Hemostasis and Anti-Inflammatory Abilities of AuNPs-Coated Chitosan Dressing for Burn Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Gold Nanoparticles (AuNPs)
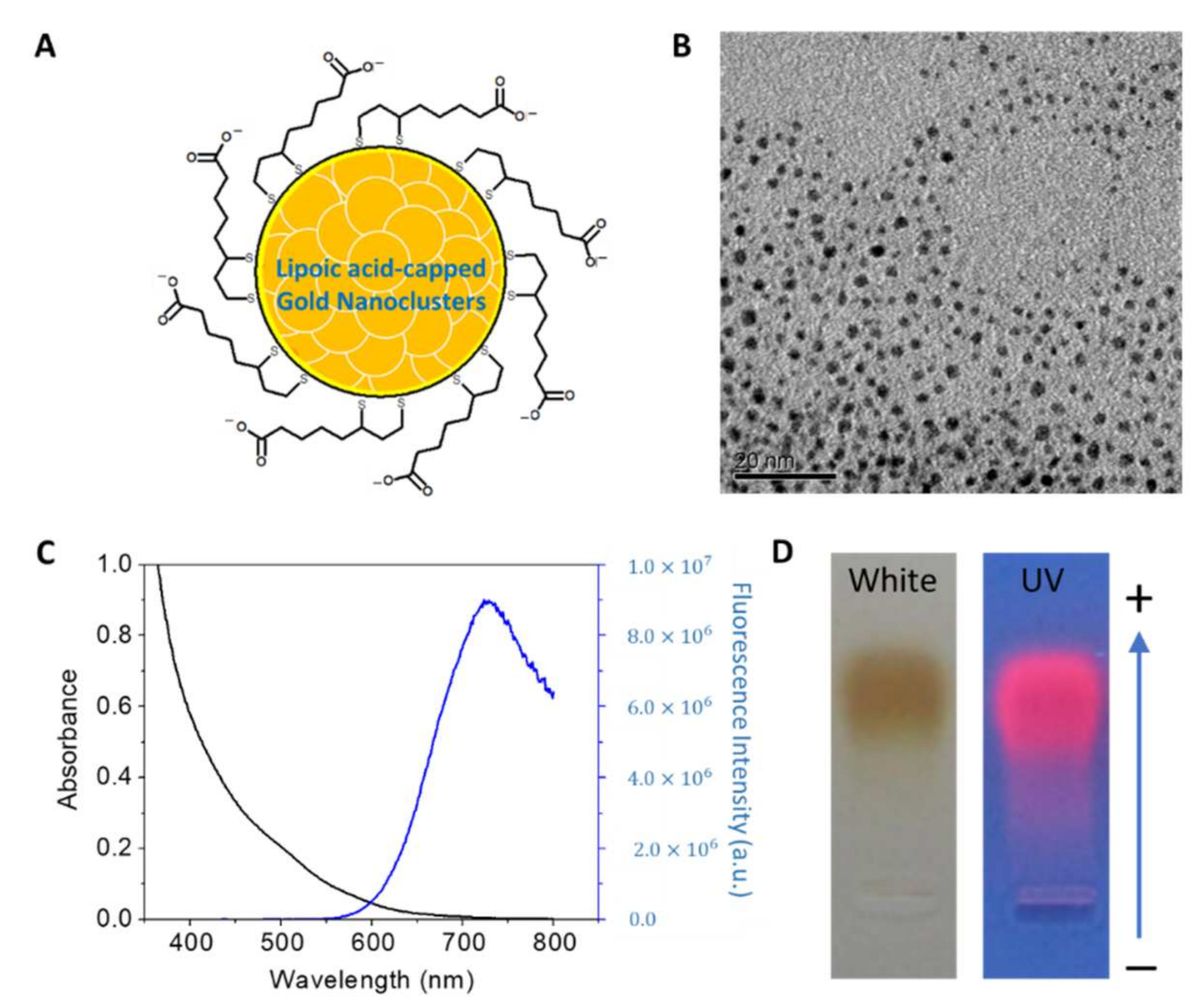
2.2.1. AuNPs Preparation
2.2.2. AuNPs Characterization
2.3. Chitosan Dressing Preparation
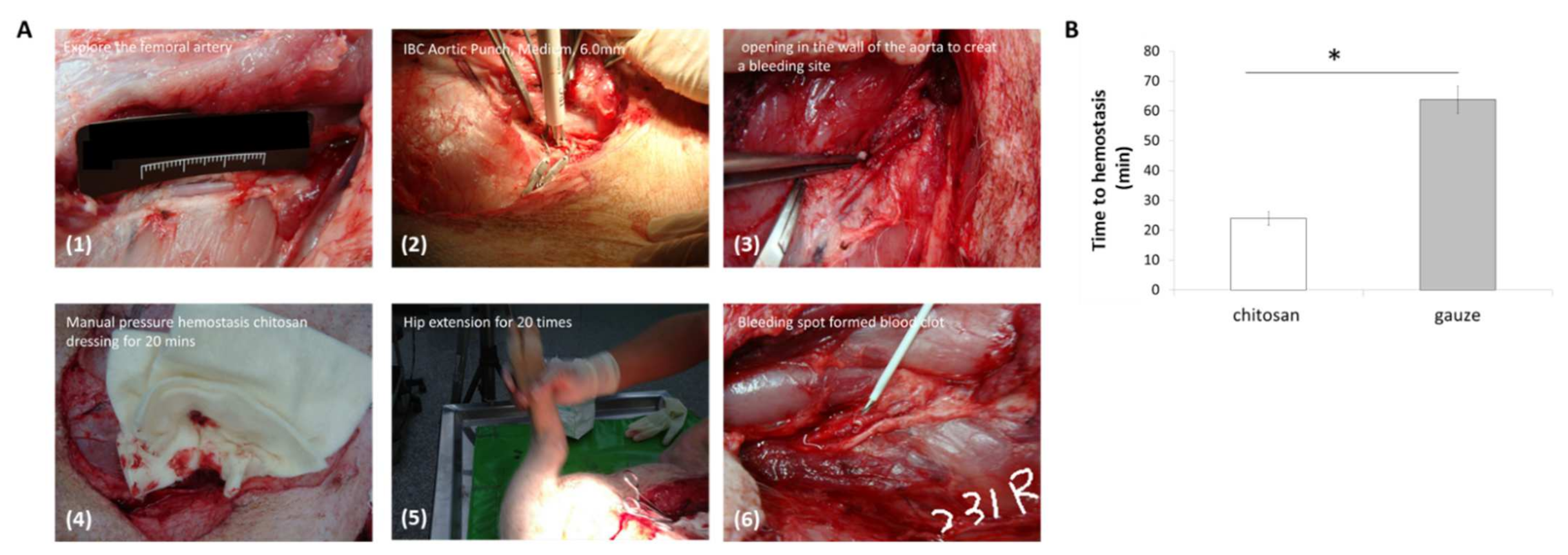
2.4. Femoral Artery Hemorrhage Swine Model
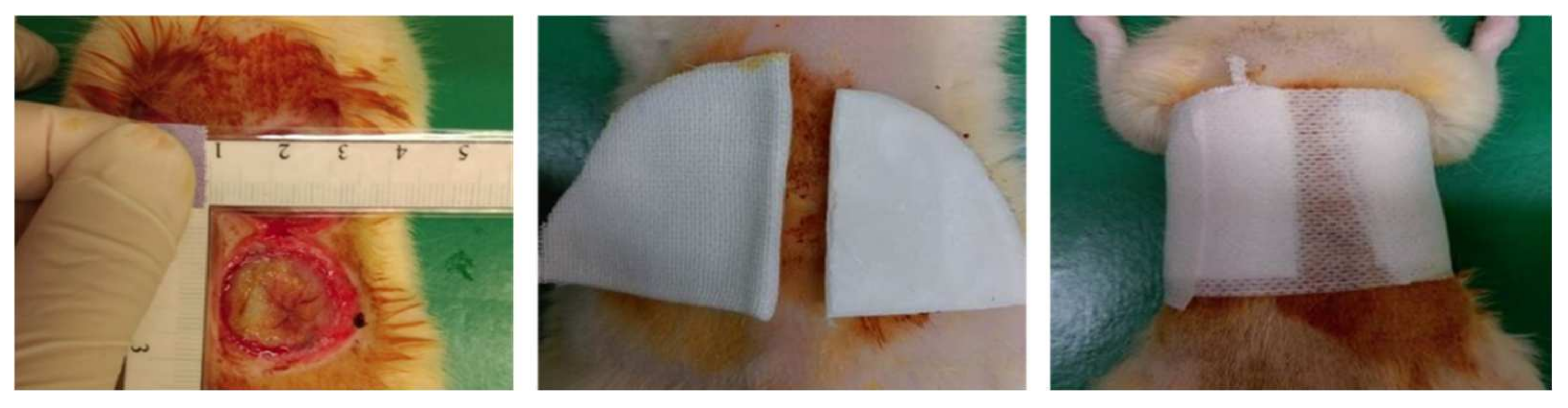
2.5. Rat Burn Model
2.6. Histological and Immunohistochemistry Analysis
2.7. Prothrombin Time (PT) and Activated Partial Thromboplastin Time (aPTT) Analysis
2.8. RNA Purification and Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
2.9. Multiplex Growth Factors in Burn Wound
2.10. Data Analysis
3. Results
3.1. Hemostasis Analysis
3.2. The Characterization of AuNPs
3.3. Histology and Immunohistochemistry (IHC) Analysis
3.4. Blood Coagulation Analysis in Burn Wound
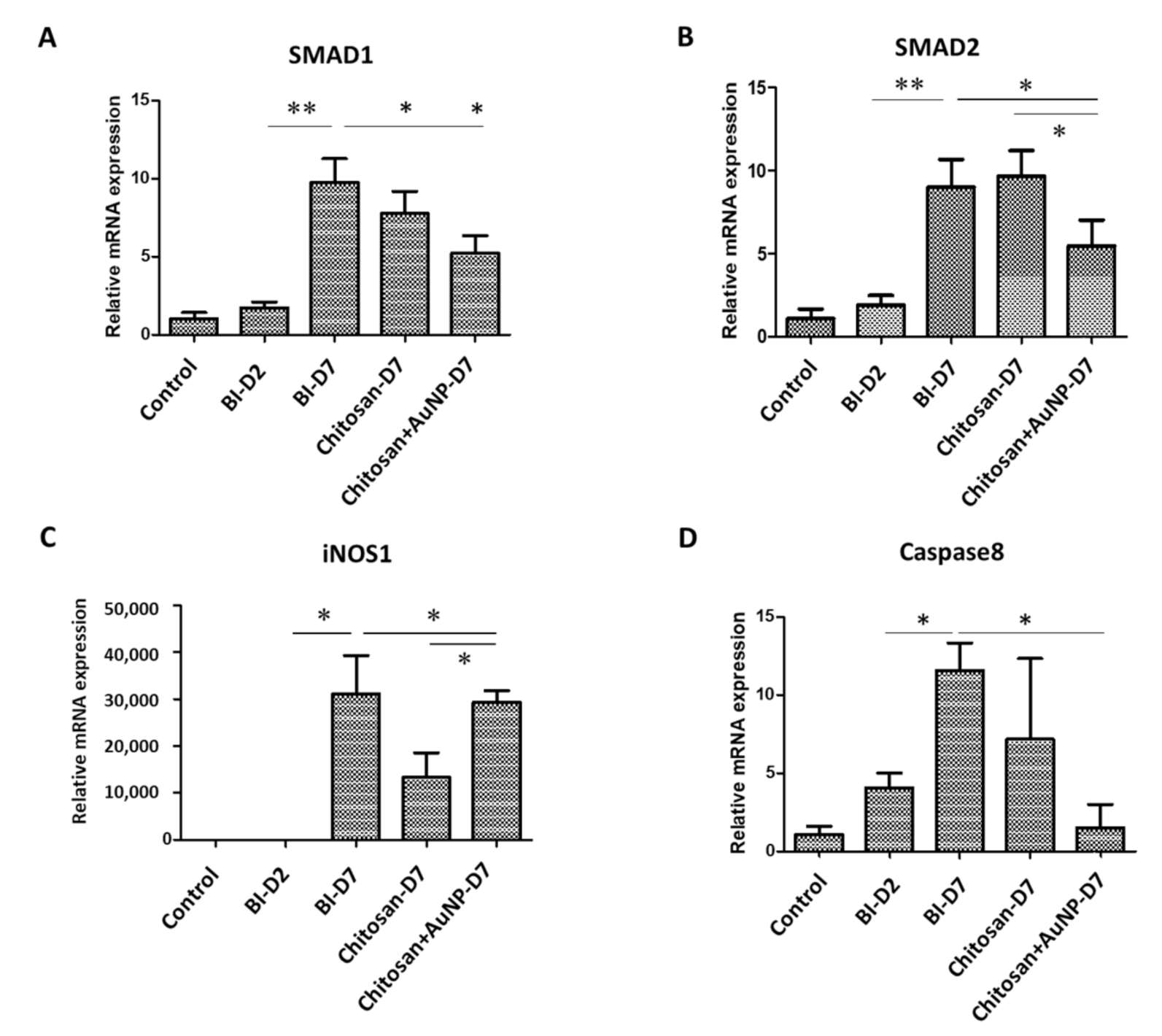
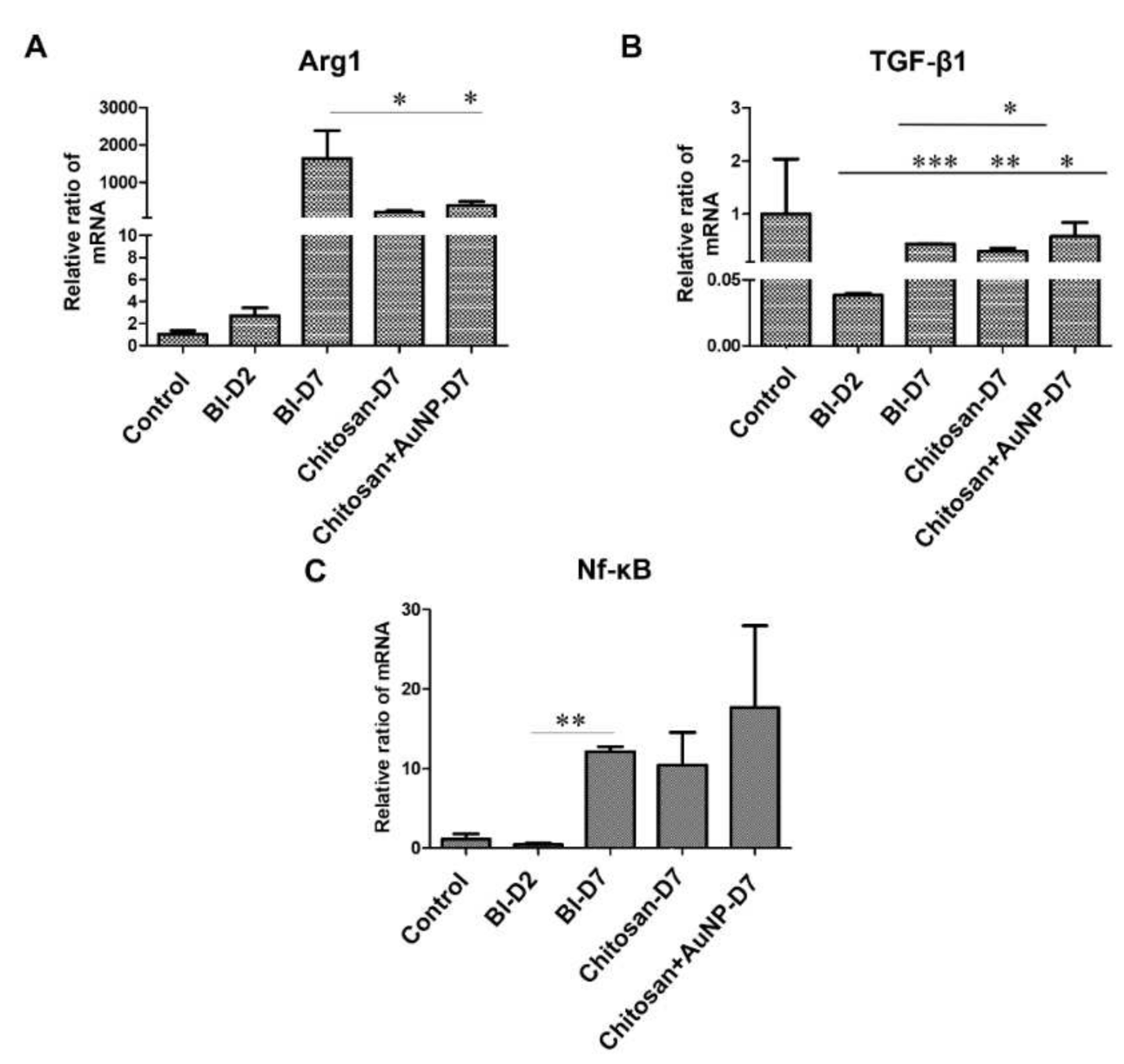
3.5. Fibrotic Formation Analysis in Burn Wound
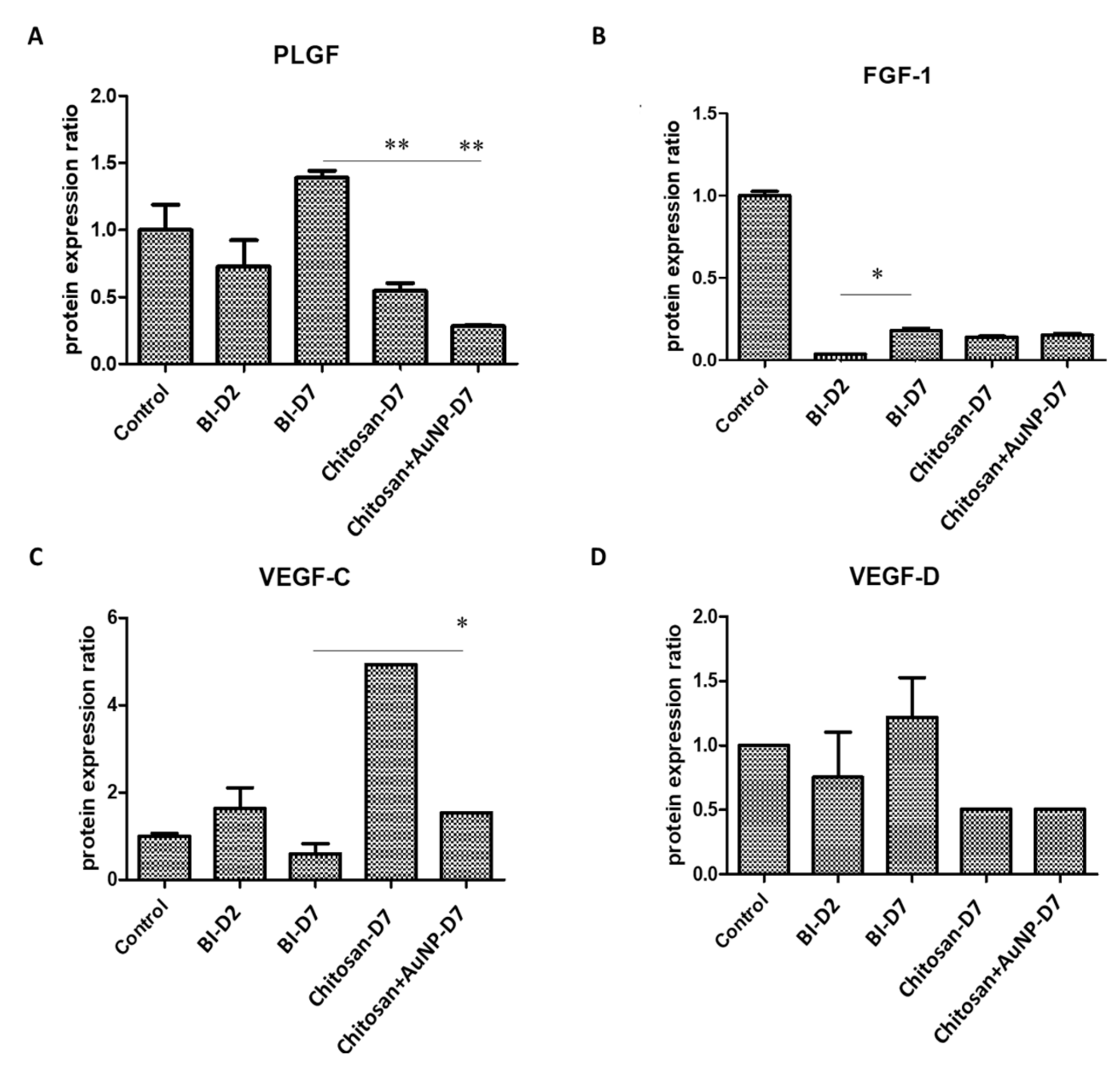
3.6. Angiogenesis Analysis in Burn Wound
3.7. Inflammation Analysis in Burn Wound
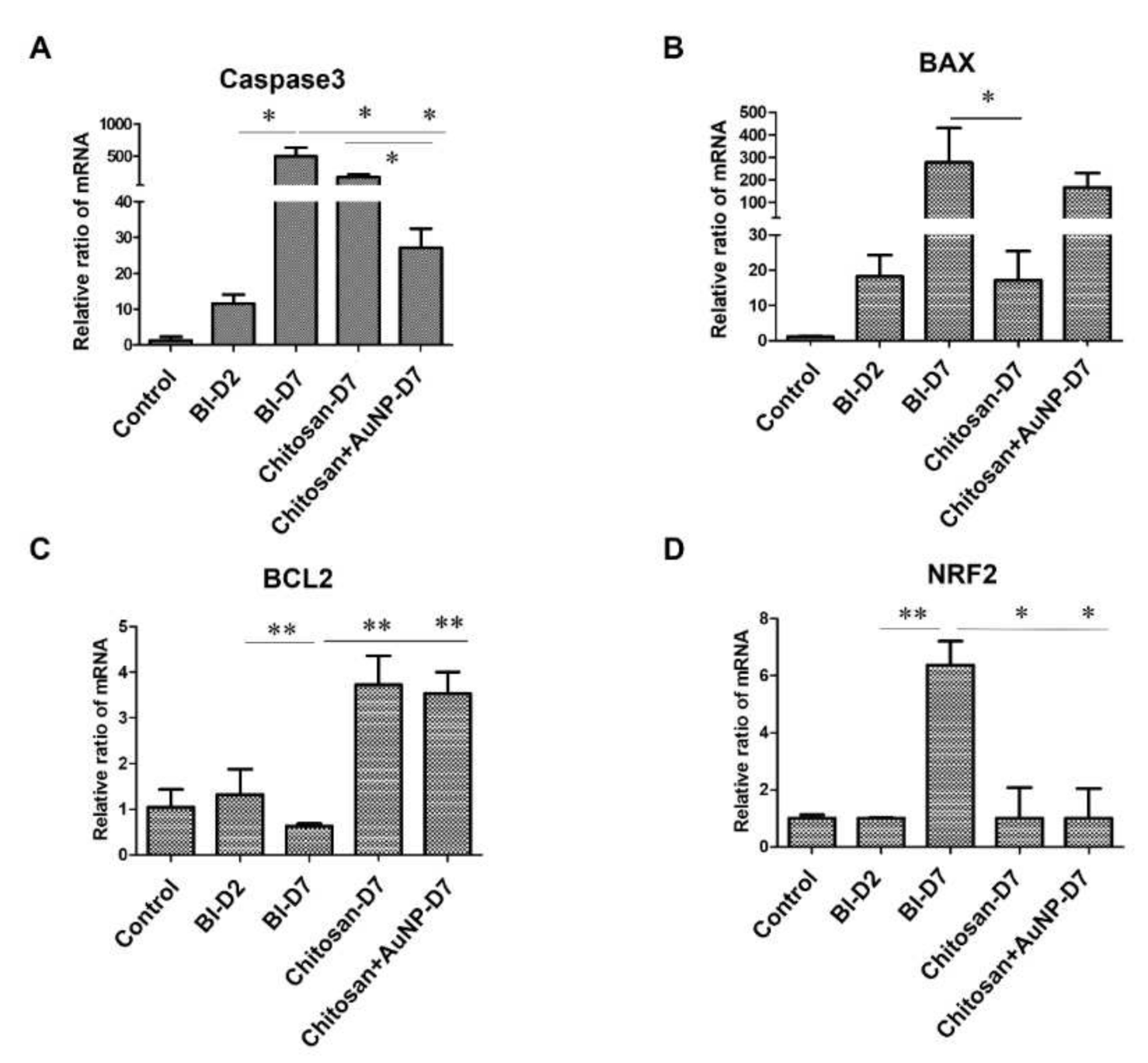
3.8. Apoptosis Analysis in Burn Wound
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burns. Available online: https://www.who.int/ (accessed on 24 March 2022).
- Ministry of Health and Welfare. Chinese People’s Cause of Death Statistics. 2017. Available online: https://www.mohw.gov.tw/ (accessed on 24 March 2022).
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent Insights into the Extraction, Characterization, and Bioactivities of Chitin and Chitosan from Insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Mujahid, M. A Review on Recent Advances in Chitosan Based Composite for Hemostatic Dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.; He, J.; Zhang, H.; Guo, B. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound Dressing to Accelerate Wound Closure and Wound Healing. Chem. Mater. 2020, 32, 9937–9953. [Google Scholar] [CrossRef]
- He, Q.; Gong, K.; Ao, Q.; Ma, T.; Yan, Y.; Gong, Y.; Zhang, X. Positive Charge of Chitosan Retards Blood Coagulation on Chitosan Films. J. Biomater. Appl. 2013, 27, 1032–1045. [Google Scholar] [CrossRef]
- Wang, Y.W.; Liu, C.C.; Cherng, J.H.; Lin, C.S.; Chang, S.J.; Hong, Z.J.; Liu, C.C.; Chiu, Y.K.; Hsu, S.D.; Chang, A.H. Biological Effects of Chitosan-Based Dressing on Hemostasis Mechanism. Polymers 2019, 11, 1906. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhu, X.K.; Xue, X.T.; Wu, D.Y. Hydrogel Sheets of Chitosan, Honey and Gelatin as Burn Wound Dressings. Carbohydr. Polym. 2012, 88, 75–83. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Hu, J.; Qian, W.; Chen, L.; Zhang, D. Recent Advances in Nanotherapeutics for the Treatment of Burn Wounds. Burns Trauma 2021, 9, tkab026. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold Nanoparticles: A New X-ray Contrast Agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional Gold Nanoparticles as Potent Antimicrobial Agents Against Multi-drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Esumi, K.; Takei, N.; Yoshimura, T. Antioxidant-Potentiality of Gold-Chitosan Nanocomposites. Colloids Surf. B Biointerfaces 2003, 32, 117–123. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, F.; Park, Y.S.; Wang, J.; Shin, M.C.; Chung, H.S.; Yang, V.C. Co-administration of Protein Drugs with Gold Nanoparticles to Enable Percutaneous Delivery. Biomaterials 2010, 31, 9086–9091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial Cellulose: A Versatile Biopolymer for Wound Dressing Applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Avalshahr, A.R.; Khorsand-Ghayeni, M.; Nokhasteh, S.; Molavi, A.M.; Naderi-Meshkin, H. Synthesis and Characterization of PLGA/Collagen Composite Scaffolds as Skin Substitute Produced by Electrospinning Through Two Different Approaches. J. Mater. Sci. Mater. Med. 2017, 28, 14. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and Advanced Technologies for Burns: Preventing Infection and Facilitating Wound Healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan Based Metallic Nanocomposite Scaffolds as Antimicrobial Wound Dressings. Bioact. Mater. 2017, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, H.; Pan, S.; Fu, C.; Chang, Y.; Li, H.; Yang, X.; Qi, Z. Evaluation of New Film Based on Chitosan/Gold Nanocomposites on Antibacterial Property and Wound-Healing Efficacy. Adv. Mater. Sci. Eng. 2020, 2020, 6212540. [Google Scholar] [CrossRef]
- Lin, C.A.; Yang, T.Y.; Lee, C.H.; Huang, S.H.; Sperling, R.A.; Zanella, M.; Li, J.K.; Shen, J.L.; Wang, H.H.; Yeh, H.I.; et al. Synthesis, Characterization, and Bioconjugation of Fluorescent Gold Nanoclusters Toward Biological Labeling Applications. ACS Nano 2009, 3, 395–401. [Google Scholar] [CrossRef]
- Aldeek, F.; Safi, M.; Zhan, N.; Palui, G.; Mattoussi, H. Understanding the Self-Assembly of Proteins onto Gold Nanoparticles and Quantum Dots Driven by Metal-Histidine Coordination. ACS Nano 2013, 7, 10197–10210. [Google Scholar] [CrossRef]
- Gaines, C.; Poranki, D.; Du, W.; Clark, R.A.; Van Dyke, M. Development of a Porcine Deep Partial Thickness Burn Model. Burns 2013, 39, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Cherng, J.H.; Liu, C.C.; Fang, T.J.; Hong, Z.J.; Chang, S.J.; Fan, G.Y.; Hsu, S.D. Procoagulant and Antimicrobial Effects of Chitosan in Wound Healing. Int. J. Mol. Sci. 2021, 22, 7067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan Modification and Pharmaceutical/Biomedical Applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maksym, P.V.; Vitalii, S. Chitosan as a Hemostatic Agent: Current State. Eur. J. Med. Ser. B 2015, 2, 24–33. [Google Scholar]
- Pérez, Y.; Mann, E.; Herradón, B. Preparation and Characterization of Gold Nanoparticles Capped by Peptide–Biphenyl Hybrids. J. Colloid Interface Sci. 2011, 359, 443–453. [Google Scholar] [CrossRef]
- Iglesias, E. Gold Nanoparticles as Colorimetric Sensors for the Detection of DNA Bases and Related Compounds. Molecules 2020, 25, 2890. [Google Scholar] [CrossRef]
- Ito, T.; Thachil, J.; Asakura, H.; Levy, J.H.; Iba, T. Thrombomodulin in Disseminated Intravascular Coagulation and Other Critical Conditions-A Multi-faceted Anticoagulant Protein with Therapeutic Potential. Crit. Care 2019, 23, 280. [Google Scholar] [CrossRef] [Green Version]
- Loghmani, H.; Conway, E.M. Exploring Traditional and Nontraditional Roles for Thrombomodulin. Blood 2018, 132, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Boehme, M.W.; Deng, Y.; Raeth, U.; Bierhaus, A.; Ziegler, R.; Stremmel, W.; Nawroth, P.P. Release of Thrombomodulin from Endothelial Cells by Concerted Action of TNF-alpha and Neutrophils: In Vivo and In Vitro Studies. Immunology 1996, 87, 134–140. [Google Scholar]
- Takano, S.; Kimura, S.; Ohdama, S.; Aoki, N. Plasma Thrombomodulin in Health and Diseases. Blood 1990, 76, 2024–2029. [Google Scholar] [CrossRef]
- Abdel-Hafez, N.M.; Saleh Hassan, Y.; El-Metwally, T.H. A Study on Biomarkers, Cytokines, and Growth Factors in Children with Burn Injuries. Ann. Burns Fire Disasters 2007, 20, 89–100. [Google Scholar] [PubMed]
- Papetti, M.; Herman, I.M. Mechanisms of Normal and Tumor-Derived Angiogenesis. Am. J. Physiol. Cell Physiol. 2002, 282, C947–C970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, M.F. TNF and TNFR Biology in Health and Disease. Cell. Mol. Biol. (Noisy-Le-Grand) 2001, 47, 619–635. [Google Scholar]
- Tsurumi, A.; Que, Y.A.; Ryan, C.M.; Tompkins, R.G.; Rahme, L.G. TNF-α/IL-10 Ratio Correlates with Burn Severity and May Serve as a Risk Predictor of Increased Susceptibility to Infections. Front. Public Health 2016, 4, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwacha, M.G. Macrophages and Post-Burn Immune Dysfunction. Burns 2003, 29, 1–14. [Google Scholar] [CrossRef]
- Au, K.; Ehrlich, H.P. When the Smad Signaling Pathway Is Impaired, Fibroblasts Advance Open Wound Contraction. Exp. Mol. Pathol. 2010, 89, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Varga, J. Transforming Growth Factor-Beta Repression of Matrix Metalloproteinase-1 in Dermal Fibroblasts Involves Smad3. J. Biol. Chem. 2001, 276, 38502–38510. [Google Scholar] [CrossRef] [Green Version]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.H.; Efron, D.; Tantry, U.; Barbul, A. Nitric Oxide in the Healing Wound: A Time-Course Study. J. Surg. Res. 2001, 101, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Laurenzana, A.; Fibbi, G.; Veiga-Villauriz, C.; Fanelli, F.; Fracassi, F.; Onzo, A.; Bianco, G.; et al. Biomolecules from Snail Mucus (Helix Aspersa) Conjugated Gold Nanoparticles, Exhibiting Potential Wound Healing and Anti-Inflammatory Activity. Soft Matter. 2020, 16, 10876–10888. [Google Scholar] [CrossRef]
- Ahn, S.; Singh, P.; Castro-Aceituno, V.; Yesmin Simu, S.; Kim, Y.; Mathiyalagan, R.; Yang, D. Gold Nanoparticles Synthesized Using Panax Ginseng Leaves Suppress Inflammatory—Mediators Production via Blockade of NF-κB Activation in Macrophages. Artif. Cells Nanomed. Biotechnol. 2017, 45, 270–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in Wound Healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuoka, K.; Ishihara, M.; Asazuma, T.; Hattori, H.; Matsui, T.; Takase, B.; Kanatani, Y.; Fujita, M.; Saito, Y.; Yura, H.; et al. The Interaction of Chitosan with Fibroblast Growth Factor-2 and Its Protection from Inactivation. Biomaterials 2005, 26, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S.; Alitalo, K. Gene Transfer as a Tool to Induce Therapeutic Vascular Growth. Nat. Med. 2003, 9, 694–701. [Google Scholar] [CrossRef]
- Saaristo, A.; Tammela, T.; Farkkilā, A.; Kärkkäinen, M.; Suominen, E.; Yla-Herttuala, S.; Alitalo, K. Vascular Endothelial Growth Factor-C Accelerates Diabetic Wound Healing. Am. J. Pathol. 2006, 169, 1080–1087. [Google Scholar] [CrossRef] [Green Version]
- Tammela, T.; Enholm, B.; Alitalo, K.; Paavonen, K. The Biology of Vascular Endothelial Growth Factors. Cardiovasc. Res. 2005, 65, 550–563. [Google Scholar] [CrossRef]
- Chen, R.R.; Mooney, D.J. Polymeric Growth Factor Delivery Strategies for Tissue Engineering. Pharm. Res. 2003, 20, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, A.A.S.; Kumar, G.S.V. PEG Grafted Chitosan Scaffold for Dual Growth Factor Delivery for Enhanced Wound Healing. Sci. Rep. 2019, 9, 19165. [Google Scholar] [CrossRef] [Green Version]
- Skobe, M.; Hamberg, L.M.; Hawighorst, T.; Schirner, M.; Wolf, G.L.; Alitalo, K.; Detmar, M. Concurrent Induction of Lymphangiogenesis, Angiogenesis, and Macrophage Recruitment by Vascular Endothelial Growth Factor-C in Melanoma. Am. J. Pathol. 2001, 159, 893–903. [Google Scholar] [CrossRef] [Green Version]
- Haiko, P.; Makinen, T.; Keskitalo, S.; Taipale, J.; Karkkainen, M.J.; Baldwin, M.E.; Stacker, S.A.; Achen, M.G.; Alitalo, K. Deletion of Vascular Endothelial Growth Factor C (VEGF-C) and VEGF-D Is Not Equivalent to VEGF Receptor 3 Deletion in Mouse Embryos. Mol. Cell. Biol. 2008, 28, 4843–4850. [Google Scholar] [CrossRef] [Green Version]
- Rutanen, J.; Rissanen, T.T.; Markkanen, J.E.; Gruchala, M.; Silvennoinen, P.; Kivelä, A.; Hedman, A.; Hedman, M.; Heikura, T.; Ordén, M.R.; et al. Adenoviral Catheter-Mediated Intramyocardial Gene Transfer Using the Mature Form of Vascular Endothelial Growth Factor-D Induces Transmural Angiogenesis in Porcine Heart. Circulation 2004, 109, 1029–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquet-Fifield, S.; Levy, S.M.; Sato, T.; Shayan, R.; Karnezis, T.; Davydova, N.; Nowell, C.J.; Roufail, S.; Ma, G.Z.; Zhang, Y.F.; et al. Vascular Endothelial Growth Factor-d Modulates Caliber and Function of Initial Lymphatics in the Dermis. J. Investig. Dermatol. 2013, 133, 2074–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, L.; Saville, C.R.; Murray, P.J.; Cruickshank, S.M.; Hardman, M.J. Local Arginase-1 Activity Is Required for Cutaneous Wound Healing. J. Investig. Dermatol. 2013, 133, 2461–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, T.J.; Martin, P. Wound Repair at a Glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Kane, S.; Ferguson, M.W. Transforming Growth Factor Betas and Wound Healing. Int. J. Biochem. Cell Biol. 1997, 29, 63–78. [Google Scholar] [CrossRef]
- Izzi, L.; Attisano, L. Regulation of the TGF Beta Signalling Pathway by Ubiquitin-Mediated Degradation. Oncogene 2004, 23, 2071–2078. [Google Scholar] [CrossRef] [Green Version]
- Saika, S. TGF-Beta Signal Transduction in Corneal Wound Healing as a Therapeutic Target. Cornea 2004, 23, S25–S30. [Google Scholar] [CrossRef]
- Saika, S.; Okada, Y.; Miyamoto, T.; Yamanaka, O.; Ohnishi, Y.; Ooshima, A.; Liu, C.Y.; Weng, D.; Kao, W.W. Role of p38 MAP Kinase in Regulation of Cell Migration and Proliferation in Healing Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Han, G.; Owens, P.; Siddiqui, Y.; Li, A.G. Role of TGF beta-mediated inflammation in cutaneous wound healing. J. Investig. Dermatol. Symp. Proc. 2006, 11, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Cañada, C.; Bernabé-García, Á.; Liarte, S.; Rodríguez-Valiente, M.; Nicolás, F.J. Chronic Wound Healing by Amniotic Membrane: TGF-β and EGF Signaling Modulation in Re-Epithelialization. Front. Bioeng. Biotechnol. 2021, 9, 689328. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Shi, T.; Lu, L. Epidermal Growth Factor (EGF)-Induced Corneal Epithelial Wound Healing Through Nuclear Factor κB Subtype-Regulated CCCTC Binding Factor (CTCF) Activation. J. Biol. Chem. 2013, 288, 24363–24371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Chen, Y.; Yang, Z.; You, B.; Ruan, Y.C.; Peng, Y. Epidermal CFTR Suppresses MAPK/NF-κB to Promote Cutaneous Wound Healing. Cell. Physiol. Biochem. 2016, 39, 2262–2274. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.C.; Jeon, E.S.; Lee, I.H.; Kim, H.S.; Kim, M.B.; Kim, J.H. Tumor Necrosis Factor-α-Activated Human Adipose Tissue-Derived Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing Through Paracrine Mechanisms. J. Investig. Dermatol. 2011, 131, 1559–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riwaldt, S.; Corydon, T.J.; Pantalone, D.; Sahana, J.; Wise, P.; Wehland, M.; Krüger, M.; Melnik, D.; Kopp, S.; Infanger, M.; et al. Role of Apoptosis in Wound Healing and Apoptosis Alterations in Microgravity. Front. Bioeng. Biotechnol. 2021, 9, 679650. [Google Scholar] [CrossRef]
- Wennersten, A.; Holmin, S.; Mathiesen, T. Characterization of Bax and Bcl-2 in Apoptosis After Experimental Traumatic Brain Injury in the Rat. Acta Neuropathol. 2003, 105, 281–288. [Google Scholar] [CrossRef]
- He, B.; Tao, H.; Liu, S.; Wei, A. Protective Effect of Carboxymethylated Chitosan on Hydrogen Peroxide-Induced Apoptosis in Nucleus Pulposus Cells. Mol. Med. Rep. 2015, 11, 1629–1638. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Gao, J.; Jiang, L.; Wang, J. Antidiabetic Nephropathy Effects of Synthesized Gold Nanoparticles Through Mitigation of Oxidative Stress. Arab. J. Chem. 2021, 14, 103007. [Google Scholar] [CrossRef]
- Hiebert, P.; Werner, S. Regulation of Wound Healing by the NRF2 Transcription Factor-More than Cytoprotection. Int. J. Mol. Sci. 2019, 20, 3856. [Google Scholar] [CrossRef] [Green Version]
- Sykiotis, G.P.; Bohmann, D. Stress-Activated Cap’n’collar Transcription Factors in Aging and Human Disease. Sci. Signal. 2010, 3, re3. [Google Scholar] [CrossRef] [Green Version]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 Pathway: Mechanisms of Activation and Dysregulation in Cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular Mechanisms of the Keap1-Nrf2 Pathway in Stress Response and Cancer Evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherng, J.-H.; Lin, C.-A.J.; Liu, C.-C.; Yeh, J.-Z.; Fan, G.-Y.; Tsai, H.-D.; Chung, C.-F.; Hsu, S.-D. Hemostasis and Anti-Inflammatory Abilities of AuNPs-Coated Chitosan Dressing for Burn Wounds. J. Pers. Med. 2022, 12, 1089. https://doi.org/10.3390/jpm12071089
Cherng J-H, Lin C-AJ, Liu C-C, Yeh J-Z, Fan G-Y, Tsai H-D, Chung C-F, Hsu S-D. Hemostasis and Anti-Inflammatory Abilities of AuNPs-Coated Chitosan Dressing for Burn Wounds. Journal of Personalized Medicine. 2022; 12(7):1089. https://doi.org/10.3390/jpm12071089
Chicago/Turabian StyleCherng, Juin-Hong, Cheng-An J. Lin, Cheng-Che Liu, Jue-Zong Yeh, Gang-Yi Fan, Hsin-Da Tsai, Chun-Fang Chung, and Sheng-Der Hsu. 2022. "Hemostasis and Anti-Inflammatory Abilities of AuNPs-Coated Chitosan Dressing for Burn Wounds" Journal of Personalized Medicine 12, no. 7: 1089. https://doi.org/10.3390/jpm12071089
APA StyleCherng, J.-H., Lin, C.-A. J., Liu, C.-C., Yeh, J.-Z., Fan, G.-Y., Tsai, H.-D., Chung, C.-F., & Hsu, S.-D. (2022). Hemostasis and Anti-Inflammatory Abilities of AuNPs-Coated Chitosan Dressing for Burn Wounds. Journal of Personalized Medicine, 12(7), 1089. https://doi.org/10.3390/jpm12071089






