The Effect of Statin on Anemia in Patients with Chronic Kidney Disease and End-Stage Kidney Disease: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database, Search Terms, and Strategies
2.2. Selection Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study and Patient Characteristics
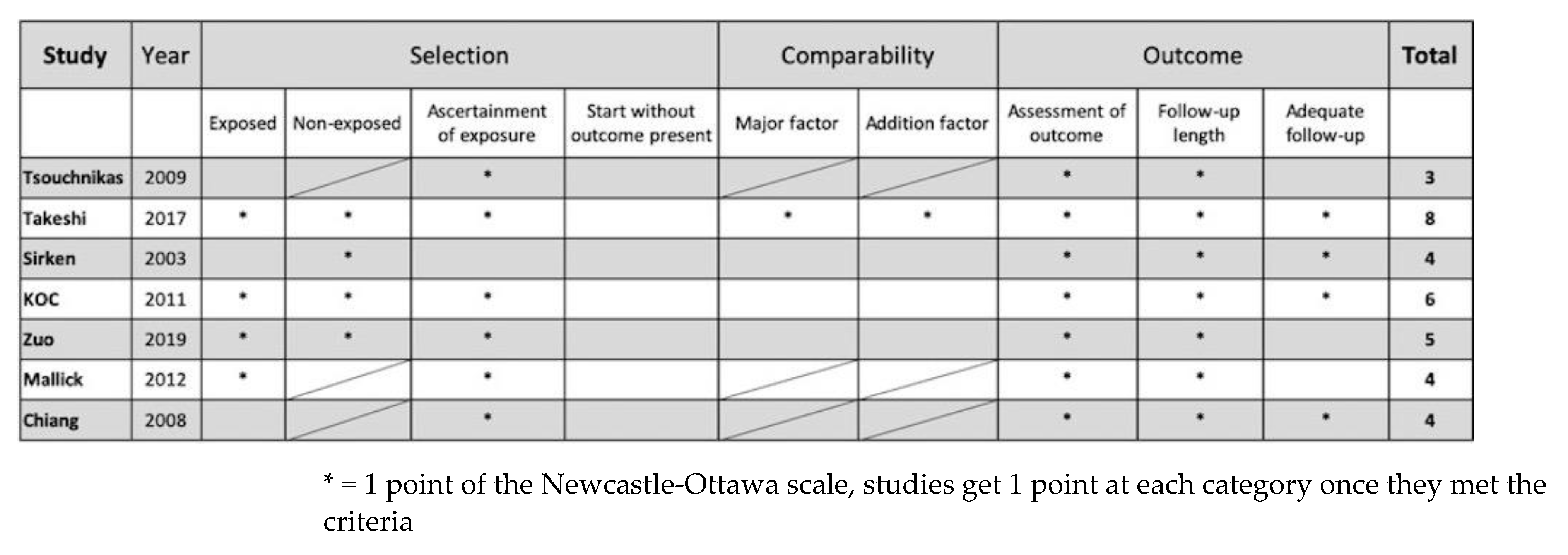
3.3. Quality Assessment
3.4. Outcomes
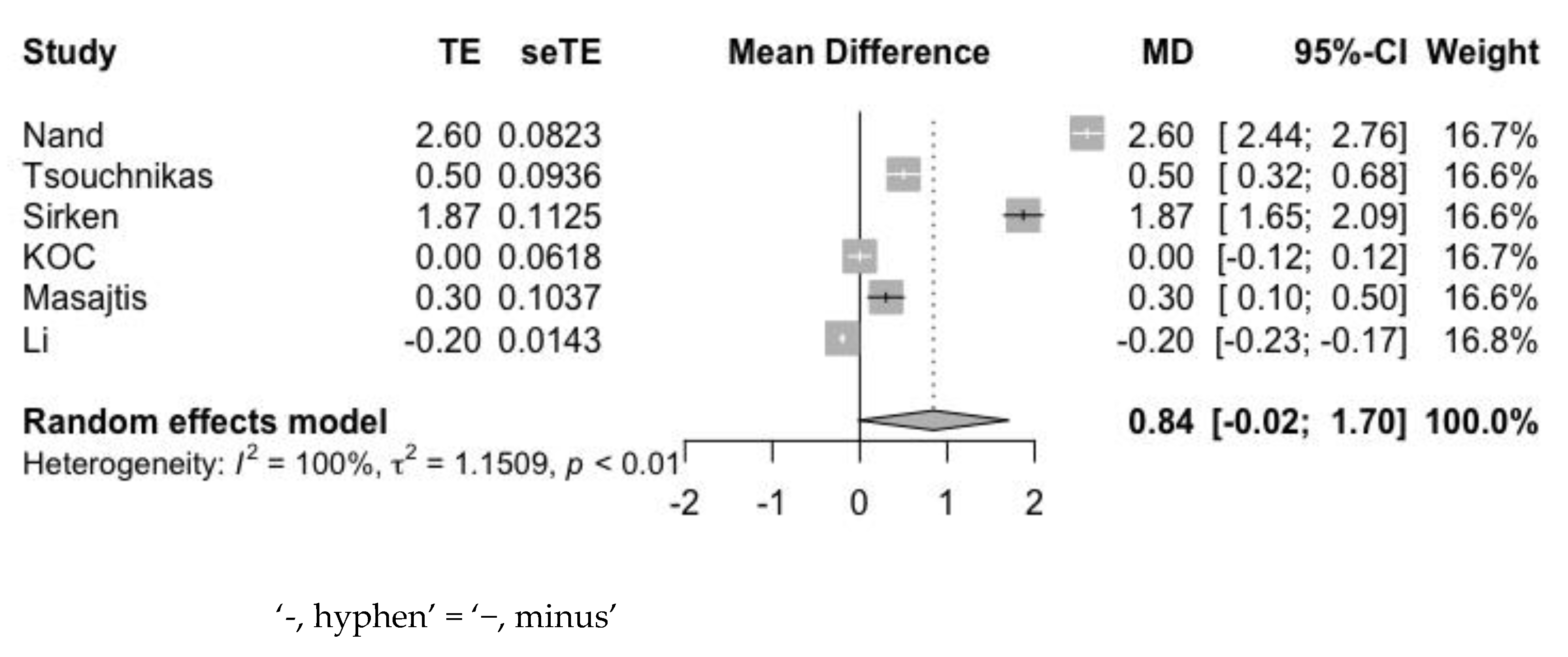
3.4.1. Hemoglobin
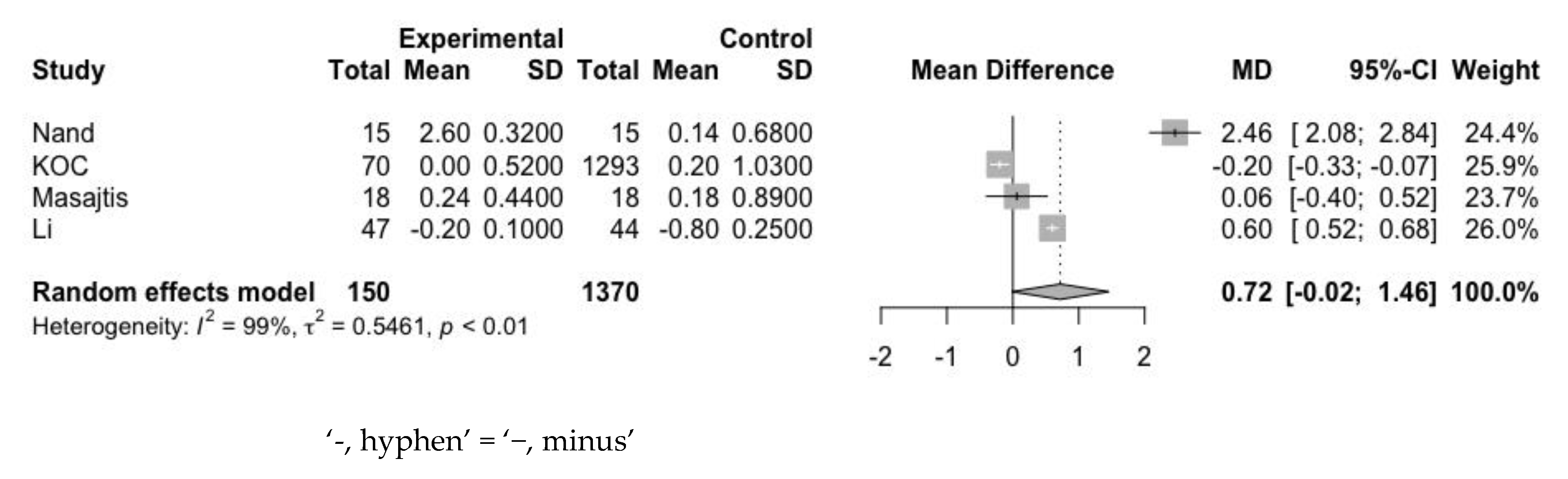
3.4.2. Ferritin
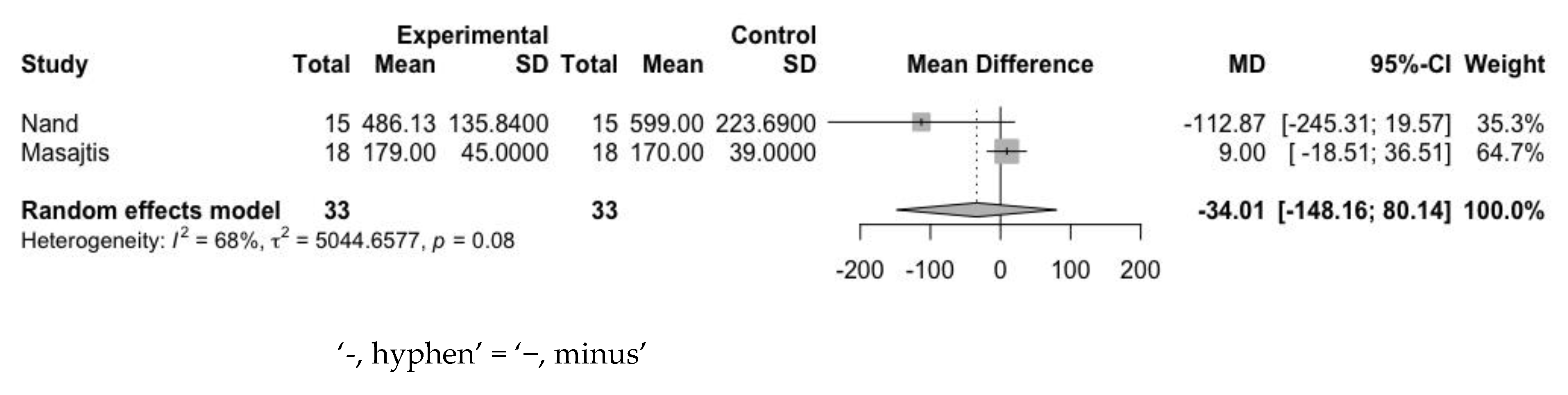
3.4.3. Erythropoietin Resistance Index
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Stauffer, M.E.; Fan, T. Prevalence of Anemia in Chronic Kidney Disease in the United States. PLoS ONE 2014, 9, e84943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.-Y.; McCulloch, C.E.; Curhan, G.C. Epidemiology of Anemia Associated with Chronic Renal Insufficiency among Adults in the United States: Results from the Third National Health and Nutrition Examination Survey. J. Am. Soc. Nephrol. 2002, 13, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Babitt, J.L.; Lin, H.Y. Mechanisms of Anemia in CKD. J. Am. Soc. Nephrol. 2012, 23, 1631–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- KDOQI. Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am. J. Kidney Dis. 2006, 47 (Suppl. 3), S11–S145. [Google Scholar] [CrossRef] [Green Version]
- Winearls, C.; Pippard, M.; Downing, M.; Oliver, D.; Reid, C.; Cotes, P.M. Effect of Human Erythropoietin Derived from Recombinant DNA on the Anaemia of Patients Maintained by Chronic Haemodialysis. Lancet 1986, 328, 1175–1178. [Google Scholar] [CrossRef]
- Eschbach, J.W.; Abdulhadi, M.H.; Browne, J.K.; Delano, B.G.; Downing, M.R.; Egrie, J.C.; Evans, R.W.; Friedman, E.A.; Graber, S.E.; Haley, N.R.; et al. Recombinant Human Erythropoietin in Anemic Patients with End-Stage Renal Disease. Ann. Intern. Med. 1989, 111, 992–1000. [Google Scholar] [CrossRef]
- Kanbay, M.; Perazella, M.A.; Kasapoglu, B.; Koroglu, M.; Covic, A. Erythropoiesis Stimulatory Agent- Resistant Anemia in Dialysis Patients: Review of Causes and Man-agement. Blood Purif. 2010, 29, 12. [Google Scholar] [CrossRef]
- Kilpatrick, R.D.; Critchlow, C.W.; Fishbane, S.; Besarab, A.; Stehman-Breen, C.; Krishnan, M.; Bradbury, B.D. Greater epoetin alfa responsiveness is associated with improved survival in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- de Francisco, A.L.M.; Stenvinkel, P.; Vaulont, S. Inflammation and its impact on anaemia in chronic kidney disease: From haemoglobin variability to hyporesponsiveness. NDT Plus 2009, 2 (Suppl. 1), i18–i26. [Google Scholar] [CrossRef] [Green Version]
- Barany, P.; Filho, J.D.; Bergström, J. High C-reactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am. J. Kidney Dis. 1997, 29, 565–568. [Google Scholar] [CrossRef]
- Goicoechea, M.; De Vinuesa, S.G.; Lahera, V.; Cachofeiro, V.; Gómez-Campderá, F.; Vega, A.; Abad, S.; Luño, J. Effects of atorvastatin on inflammatory and fibrinolytic parameters in patients with chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17 (Suppl. 3), S231–S235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deedwania, P.C. Statins in Chronic Kidney Disease: Cardiovascular Risk and Kidney Function. Postgrad. Med. 2014, 126, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Corpataux, J.-M.; Naik, J.; Porter, K.E.; London, N.J.M. The Effect of Six Different Statins on the Proliferation, Migration, and Invasion of Human Smooth Muscle Cells. J. Surg. Res. 2005, 129, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, C.; Cioppa, A.; Stabile, E.; Di Lorenzo, E.; Esposito, G.; Pisani, A.; Leccia, A.; Cavuto, L.; Stingone, A.M.; Chieffo, A.; et al. Effects of hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin on smooth muscle cell pro-liferation in vitro and neointimal formation in vivo after vascular injury. J. Am. Coll. Cardiol. 2000, 35, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Wassmann, S.; Laufs, U.; Müller, K.; Konkol, C.; Ahlbory, K.; Bäumer, A.T.; Linz, W.; Böhm, M.; Nickenig, G. Cellular Antioxidant Effects of Atorvastatin In Vitro and In Vivo. Arter. Thromb. Vasc. Biol. 2002, 22, 300–305. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, M.; Komatsu, M.; Kawaguchi, H.; Tsuchiya, K.; Nitta, K. Erythropoietin Resistance Index and the All-Cause Mortality of Chronic Hemodialysis Patients. Blood Purif. 2014, 37, 106–112. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Nand, N.; Mittal, A. Evaluation of Effect of Statins on Erythropoietin Resistance in Patients of Chronic Kidney Disease on Maintenance Haemodialysis. J. Assoc. Physicians India 2018, 66, 29–32. [Google Scholar]
- Masajtis-Zagajewska, A.; Nowicki, M. Effect of atorvastatin on iron metabolism regulation in patients with chronic kidney disease—A randomized double blind crossover study. Ren. Fail. 2018, 40, 700–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, T.; Zhao, J.; Fuller, D.S.; Bieber, B.; Zee, J.; Morgenstern, H.; Hanafusa, N.; Nangaku, M. Erythropoietin Hyporesponsiveness in Dialysis Patients: Possible Role of Statins. Am. J. Nephrol. 2017, 46, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Wang, M.; Zuo, L. Statin therapy and erythropoiesis-stimulating agent hyporesponsiveness in patients with nondialysis chronic kidney disease: A retrospective study in Beijing, China. Medicine 2019, 98, e13981. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-K.; Yang, S.-Y.; Peng, Y.-S.; Hsu, S.-P.; Pai, M.-F.; Huang, J.-W.; Hung, K.-Y.; Wu, K.-D. Atorvastatin increases erythropoietin-stimulating agent hyporesponsiveness in maintenance hemodi-alysis patients: Role of anti-inflammation effects. Am. J. Nephrol. 2009, 29, 392–397. [Google Scholar] [CrossRef]
- Tsouchnikas, I.; Dounousi, E.; Papakonstantinou, S.; Ioannou, K.; Kelesidis, A.; Kotzadamis, N.; Xanthopoulou, K.; Tsakiris, D. Beneficial effect of atorvastatin on erythropoietin responsiveness in maintenance haemodialysis pa-tients. Nephrology 2009, 14, 560–564. [Google Scholar] [CrossRef]
- Koc, M.; Dogan, C.; Arinsoy, T.; Tonbul, Z.; Ayli, D.; Cirit, M.; Sever, M.S.; Yilmaz, M.E.; Unsal, A.; Suleymanlar, G.; et al. Statin use is associated with lower inflammation and erythropoietin responsiveness index in hemodialysis patients. Hemodial. Int. 2011, 15, 366–373. [Google Scholar] [CrossRef]
- Sirken, G.; Kung, S.-C.; Raja, R. Decreased erythropoietin requirements in maintenance hemodialysis patients with statin therapy. ASAIO J. 2003, 49, 422–425. [Google Scholar] [CrossRef]
- Mallick, S.; Rafiroiu, A.; Kanthety, R.; Iqbal, S.; Malik, R.; Rahman, M. Factors Predicting Erythropoietin Resistance among Maintenance Hemodialysis Patients. Blood Purif. 2012, 33, 238–244. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, G.Z.; Chen, W.L. Protective Effection of Atorvastatin on Cardiovascular in Maintenance Hemodialysis Patients. Chin. J. Microcirc. 2019, 1, 60–63. [Google Scholar]
- Locatelli, F.; Andrulli, S.; Memoli, B.; Maffei, C.; Del Vecchio, L.; Aterini, S.; De Simone, W.; Mandalari, A.; Brunori, G.; Amato, M.; et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol. Dial. Transplant. 2006, 21, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Kooistra, M.P.; Niemantsverdriet, E.C.; Van Es, A.; Mol-Beermann, N.M.; Struyvenberg, A.; Marx, J.J.M. Iron absorption in erythropoietin-treated haemodialysis patients: Effects of iron availability, inflammation and aluminium. Nephrol. Dial. Transplant. 1998, 13, 82–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolic, D.; Nikfar, S.; Salari, P.; Rizzo, M.; Ray, K.K.; Pencina, M.J.; Mikhailidis, D.P.; Toth, P.P.; Nicholls, S.J.; Rysz, J.; et al. Effects of statins on lipid profile in chronic kidney disease patients: A meta-analysis of randomized controlled trials. Curr. Med. Res. Opin. 2013, 29, 435–451. [Google Scholar] [CrossRef] [PubMed]






| Study | N (Total N = 5258) |
Statin (Dose) |
Baseline Hb (g/dL) | Baseline ERI |
Baseline Ferritin (ng/mL) |
Patient Characteristics | Follow-Up | Study Type | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (M:F) | CKD Stage | ||||||||||
| N Nand 2018 | N = 30 | Atorvastatin (20 mg/d) | A 1 | 6.36 ± 0.81 | 38.67 ± 11.33 | 615.60 ± 261.04 | NR | ESKD (HD) | 4 months | RCT | |
| A 1 | B 2 | ||||||||||
| B 2 | 6.50 ± 1.05 | 43.62 ± 12.18 | 614.00 ± 407.11 | ||||||||
| 15 | 15 | ||||||||||
| Masajtis 2018 | N = 36 | Atorvastatin (20 mg) | A 1 | 11.6 ± 1.6 | NR | 182 ± 23 | 17:1 | CKD stage III/IV | 15 months | RCT | |
| A 1 | B 2 | ||||||||||
| B 2 | 11.7 ± 1.3 | 172 ± 24 | |||||||||
| 18 | 18 | ||||||||||
|
Li 2013 | N = 91 | Atorvastatin (20 mg/d) | A 1 | 11.7 ± 3.3 | NR | NR | 26:21 | ESKD (HD) | 6 months | RCT | |
| A 1 | B 2 | ||||||||||
| B 2 | 11.5 ± 3.8 | NR | NR | 24:20 | |||||||
| 47 | 44 | ||||||||||
|
Chiang 2008 | N = 30 | Atorvastatin (10 mg/d) | NR | NR | 641.8 ± 223 | 12:18 | ESKD (HD) | 12 weeks | Prospective cohort (Single arm) | ||
| Tsouchnikas 2009 | N = 25 | Atorvastatin (20 mg/d) (40 mg/d) 4 | 12.1 ± 1.1 | 8.34 ± 3.70 | 443.3± 203.3 | 14:11 | ESKD (HD) | 9 months | Prospective cohort (Single arm) | ||
|
Takeshi 2017 | N = 3602 | Atorvastatin Fluvastatin Lovastatin Pravastatin Rosuvastatin Simvastatin | A 1 | NR | NR | 87.0 (37.0–185.0) 3 | 304:281 | ESKD (HD) | 4 months | Prospective cohort | |
| A 1 | B 2 | B 2 | NR | NR | 99.5 (41.9–213.0) 3 | 1901:1116 | |||||
| 585 | 3017 | ||||||||||
|
Sirken 2003 | N = 38 | Atorvastatin (mean = 18.1 mg) Simvastatin (mean = 24 mg) Cerivastatin (mean = 0.4 mg) Lovastatin (mean = 20 mg) Pravastatin (mean = 20 mg) | A 1 | 10.61 ± 1.2 | NR | 618 ± 334.1 | 9:10 | ESKD (HD) | 4.7 months (mean) | Retrospective cohort | |
| A 1 | B 2 | ||||||||||
| 19 | 19 | B 2 | 11.64 ± 0.98 | NR | 470.2 ± 287 | 13:6 | |||||
|
KOC 2011 | N = 1363 | NR | A 1 | 11.1 ± 1.4 | NR | 625 (388–761) 3 | 35:35 | ESKD (HD) | NR | Retrospective cohort | |
| A 1 | B 2 | ||||||||||
| B 2 | 10.8 ± 1.6 | NR | 612 (337–1000) 3 | 737:556 | |||||||
| 70 | 1293 | ||||||||||
|
Zuo 2019 | N = 200 | Atorvastatin (20 mg, N = 35) Atorvastatin (10 mg, N = 11) Rosuvastatin (10 mg, N = 7) Simvastatin (20 mg, N = 6) Simvastatin (40 mg, N = 11) | A 1 | 7.9 ± 1.4 | NR | 231.1 (89.8–411.6) 3 | 34:43 | CKD stage III-V | 23.6 ± 13.4 months (6–56 month) | Retrospective cohort | |
| B 2 | 7.7 ± 1.7 | NR | 235.3 (81.1–453.7) 3 | 48:75 | |||||||
| A 1 | B 2 | ||||||||||
| 77 | 123 | ||||||||||
|
Mallick 2012 | N = 1305 | NR | 11.8 ± 0.95 | Mean 15 ± 14.08 Male 13.5 ± 13.2 Female 17.0 ± 14.8 | 509.6 ± 228.19 | 704:601 | ESKD (HD) | 2 years | Retrospective cohort (Single arm) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-H.; Su, F.-Y.; Chang, H.-Y.; Su, P.-C.; Chiu, L.-Y.; Nowicki, M.; Kao, C.-C.; Lin, Y.-C. The Effect of Statin on Anemia in Patients with Chronic Kidney Disease and End-Stage Kidney Disease: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 1175. https://doi.org/10.3390/jpm12071175
Tsai M-H, Su F-Y, Chang H-Y, Su P-C, Chiu L-Y, Nowicki M, Kao C-C, Lin Y-C. The Effect of Statin on Anemia in Patients with Chronic Kidney Disease and End-Stage Kidney Disease: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2022; 12(7):1175. https://doi.org/10.3390/jpm12071175
Chicago/Turabian StyleTsai, Meng-Hsu, Fu-You Su, Hao-Yun Chang, Po-Cheng Su, Li-Yun Chiu, Michal Nowicki, Chih-Chin Kao, and Yen-Chung Lin. 2022. "The Effect of Statin on Anemia in Patients with Chronic Kidney Disease and End-Stage Kidney Disease: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 12, no. 7: 1175. https://doi.org/10.3390/jpm12071175
APA StyleTsai, M.-H., Su, F.-Y., Chang, H.-Y., Su, P.-C., Chiu, L.-Y., Nowicki, M., Kao, C.-C., & Lin, Y.-C. (2022). The Effect of Statin on Anemia in Patients with Chronic Kidney Disease and End-Stage Kidney Disease: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 12(7), 1175. https://doi.org/10.3390/jpm12071175







