Accelerated Partial Breast Irradiation with Intraoperative Radiotherapy Is Effective in Luminal Breast Cancer Patients Aged 60 Years and Older
Abstract
1. Introduction
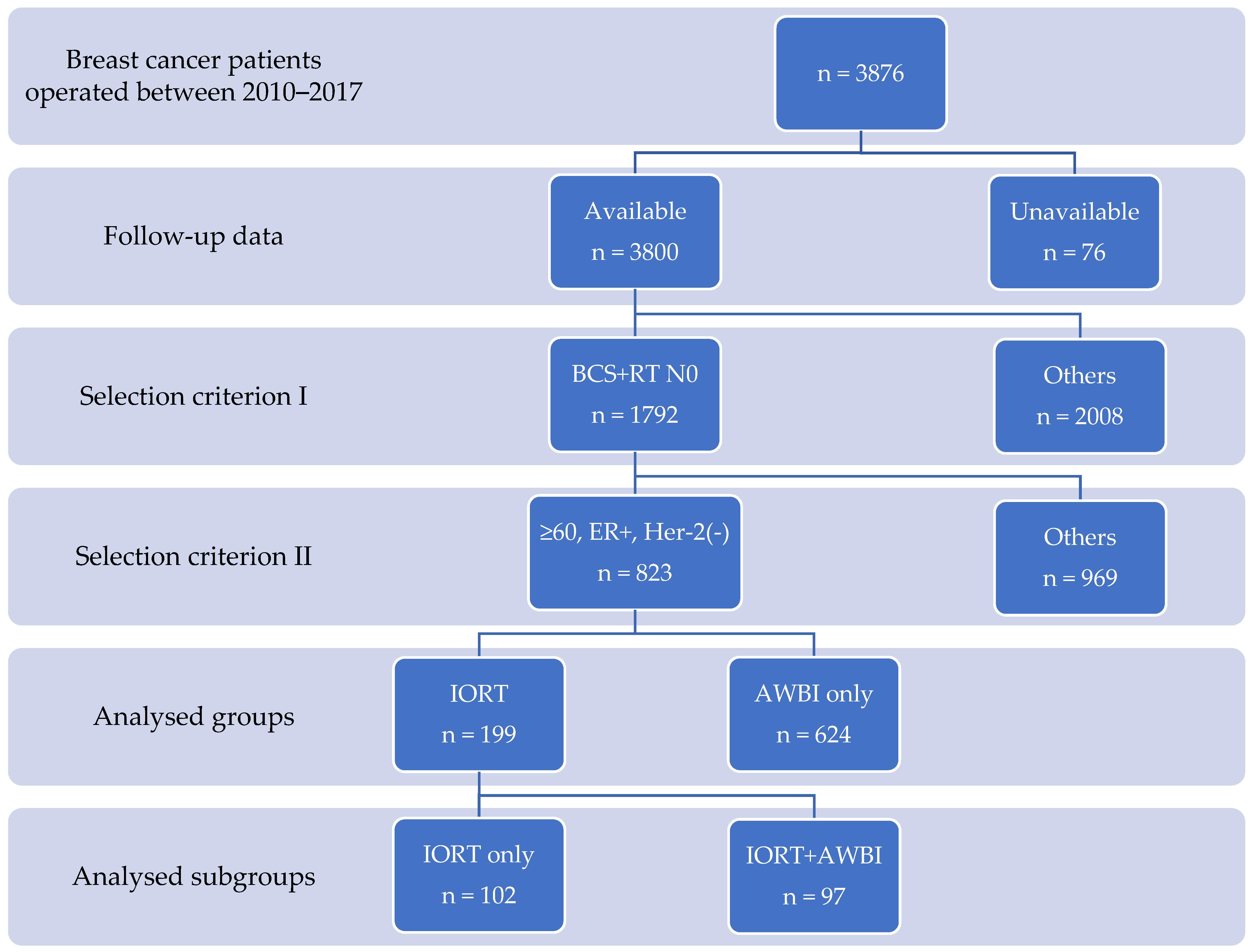
2. Materials and Methods
2.1. Adjuvant Whole Breast Radiotherapy
2.2. IORT Protocol
2.3. Studied Groups
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviation
| APBI | accelerated partial breast irradiation |
| ASTRO | American Society for Therapeutic Radiation Oncology |
| AWBI | adjuvant whole breast irradiation |
| BCS | breast-conserving surgery |
| BCSS | breast cancer-specific survival |
| DMFS | distant metastasis free survival |
| DCIS | ductal carcinoma in situ |
| ER | estrogen receptor |
| IBRFS | breast relapse-free survival |
| IORT | intraoperative radiotherapy |
| LRRFS | locoregional relapse-free survival |
| LVI | lymphatic vessels invasion |
| OS | overall survival |
| SLN | sentinel lymph node |
References
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [PubMed]
- Sjöström, M.; Lundstedt, D.; Hartman, L.; Holmberg, E.; Killander, F.; Kovács, A.; Malmström, P.; Niméus, E.; Rönnerman, E.W.; Fernö, M.; et al. Response to radiotherapy after breast-conserving surgery in different breast cancer subtypes in the Swedish Breast Cancer Group 91 Radiotherapy Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Killander, F.; Karlsson, P.; Anderson, H.; Mattsson, J.; Holmberg, E.; Lundstedt, D.; Malmström, P. No breast cancer subgroup can be spared postoperative radiotherapy after breast-conserving surgery. Fifteen-year results from the Swedish Breast Cancer Group randomised trial, SweBCG 91 RT. Eur. J. Cancer 2016, 67, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.L.; A Cameron, D.; Dixon, J.M. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015, 16, 266–273. [Google Scholar] [CrossRef]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef]
- Martelli, G.; Boracchi, P.; Guzzetti, E.; Marano, G.; Lozza, L.; Agresti, R.; Ferraris, C.; Piromalli, D.; Greco, M. Omission of radiotherapy in elderly patients with early breast cancer: 15-Year results of a prospective non-randomised trial. Eur. J. Cancer 2015, 51, 1358–1364. [Google Scholar] [CrossRef]
- Veronesi, U.; Orecchia, R.; Maisonneuve, P.; Viale, G.; Rotmensz, N.; Sangalli, C.; Luini, A.; Veronesi, P.; Galimberti, V.; Zurrida, S.; et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): A randomised controlled equivalence trial. Lancet Oncol. 2013, 14, 1269–1277. [Google Scholar] [CrossRef]
- Leonardi, M.C.; Maisonneuve, P.; Mastropasqua, M.G.; Morra, A.; Lazzari, R.; Dell’Acqua, V.; Ferrari, A.; Rotmensz, N.; Sangalli, C.; Luini, A.; et al. Accelerated partial breast irradiation with intraoperative electrons: Using GEC-ESTRO recommendations as guidance for patient selection. Radiother. Oncol. 2013, 106, 21–27. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Wenz, F.; Bulsara, M.; Tobias, J.S.; Joseph, D.J.; Keshtgar, M.; Flyger, H.L.; Massarut, S.; Alvarado, M.; Saunders, C.; et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 2014, 383, 603–613. [Google Scholar] [CrossRef]
- Hannoun-Lévi, J.-M.; Kee, D.L.C.; Gal, J.; Schiappa, R.; Hannoun, A.; Gautier, M.; Boulahssass, R.; Peyrottes, I.; Barranger, E.; Ferrero, J.-M.; et al. Accelerated partial breast irradiation for suitable elderly women using a single fraction of multicatheter interstitial high-dose-rate brachytherapy: Early results of the Single-Fraction Elderly Breast Irradiation (SiFEBI) Phase I/II trial. Brachytherapy 2018, 17, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Strnad, V.; Ott, O.J.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; Guinot, J.L.; Dunst, J.; Miguelez, C.G.; et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet 2016, 387, 229–238. [Google Scholar] [PubMed]
- Polgár, C.; Fodor, J.; Major, T.; Sulyok, Z.; Kásler, M. Breast-conserving therapy with partial or whole breast irradiation: Ten-year results of the Budapest randomized trial. Radiother. Oncol. 2013, 108, 197–202. [Google Scholar] [CrossRef] [PubMed]
- E Coles, C.; Griffin, C.L.; Kirby, A.M.; Titley, J.; Agrawal, R.K.; Alhasso, A.; Bhattacharya, I.S.; Brunt, A.M.; Ciurlionis, L.; Chan, C.; et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017, 390, 1048–1060. [Google Scholar] [CrossRef]
- Livi, L.; Meattini, I.; Marrazzo, L.; Simontacchi, G.; Pallotta, S.; Saieva, C.; Paiar, F.; Scotti, V.; Cardillo, C.D.L.; Bastiani, P.; et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur. J. Cancer 2015, 51, 451–463. [Google Scholar] [CrossRef]
- Polgár, C.; Van Limbergen, E.; Pötter, R.; Kovács, G.; Polo, A.; Lyczek, J.; Hildebrandt, G.; Niehoff, P.; Guinot, J.L.; Guedea, F.; et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: Recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother. Oncol. 2010, 94, 264–273. [Google Scholar]
- Correa, C.; Harris, E.E.; Leonardi, M.C.; Smith, B.D.; Taghian, A.G.; Thompson, A.M.; White, J.; Harris, J.R. Accelerated partial breast irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract. Radiat Oncol. 2017, 7, 73–79. [Google Scholar] [CrossRef]
- Marta, G.N.; Macedo, C.R.; Carvalho, H.D.A.; Hanna, S.A.; da Silva, J.L.F.; Riera, R. Accelerated partial irradiation for breast cancer: Systematic review and meta-analysis of 8653 women in eight randomized trials. Radiother. Oncol. 2015, 114, 42–49. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Baum, M.; Tobias, J.S.; Morgan, S.; D’Souza, D. The novel technique of delivering targeted intraoperative radiotherapy (Targit) for early breast cancer. Eur. J. Surg. Oncol. 2002, 28, 447–454. [Google Scholar] [CrossRef][Green Version]
- Falco, M.; Masojć, B.; Milchert-Leszczyńska, M.; Kram, A. Frequency of whole breast irradiation (WBRT) after intraoperative radiotherapy (IORT) is strongly influenced by institutional protocol qualification criteria. Rep. Pract. Oncol. Radiother. 2018, 23, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.; Zebak, S.; Alba, V.; Mah, K.; Au, C.; Vos, L.; Ghosh, S.; Abraham, A.; Chafe, S.; Wiebe, E.; et al. Adjuvant breast radiotherapy, endocrine therapy, or both after breast conserving surgery in older women with low-risk breast cancer: Results from a population-based study. Radiother. Oncol. 2021, 154, 93–100. [Google Scholar] [CrossRef] [PubMed]
- van der Leij, F.; van Werkhoven, E.; Bosma, S.C.; Linn, S.; J.Rutgers, E.; J.van de Vijver, M.; Bartelink, H.; Elkhuizen, P.H.M.; Scholten, A. Low risk of recurrence in elderly patients treated with breast conserving therapy in a single institute. Breast 2016, 30, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Fyles, A.W.; McCready, D.R.; Manchul, L.A.; Trudeau, M.E.; Merante, P.; Pintilie, M.; Weir, L.M.; Olivotto, I.A. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N. Engl. J. Med. 2004, 351, 963–970. [Google Scholar] [CrossRef]
- Blamey, R.; Bates, T.; Chetty, U.; Duffy, S.; Ellis, I.; George, D.; Mallon, E.; Mitchell, M.; Monypenny, I.; Morgan, D.; et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur. J. Cancer 2013, 49, 2294–2302. [Google Scholar] [CrossRef]
- Meattini, I.; Saieva, C.; Marrazzo, L.; Di Brina, L.; Pallotta, S.; Mangoni, M.; Meacci, F.; Bendinelli, B.; Francolini, G.; Desideri, I.; et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy technique compared to whole breast irradiation for patients aged 70 years or older: Subgroup analysis from a randomized phase 3 trial. Breast Cancer Res. Treat. 2015, 153, 539–547. [Google Scholar] [CrossRef]
- Falco, M.; Masojć, B.; Kram, A. Locoregional relapse is a strong prognostic indicator of distant metastatic progression in breast cancer patients after negative sentinel lymph node biopsy. Breast. J. 2020, 26, 2364–2370. [Google Scholar] [CrossRef]
- Buszek, S.M.; Lin, H.Y.; Bedrosian, I.; Tamirisa, N.; Babiera, G.V.; Shen, Y.; Shaitelman, S.F. Lumpectomy plus hormone or radiation therapy alone for women aged 70 years or older with hormone receptor-positive early stage breast cancer in the modern era: An analysis of the National Cancer Database. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 795–802. [Google Scholar] [CrossRef]
- Herskovic, A.; Wu, X.; Christos, P.; Nagar, H. Omission of adjuvant radiotherapy in the elderly breast cancer patient: Missed opportunity? Clin. Breast Cancer 2018, 18, 418–431. [Google Scholar] [CrossRef]
- Jhawar, S.R.; Alpert, N.; Taioli, E.; Sayan, M.; Bazan, J.; Park, K.U.; Stover, D.; Cherian, M.; White, J.; Haffty, B.; et al. Adjuvant radiation therapy alone is associated with improved overall survival compared to hormonal therapy alone in older women with estrogen receptor positive early stage breast cancer. Cancer Med. 2020, 9, 8345–8354. [Google Scholar] [CrossRef]
- Goldberg, M.; Sutradhar, R.; Paszat, L.; Whelan, T.J.; Gu, S.; Fong, C.; Rakovitch, E. Patterns of adjuvant care and outcomes of elderly women with stage I breast cancer after breast-conserving surgery: A population-based analysis. Breast Cancer Res. Treat. 2019, 176, 657–667. [Google Scholar] [CrossRef] [PubMed]

| n | IBRFS | p | LRRFS | p | DMFS | p | BCSS | p | OS | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ALL | 823 | 99.6% | 99.4% | 96.8% | 97.7% | 88.8% | |||||
| IORT protocol | 199 | 99.0% | NS | 98.5% | 0.048 | 98.0% | NS | 98.0% | NS | 93.0% | p = 0.047 |
| AWBI only | 624 | 99.8% | 99.7% | 96.5% | 97.6% | 87.5% |
| IORT Protocol | AWBI only | p | ||
|---|---|---|---|---|
| 199 | 624 | |||
| Age (years) | Mean value | 67.4 (66.6–68.2) | 67.6 (67.1–68.1) | NS |
| pT | 1a-b | 85 (10.4%) | 179 (21.9%) | 0.002 |
| 1c | 94 (11.5%) | 314 (38.5%) | ||
| 2 | 20 (2.5%) | 124 (15.2%) | ||
| pSLN | Negative | 174 (21.1%) | 500 (60.7%) | 0.02 |
| Positive | 25 (3%) | 124 (15.1%) | ||
| Molecular type | Luminal A | 115 (18.5%) | 268 (43%) | 0.03 |
| Luminal B | 46 (7.4%) | 194 (31.1%) | ||
| Hormonotherapy | No | 6 (0.7%) | 51 (6.2%) | 0.013 |
| Yes | 193 (23.5%) | 573 (69.6%) | ||
| Chemotherapy | No | 5 (6.1%) | 40 (4.9%) | 0.035 |
| Yes | 194 (23.6%) | 584 (70.1%) |
| n | IBRFS | p | LRRFS | p | DMFS | p | BCSS | p | OS | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IORT only | 102 | 98.0% | NS | 98.0% | NS | 100.0% | 0.04 | 100.0% | 0.04 | 93.1% | NS |
| IORT + AWBI | 97 | 100.0% | 99.0% | 95.9% | 95.9% | 92.8% |
| IORT Only | IORT + AWBI | p | ||
|---|---|---|---|---|
| 102 | 97 | |||
| Age (years) | Mean value | 68.18 (66.89–69.48) | 66.55 (65.54–67.55) | NS |
| pT | 1a–b | 52 (26.1%) | 33 (16.6%) | 0.005 |
| 1c | 44 (22.1%) | 50 (25.1%) | ||
| 2 | 6 (3%) | 14 (7%) | ||
| pSLN | Negative | 102 (51.3%) | 72 (36.2%) | <0.0001 |
| Positive | 0 (0%) | 25 (12.5%) | ||
| Lobular | Negative | 101 (50.7%) | 81 (40.7%) | 0.0001 |
| Present | 1 (0.5%) | 16 (8%) | ||
| Molecular type | Luminal A | 56 (34.8%) | 59 (36.6%) | NS |
| Luminal B | 21 (13%) | 25 (15.5%) | ||
| LVI | No | 102 (51.3%) | 86 (43.2%) | 0.0005 |
| Yes | 0 (0%) | 11 (5.5%) | ||
| DCIS | No | 78 (39.2%) | 44 (22.1%) | <0.0001 |
| <20% | 23 (11.6%) | 38 (91.1%) | ||
| >20% | 1 (0.5%) | 15 (7.5%) | ||
| Margin | >2 mm | 88 (44.2%) | 57 (28.6%) | <0.0001 |
| ≤2 mm | 14 (7%) | 40 (20.1%) | ||
| Hormonotherapy | No | 3 (1.5%) | 3 (1.5%) | NS |
| Yes | 99 (49.7%) | 94 (47.2%) | ||
| Chemotherapy | No | 102 (51.3%) | 92 (46.2%) | 0.02 |
| Yes | 0 | 5 (2.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falco, M.; Masojć, B.; Rolla, M.; Czekała, A.; Milchert-Leszczyńska, M.; Pietruszewska, J. Accelerated Partial Breast Irradiation with Intraoperative Radiotherapy Is Effective in Luminal Breast Cancer Patients Aged 60 Years and Older. J. Pers. Med. 2022, 12, 1116. https://doi.org/10.3390/jpm12071116
Falco M, Masojć B, Rolla M, Czekała A, Milchert-Leszczyńska M, Pietruszewska J. Accelerated Partial Breast Irradiation with Intraoperative Radiotherapy Is Effective in Luminal Breast Cancer Patients Aged 60 Years and Older. Journal of Personalized Medicine. 2022; 12(7):1116. https://doi.org/10.3390/jpm12071116
Chicago/Turabian StyleFalco, Michał, Bartłomiej Masojć, Magdalena Rolla, Agnieszka Czekała, Marta Milchert-Leszczyńska, and Jolanta Pietruszewska. 2022. "Accelerated Partial Breast Irradiation with Intraoperative Radiotherapy Is Effective in Luminal Breast Cancer Patients Aged 60 Years and Older" Journal of Personalized Medicine 12, no. 7: 1116. https://doi.org/10.3390/jpm12071116
APA StyleFalco, M., Masojć, B., Rolla, M., Czekała, A., Milchert-Leszczyńska, M., & Pietruszewska, J. (2022). Accelerated Partial Breast Irradiation with Intraoperative Radiotherapy Is Effective in Luminal Breast Cancer Patients Aged 60 Years and Older. Journal of Personalized Medicine, 12(7), 1116. https://doi.org/10.3390/jpm12071116





