2198. [Google Scholar] [CrossRef]
- Choi, J.K.; Paek, D.; Lee, J.O. Normal predictive values of spirometry in Korean population. Tuberc. Respir. Dis. 2005, 58, 230–242. [Google Scholar] [CrossRef]
- Park, J.O.; Choi, I.S.; Park, K.O. Normal predicted standards of single breath carbon monoxide diffusing capacity of lung in healthy nonsmoking adults. Korean J. Intern. Med. 1985, 28, 176–183. [Google Scholar]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; van der Grinten, C.P.; Gustafsson, P.; Hankinson, J.; et al. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Plaza, V.; Alvarez, F.; Calle, M.; Casanova, C.; Cosio, B.G.; Lopez-Vina, A.; de Llano, L.P.; Quirce, S.; Roman-Rodriguez, M.; Soler-Cataluna, J.J.; et al. Consensus on the Asthma-COPD Overlap Syndrome (ACOS) between the Spanish COPD Guidelines (GesEPOC) and the Spanish Guidelines on the Management of Asthma (GEMA). Arch. Bronconeumol. 2017, 53, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Çolak, Y.; Afzal, S.; Nordestgaard, B.G.; Vestbo, J.; Lange, P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: A prospective cohort study. Lancet Respir. Med. 2017, 5, 426–434. [Google Scholar] [CrossRef]
- Diab, N.; Gershon, A.S.; Sin, D.D.; Tan, W.C.; Bourbeau, J.; Boulet, L.P.; Aaron, S.D. Underdiagnosis and Overdiagnosis of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1130–1139. [Google Scholar] [CrossRef]
- Gershon, A.S.; Thiruchelvam, D.; Chapman, K.R.; Aaron, S.D.; Stanbrook, M.B.; Bourbeau, J.; Tan, W.; To, T.; Canadian Respiratory Research, N. Health Services Burden of Undiagnosed and Overdiagnosed COPD. Chest 2018, 153, 1336–1346. [Google Scholar] [CrossRef]
- Morice, A.H.; Celli, B.; Kesten, S.; Lystig, T.; Tashkin, D.; Decramer, M. COPD in young patients: A pre-specified analysis of the four-year trial of tiotropium (UPLIFT). Respir. Med. 2010, 104, 1659–1667. [Google Scholar] [CrossRef]
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Mullerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; Macnee, W.; et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef]
- Izquierdo Alonso, J.L.; Rodríguez Glez-Moro, J.M. The excessive use of inhaled corticosteroids in chronic obstructive pulmonary disease. Arch. Bronconeumol. 2012, 48, 207–212. [Google Scholar] [CrossRef]
- Barrecheguren, M.; Monteagudo, M.; Ferrer, J.; Borrell, E.; Llor, C.; Esquinas, C.; Miravitlles, M. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir. Med. 2016, 111, 47–53. [Google Scholar] [CrossRef]
- Jo, Y.S.; Yoo, K.H.; Park, Y.B.; Rhee, C.K.; Jung, K.S.; Jang, S.H.; Park, J.Y.; Kim, Y.; Kim, B.Y.; Ahn, S.I.; et al. Relationship Between Changes in Inhalation Treatment Level and Exacerbation of Chronic Obstructive Pulmonary Disease: Nationwide the Health Insurance and Assessment Service Database. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Calverley, P.M.; Tetzlaff, K.; Dusser, D.; Wise, R.A.; Mueller, A.; Metzdorf, N.; Anzueto, A. Determinants of exacerbation risk in patients with COPD in the TIOSPIR study. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3391–3405. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rhee, C.K.; Lee, B.J.; Choi, D.C.; Kim, J.A.; Kim, S.H.; Jeong, Y.; Kim, T.H.; Chon, G.R.; Jung, K.S.; et al. Impacts of coexisting bronchial asthma on severe exacerbations in mild-to-moderate COPD: Results from a national database. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 775–783. [Google Scholar]
- Menezes, A.M.B.; de Oca, M.M.; Pérez-Padilla, R.; Nadeau, G.; Wehrmeister, F.C.; Lopez-Varela, M.V.; Muiño, A.; Jardim, J.R.B.; Valdivia, G.; Tálamo, C. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest 2014, 145, 297–304. [Google Scholar] [CrossRef]
- Gonzalez, A.V.; Coulombe, J.; Ernst, P.; Suissa, S. Long-term Use of Inhaled Corticosteroids in COPD and the Risk of Fracture. Chest 2018, 153, 321–328. [Google Scholar] [CrossRef]
- Price, D.; Yawn, B.; Brusselle, G.; Rossi, A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim. Care Respir. J. 2013, 22, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Lettis, S.; Locantore, N.; Pascoe, S.; Jones, P.W.; Wedzicha, J.A.; Barnes, N.C. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax 2016, 71, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Suissa, S.; Dell’Aniello, S.; Ernst, P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: A population-based cohort study. Lancet Respir. Med. 2018, 6, 855–862. [Google Scholar] [CrossRef]
- Papi, A.; Vestbo, J.; Fabbri, L.; Corradi, M.; Prunier, H.; Cohuet, G.; Guasconi, A.; Montagna, I.; Vezzoli, S.; Petruzzelli, S.; et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet 2018, 391, 1076–1084. [Google Scholar] [CrossRef]
- Lipson, D.A.; Barnhart, F.; Brealey, N.; Brooks, J.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Jones, C.E.; et al. Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD. N. Engl. J. Med. 2018, 378, 1671–1680. [Google Scholar] [CrossRef]
- Rabe, K.F.; Martinez, F.J.; Ferguson, G.T.; Wang, C.; Singh, D.; Wedzicha, J.A.; Trivedi, R.; St Rose, E.; Ballal, S.; McLaren, J.; et al. Triple Inhaled Therapy at Two Glucocorticoid Doses in Moderate-to-Very-Severe COPD. N. Engl. J. Med. 2020, 383, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.A.; Crim, C.; Criner, G.J.; Day, N.C.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Jones, C.E.; Kilbride, S.; Lange, P.; et al. Reduction in All-Cause Mortality with Fluticasone Furoate/Umeclidinium/Vilanterol in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2020, 201, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.J.; Rabe, K.F.; Ferguson, G.T.; Wedzicha, J.A.; Singh, D.; Wang, C.; Rossman, K.; St Rose, E.; Trivedi, R.; Ballal, S.; et al. Reduced All-Cause Mortality in the ETHOS Trial of Budesonide/Glycopyrrolate/Formoterol for Chronic Obstructive Pulmonary Disease. A Randomized, Double-Blind, Multicenter, Parallel-Group Study. Am. J. Respir. Crit. Care Med. 2021, 203, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Verhamme, K.; Calverley, P.M.A.; Dreher, M.; Bayer, V.; Gardev, A.; de la Hoz, A.; Wedzicha, J.; Price, D. A Pooled Analysis of Mortality in Patients with COPD Receiving Dual Bronchodilation with and without Additional Inhaled Corticosteroid. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 545–558. [Google Scholar] [CrossRef]
- Pascoe, S.; Barnes, N.; Brusselle, G.; Compton, C.; Criner, G.J.; Dransfield, M.T.; Halpin, D.M.G.; Han, M.K.; Hartley, B.; Lange, P.; et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: Analysis of the IMPACT trial. Lancet Respir. Med. 2019, 7, 745–756. [Google Scholar] [CrossRef]
- Barnes, N.C.; Sharma, R.; Lettis, S.; Calverley, P.M. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur. Respir. J. 2016, 47, 1374–1382. [Google Scholar] [CrossRef]
- Jo, Y.S.; Hwang, Y.I.; Yoo, K.H.; Kim, T.H.; Lee, M.G.; Lee, S.H.; Shin, K.C.; In, K.H.; Yoon, H.K.; Rhee, C.K. Effect of Inhaled Corticosteroids on Exacerbation of Asthma-COPD Overlap According to Different Diagnostic Criteria. J. Allergy Clin. Immunol. Pract. 2020, 8, 1625–1633.e6. [Google Scholar] [CrossRef]
- Bjermer, L.; Boucot, I.H.; Maltais, F.; Kerwin, E.M.; Naya, I.P.; Tombs, L.; Jones, P.W.; Compton, C.; Lipson, D.A.; Vogelmeier, C.F. Dual Bronchodilator Therapy as First-Line Treatment in Maintenance-Naïve Patients with Symptomatic COPD: A Pre-Specified Analysis of the EMAX Trial. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 1939–1956. [Google Scholar] [CrossRef]
- Jones, P.W.; Nadeau, G.; Small, M.; Adamek, L. Characteristics of a COPD population categorised using the GOLD framework by health status and exacerbations. Respir. Med. 2014, 108, 129–135. [Google Scholar] [CrossRef][Green Version]
- Oishi, K.; Hirano, T.; Hamada, K.; Uehara, S.; Suetake, R.; Yamaji, Y.; Ito, K.; Asami-Noyama, M.; Edakuni, N.; Matsunaga, K. Characteristics of 2017 GOLD COPD group A: A multicenter cross-sectional CAP study in Japan. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3901–3907. [Google Scholar] [CrossRef]
- Park, Y.B.; Yoo, K.H. The current status of chronic obstructive pulmonary disease awareness, treatments, and plans for improvement in South Korea: A narrative review. J. Thorac. Dis. 2021, 13, 3898–3906. [Google Scholar] [CrossRef] [PubMed]
| Overall (N = 286) | Without ICS (N = 191) | With ICS (N = 95) | p Value | |
|---|---|---|---|---|
| Age (years) | 68.3 ± 7.4 | 68.2 ± 7.5 | 68.5 ± 7.3 | 0.701 |
| Male sex | 269 (94.1) | 179 (93.7) | 90 (94.7) | 0.731 |
| Smoking (N = 287) | 0.302 | |||
| Never | 29 (10.2) | 17 (9.0) | 12 (12.6) | |
| Former smoker | 194 (68.1) | 135 (71.1) | 59 (62.1) | |
| Current smoker | 62 (21.7) | 38 (20.0) | 24 (25.3) | |
| Pack years (N = 245) | 42.1 ± 23.2 | 42.0 ± 23.6 | 42.2 ± 22.6 | 0.956 |
| BMI (kg/m2) | 23.5 ± 3.3 | 23.4 ± 3.4 | 23.6 ± 3.2 | 0.700 |
| Education (above high school) (N = 284) | 52 (18.3) | 36 (18.9) | 16 (17.2) | 0.737 |
| mMRC dyspnoea scale | 0.046 | |||
| 0 | 98 (34.3) | 73 (38.2) | 25 (26.3) | |
| 1 | 188 (65.7) | 118 (61.8) | 70 (73.7) | |
| Quality of life | ||||
| SGRQ score | 16.4 ± 9.6 | 15.8 ± 9.2 | 17.8 ± 10.4 | 0.091 |
| CAT score | 5.8 ± 2.3 | 5.8 ± 2.4 | 5.7 ± 2.3 | 0.744 |
| Moderate exacerbation in the prior year | 24 (8.4) | 16 (8.4) | 8 (8.4) | 0.990 |
| Comorbidities | ||||
| Hypertension | 111 (38.8) | 74 (38.7) | 37 (39.0) | 0.973 |
| Congestive heart failure | 5 (1.8) | 5 (2.6) | 0 (0) | 0.112 |
| Ischemic heart disease | 16 (5.6) | 11 (5.8) | 5 (5.3) | 0.864 |
| Dyslipidaemia | 46 (16.1) | 30 (15.7) | 16 (16.8) | 0.350 |
| Diabetes mellitus | 52 (18.2) | 33 (17.3) | 19 (20.0) | 0.574 |
| Gastro-oesophageal reflux | 36 (12.6) | 26 (13.6) | 10 (10.5) | 0.459 |
| Osteoporosis | 6 (2.1) | 6 (3.1) | 0 (0) | 0.168 |
| Tuberculosis | 73 (25.5) | 53 (27.8) | 20 (21.1) | 0.221 |
| Asthma | 103 (36.0) | 60 (31.4) | 43 (45.3) | 0.022 |
| Asthma–COPD overlap * (N = 229) | 44 (19.2) | 26 (17.0) | 18 (23.7) | 0.226 |
| Spirometry | ||||
| Post-BD FVC, L | 3.50 ± 0.76 | 3.54 ± 0.75 | 3.33 ± 0.77 | 0.025 |
| Post-BD FVC, %predicted | 83.5 ± 15.5 | 84.4 ± 14.8 | 81.7 ± 16.7 | 0.164 |
| Post-BD FEV1, L | 1.84 ± 0.51 | 1.91 ± 0.51 | 1.69 ± 0.47 | <0.001 |
| Post-BD FEV1, %predicted | 62.2 ± 15.2 | 64.0 ± 15.2 | 58.6 ± 14.6 | 0.005 |
| Post-BD FEV1/FVC | 53.3 ± 10.6 | 54.4 ± 10.7 | 51.0 ± 10.0 | 0.012 |
| BDR positivity † | 23 (8.0) | 9 (4.7) | 14 (14.7) | 0.003 |
| DLco, %predicted (N = 226) | 70.5 ± 20.8 | 70.0 ± 21.9 | 71.4 ± 18.5 | 0.631 |
| Exercise capacity, 6MWD (m) (N = 223) | 425.2 ± 120.5 | 426.6 ± 113.6 | 422.2 ± 135.1 | 0.800 |
| Blood eosinophil count (N = 229) | 213.3 ± 220.3 | 204.4 ± 205.6 | 231.3 ± 247.7 | 0.386 |
| No (%) of Patients with Moderate or Severe Exacerbation during 1-Year Follow-Up Period | Odds Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|---|
| Crude | Model 1 | Model 2 | Model 3 | ||
| Without ICS (N = 191) | 41 (21.5) | Reference | Reference | Reference | Reference |
| With ICS (N = 95) | 25 (26.3) | 1.31 (0.74–2.32) | 1.22 (0.66–2.26) | 1.24 (0.67–2.31) | 1.37 (0.67–2.78) |
| No (%) of Patients with Moderate or Severe Exacerbation during 1-Year Follow-Up Period | Odds Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|---|
| Crude | Model 1 | p for Interaction | |||
| Without ICS | With ICS | ||||
| Overall (N = 286) | 41/191 (21.5) | 25/95 (26.3) | 1.31 (0.74–2.32) | 1.22 (0.66–2.26) | |
| Past history of moderate AE | 0.100 | ||||
| No (N = 262) | 33/175 (18.9) | 23/87 (26.4) | 1.55 (0.84–2.84) | 1.53 (0.80–2.94) | |
| Yes (N = 24) | 8/16 (50.0) | 2/8 (25.0) | 0.33 (0.05–2.18) | 0.36 (0.02–5.35) | |
| Blood eosinophil count (N = 229) | 0.501 | ||||
| <300 cells/μL (N = 187) | 23/127 (18.1) | 16/60 (26.7) | 1.64 (0.79–3.41) | 1.52 (0.68–3.43) | |
| ≥300 cells/μL (N = 42) | 6/26 (23.1) | 4/16 (25.0) | 1.11 (0.26–4.75) | 1.04 (0.17–6.49) | |
| Self-reported asthma | 0.901 | ||||
| No (N = 183) | 25/131(19.1) | 13/52 (25.0) | 1.41 (0.66–3.03) | 1.38 (0.60–3.16) | |
| Yes (N = 103) | 16/60 (26.7) | 12/43 (27.9) | 1.06 (0.44–2.56) | 1.27 (0.45–3.58) | |
| Postbronchodilator FEV1, %predicted * | 0.037 | ||||
| ≥50% (N = 225) | 32/158 (20.3) | 14/67 (20.9) | 1.04 (0.51–2.11) | 1.11 (0.53–2.32) | |
| <50% (N = 61) | 9/33 (27.3) | 11/28 (39.3) | 1.73 (0.59–5.07) | 1.98 (0.60–6.58) | |
| No (%) of Patients with Moderate or Severe Exacerbation during 1-Year Follow-Up Period | Odds Ratio (95% Confidence Interval) | ||||
|---|---|---|---|---|---|
| Crude | Model 1 | Model 2 | Model 3 | ||
| Mono bronchodilator (N = 108) | 25 (23.2) | Ref | Ref | Ref | Ref |
| Dual bronchodilator (N = 83) | 16 (19.3) | 0.79 (0.39–1.60) | 0.59 (0.27–1.28) | 0.62 (0.29–1.35) | 0.60 (0.24–1.47) |
| ICS/LABA (N = 44) | 11 (25.0) | 1.11 (0.49–2.50) | 1.03 (0.43–2.43) | 1.11 (0.47–2.65) | 1.29 (0.49–3.40) |
| Triple therapy (N = 50) | 13 (26.0) | 1.17 (0.54–2.53) | 0.85 (0.36–2.02) | 0.85 (0.35–2.04) | 0.80 (0.28–2.27) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.H.; Kim, D.K.; Kim, S.-H.; Shin, T.R.; Jung, K.-S.; Yoo, K.H.; Hwang, K.-E.; Park, H.Y.; Jo, Y.S.; on behalf of the KOCOSS Cohort. Lack of Association between Inhaled Corticosteroid Use and the Risk of Future Exacerbation in Patients with GOLD Group A Chronic Obstructive Pulmonary Disease. J. Pers. Med. 2022, 12, 916. https://doi.org/10.3390/jpm12060916
Shin SH, Kim DK, Kim S-H, Shin TR, Jung K-S, Yoo KH, Hwang K-E, Park HY, Jo YS, on behalf of the KOCOSS Cohort. Lack of Association between Inhaled Corticosteroid Use and the Risk of Future Exacerbation in Patients with GOLD Group A Chronic Obstructive Pulmonary Disease. Journal of Personalized Medicine. 2022; 12(6):916. https://doi.org/10.3390/jpm12060916
Chicago/Turabian StyleShin, Sun Hye, Deog Kyeom Kim, Sang-Heon Kim, Tae Rim Shin, Ki-Suck Jung, Kwang Ha Yoo, Ki-Eun Hwang, Hye Yun Park, Yong Suk Jo, and on behalf of the KOCOSS Cohort. 2022. "Lack of Association between Inhaled Corticosteroid Use and the Risk of Future Exacerbation in Patients with GOLD Group A Chronic Obstructive Pulmonary Disease" Journal of Personalized Medicine 12, no. 6: 916. https://doi.org/10.3390/jpm12060916
APA StyleShin, S. H., Kim, D. K., Kim, S.-H., Shin, T. R., Jung, K.-S., Yoo, K. H., Hwang, K.-E., Park, H. Y., Jo, Y. S., & on behalf of the KOCOSS Cohort. (2022). Lack of Association between Inhaled Corticosteroid Use and the Risk of Future Exacerbation in Patients with GOLD Group A Chronic Obstructive Pulmonary Disease. Journal of Personalized Medicine, 12(6), 916. https://doi.org/10.3390/jpm12060916






