The Association between Muscle Quantity and Overall Survival Depends on Muscle Radiodensity: A Cohort Study in Non-Small-Cell Lung Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Patient Inclusion
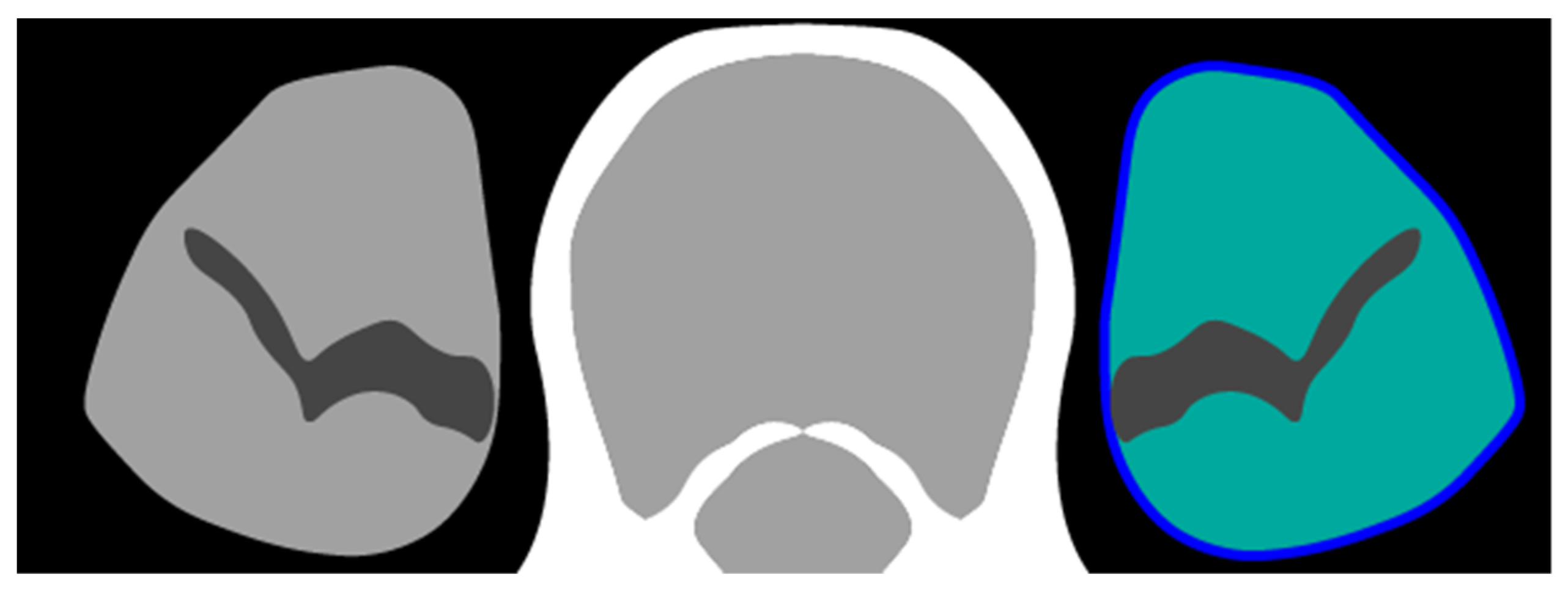
2.3. Definition of Determinants and Outcome
3. Statistical Analysis
3.1. Model Definition
3.2. Sample Size Calculation
3.3. Missing Data
3.4. Hypothesis Testing
3.5. Implementation
3.6. Reporting
4. Results
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Overall survival | (OS) |
| Non-Small Cell Lung Cancer | (NSCLC) |
| Computed tomography | (CT) |
| Psoas muscle index | (PMI) |
| Skeletal muscle index | (SMI) |
| Psoas muscle radiodensity | (PMD) |
| Skeletal muscle radiodensity | (SMD) |
| Electronic health records | (EHR) |
| Positron Emission Tomography | (PET) |
| American Joint Committee on Cancer Tumor-Node-Metastasis | (TNM) staging protocol |
| Hounsfield Units | (HU) |
| Intravenous Contrast | (IV) |
| Body mass index | (BMI) |
| Standard Deviation | (SD) |
References
- Srdic, D.; Plestina, S.; Sverko-Peternac, A.; Nikolac, N.; Simundic, A.-M.; Samarzija, M. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer—Chemotherapy toxicity and prognostic value. Supportive Care Cancer 2016, 24, 4495–4502. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.; Kazemi-Bajestani, S.M.R. Clinical outcomes related to muscle mass in humans with cancer and catabolic illnesses. Int. J. Biochem. Cell Biol. 2013, 45, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Prado, C.M.; Sullivan, E.S.; Power, D.G.; Daly, L.E. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life and survival. Nutrition 2019, 67–68, 110539. [Google Scholar] [CrossRef] [PubMed]
- Topkan, E.; Parlak, C.; Topuk, S.; Pehlivan, B. Influence of oral glutamine supplementation on survival outcomes of patients treated with concurrent chemoradiotherapy for locally advanced non-small cell lung cancer. BMC Cancer 2012, 12, 502. [Google Scholar] [CrossRef] [Green Version]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Portal, D.; Hofstetter, L.; Eshed, I.; Dan-Lantsman, C.; Sella, T.; Urban, D.; Onn, A.; Bar, J.; Segal, G. L3 skeletal muscle index (L3SMI) is a surrogate marker of sarcopenia and frailty in non-small cell lung cancer patients. Cancer Manag. Res. 2019, 11, 2579–2588. [Google Scholar] [CrossRef] [Green Version]
- Shoji, F.; Matsubara, T.; Kozuma, Y.; Haratake, N.; Akamine, T.; Takamori, S.; Katsura, M.; Toyokawa, G.; Okamoto, T.; Maehara, Y. Relationship between preoperative sarcopenia status and immuno-nutritional parameters in patients with early-stage non-small cell lung cancer. Anticancer Res. 2017, 37, 6997–7003. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Okamoto, T.; Fujishita, T.; Katsura, M.; Akamine, T.; Takamori, S.; Morodomi, Y.; Tagawa, T.; Shoji, F.; Maehara, Y. Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer 2016, 101, 92–97. [Google Scholar] [CrossRef]
- Takada, K.; Yoneshima, Y.; Tanaka, K.; Okamoto, I.; Shimokawa, M.; Wakasu, S.; Takamori, S.; Toyokawa, G.; Oba, T.; Osoegawa, A.; et al. Clinical impact of skeletal muscle area in patients with non-small cell lung cancer treated with anti-PD-1 inhibitors. J. Cancer Res. Clin. Oncol. 2020, 146, 1217–1225. [Google Scholar] [CrossRef]
- Stene, G.B.; Helbostad, J.L.; Amundsen, T.; Sørhaug, S.; Hjelde, H.; Kaasa, S.; Grønberg, B.H. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol. 2015, 54, 340–348. [Google Scholar] [CrossRef]
- Nattenmüller, J.; Wochner, R.; Muley, T.; Steins, M.; Hummler, S.; Teucher, B.; Wiskemann, J.; Kauczor, H.; Wielpütz, M.O.; Heussel, C.P. Prognostic impact of CT-quantified muscle and fat distribution before and after first-line-chemotherapy in lung cancer patients. PLoS ONE 2017, 12, e0169136. [Google Scholar] [CrossRef] [PubMed]
- Sjøblom, B.; Grønberg, B.H.; Wentzel-Larsen, T.; Baracos, V.E.; Hjermstad, M.J.; Aass, N.; Bremnes, R.M.; Fløtten, Ø.; Bye, A.; Jordhøy, M. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin. Nutr. 2016, 35, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, N.; Naito, T.; Notsu, A.; Mori, K.; Kodama, H.; Miyawaki, E.; Miyawaki, T.; Mamesaya, N.; Kobayashi, H.; Omori, S.; et al. Unfavorable impact of decreased muscle quality on the efficacy of immunotherapy for advanced non-small cell lung cancer. Cancer Med. 2021, 10, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Abbass, T.; Dolan, R.D.; MacLeod, N.; Horgan, P.G.; Laird, B.J.; McMillan, D.C. Comparison of the prognostic value of MUST, ECOG-PS, mGPS and CT derived body composition analysis in patients with advanced lung cancer. Clin. Nutr. ESPEN 2020, 40, 349–356. [Google Scholar] [CrossRef]
- Bowden, J.C.S.; Williams, L.J.; Simms, A.; Price, A.; Campbell, S.; Fallon, M.; Fearon, K. Prediction of 90 Day and Overall Survival after Chemoradiotherapy for Lung Cancer: Role of Performance Status and Body Composition. Clin. Oncol. 2017, 29, 576–584. [Google Scholar] [CrossRef]
- Cortellini, A.; Bozzetti, F.; Palumbo, P.; Brocco, D.; di Marino, P.; Tinari, N.; de Tursi, M.; Agostinelli, V.; Patruno, L.; Valdesi, C.; et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: A multicenter real-life study. Sci. Rep. 2020, 10, 1456. [Google Scholar] [CrossRef] [Green Version]
- Dolan, R.D.; Maclay, J.D.; Abbass, T.; Colville, D.; Buali, F.; MacLeod, N.; McSorley, S.T.; Horgan, P.G.; McMillan, D.C. The relationship between 18F-FDG-PETCT-derived tumour metabolic activity, nutritional risk, body composition, systemic inflammation and survival in patients with lung cancer. Sci. Rep. 2020, 10, 20819. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Takenaka, Y.; Oya, R.; Takemoto, N.; Inohara, H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: A meta-analysis. J. Cachexia Sarcopenia Muscle 2021, 12, 1122–1135. [Google Scholar] [CrossRef]
- TNM Classification of Malignant Tumours, 6th Edition. Available online: https://www.wiley.com/en-nl/TNM+Atlas%2C+6th+Edition-p-9781118695609 (accessed on 1 December 2020).
- TNM Classification of Malignant Tumours, 7th Edition. Available online: https://www.wiley.com/en-nl/TNM+Classification+of+Malignant+Tumours%2C+7th+Edition-p-9781444358964 (accessed on 1 December 2020).
- Van Erck, D.; Moeskops, P.; Schoufour, J.; Weijs, P.J.M.; Scholte Op Reimer, W.J.M.; Van Mourik, M.S.; Janmaat, Y.C.; Planken, R.N.; Vis, M.; Baan, J.; et al. Evaluation of a fully automatic deep learning-based method for the measurement of psoas muscle area. Front. Nutr. 2022, 9, 781860. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.; McFadden, E.; Carbone, P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Ettinger DS (2020) NCCN Non-Small Cell Lung Cancer Guideline, Version 1. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 15 February 2021).
- NVALT Niet Kleincellig Longcarcinoom—Resectabel en Lokaal Uitgebreid NSCLC—Richtlijn—Richtlijnendatabase. Available online: https://richtlijnendatabase.nl/index.php/richtlijn/niet_kleincellig_longcarcinoom/resectabel_en_lokaal_uitgebreid_nsclc.html (accessed on 16 July 2022).
- Little, R.J.A.; Rubin, D.B. Complete-Case and Available-Case Analysis, Including Weighting Methods. In Statistical Analysis with Missing Data; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 41–58. [Google Scholar]
- Little, R.J.A.; Rubin, D.B. Estimation of Imputation Uncertainty. In Statistical Analysis with Missing Data; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 75–93. [Google Scholar]
- Bartlett, J.W.; Seaman, S.R.; White, I.R.; Carpenter, J.R. Multiple imputation of covariates by fully conditional specification: Accommodating the substantive model. Stat. Methods Med. Res. 2015, 24, 462–487. [Google Scholar] [CrossRef] [PubMed]
- Li, K.H.; Raghunathan, T.E.; Rubin, D.B. Large-Sample Significance Levels from Multiply Imputed Data Using Moment-Based Statistics and an F Reference Distribution. J. Am. Stat. Assoc. 1991, 86, 1065–1073. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E. Reporting recommendations for tumour marker prognostic studies (REMARK). Br. J. Cancer 2005, 93, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Schomaker, M.; Heumann, C. Bootstrap Inference When Using Multiple Imputation. Stat. Med. 2018, 37, 2252–2266. [Google Scholar] [CrossRef]
- Kay, F.U.; Kandathil, A.; Batra, K.; Saboo, S.S.; Abbara, S.; Rajiah, P. Revisions to the tumor, node, metastasis staging of lung cancer (8th edition): Rationale, radiologic findings and clinical implications. World J. Radiol. 2017, 9, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Yerokun, B.A.; Yang, C.-F.J.; Gulack, B.C.; Li, X.; Mulvihill, M.S.; Gu, L.; Wang, X.; Harpole, D.H.; D’Amico, T.A.; Berry, M.F.; et al. A national analysis of wedge resection versus stereotactic body radiation therapy for stage IA non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2017, 154, 675–686.e4. [Google Scholar] [CrossRef] [PubMed]
- Rowell, N.P.; Williams, C. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable). Cochrane Database Syst. Rev. 2001, 1, CD002935. [Google Scholar] [CrossRef]
- Van Amsterdam, W.A.C.; Verhoeff, J.J.C.; Harlianto, N.I.; Bartholomeus, G.A.; Puli, A.M.; de Jong, P.A.; Leiner, T.; van Lindert, A.S.R.; Eijkemans, M.J.C.; Ranganath, R. Individual treatment effect estimation in the presence of unobserved confounding using proxies: A cohort study in stage III non-small cell lung cancer. Sci. Rep. 2022, 12, 5848. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.A.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med. 2015, 162, W1. [Google Scholar] [CrossRef] [Green Version]
- Altman, D.G.; McShane, L.M.; Sauerbrei, W.; Taube, S.E. Reporting recommendations for tumor marker prognostic studies (REMARK): Explanation and elaboration. BMC Med. 2012, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hippel, P.T. How Many Imputations Do You Need? A Two-stage Calculation Using a Quadratic Rule. Sociol. Methods Res. 2020, 49, 699–718. [Google Scholar] [CrossRef] [Green Version]
- Simmons, C.P.; Koinis, F.; Fallon, M.T.; Fearon, K.C.; Solheim, T.S.; Gronberg, B.H.; McMillan, D.C.; Gioulbasanis, I.; Laird, B.J. Prognosis in advanced lung cancer—A prospective study examining key clinicopathological factors. Lung Cancer 2015, 88, 304–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolan, R.D.; Daly, L.; Sim, W.M.J.; Fallon, M.; Ryan, A.; McMillan, D.C.; Laird, B.J. Comparison of the prognostic value of ECOG-PS, mGPS and BMI/WL: Implications for a clinically important framework in the assessment and treatment of advanced cancer. Clin. Nutr. 2020, 39, 2889–2895. [Google Scholar] [CrossRef]
- Forrest, L.M.; McMillan, D.C.; McArdle, C.S.; Angerson, W.J.; Dunlop, D.J. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br. J. Cancer 2003, 89, 1028–1030. [Google Scholar] [CrossRef] [Green Version]


| Overall | Stage I | Stage II | Stage III | Stage IV | Missing | |
|---|---|---|---|---|---|---|
| n | 2840 | 714 | 145 | 871 | 343 | 767 |
| age (mean (SD)) | 68.95 (10.44) | 72.65 (9.18) | 71.63 (10.47) | 66.53 (10.24) | 66.26 (10.21) | 68.97 (10.73) |
| male sex (%) | 1692 (59.6) | 422 (59.1) | 89 (61.4) | 531 (61.0) | 211 (61.5) | 439 (57.2) |
| histology (%) | ||||||
| adenocarcinoma | 595 (21.0) | 81 (11.3) | 32 (22.1) | 272 (31.2) | 136 (39.7) | 74 (9.6) |
| no examination | 1402 (49.4) | 482 (67.5) | 55 (37.9) | 190 (21.8) | 83 (24.2) | 592 (77.2) |
| other | 259 (9.1) | 46 (6.4) | 13 (9.0) | 121 (13.9) | 56 (16.3) | 23 (3.0) |
| squamous cell | 508 (17.9) | 74 (10.4) | 43 (29.7) | 278 (31.9) | 59 (17.2) | 54 (7.0) |
| missing | 76 (2.7) | 31 (4.3) | 2 (1.4) | 10 (1.1) | 9 (2.6) | 24 (3.1) |
| PS (%) | ||||||
| 0 | 872 (30.7) | 177 (24.8) | 23 (15.9) | 206 (23.7) | 64 (18.7) | 402 (52.4) |
| 1 | 553 (19.5) | 154 (21.6) | 29 (20.0) | 239 (27.4) | 61 (17.8) | 70 (9.1) |
| >=2 | 446 (15.7) | 102 (14.3) | 31 (21.4) | 153 (17.6) | 80 (23.3) | 80 (10.4) |
| missing | 969 (34.1) | 281 (39.4) | 62 (42.8) | 273 (31.3) | 138 (40.2) | 215 (28.0) |
| BMI (mean (SD)) | 25.66 (6.07) | 25.57 (5.96) | 25.57 (5.26) | 25.73 (5.64) | 26.42 (7.75) | 25.32 (6.23) |
| BMI missing (%) | 1500 (52.8) | 309 (43.3) | 64 (44.1) | 417 (47.9) | 212 (61.8) | 498 (64.9) |
| PMI (mean (SD)) | 6.28 (1.64) | 6.27 (1.74) | 6.09 (1.41) | 6.41 (1.59) | 6.21 (1.74) | 43.59 (8.43) |
| PMI missing (%) | 1851 (65.2) | 386 (54.1) | 79 (54.5) | 525 (60.3) | 266 (77.6) | 595 (77.6) |
| PMD (mean (SD)) | 27.93 (10.89) | 25.81 (12.28) | 26.99 (11.32) | 30.99 (9.21) | 29.33 (10.07) | 7.16 (13.88) |
| PMD missing (%) | 1637 (57.6) | 314 (44.0) | 68 (46.9) | 442 (50.7) | 262 (76.4) | 551 (71.8) |
| RT target (%) | ||||||
| lung | 1520 (53.5) | 667 (93.4) | 92 (63.4) | 179 (20.6) | 126 (36.7) | 456 (59.5) |
| multi-site | 1040 (36.6) | 29 (4.1) | 37 (25.5) | 618 (71.0) | 146 (42.6) | 210 (27.4) |
| other | 114 (4.0) | 12 (1.7) | 7 (4.8) | 16 (1.8) | 31 (9.0) | 48 (6.3) |
| mediastinum | 97 (3.4) | 5 (0.7) | 0 (0.0) | 43 (4.9) | 19 (5.5) | 30 (3.9) |
| hilus | 37 (1.3) | 0 (0.0) | 7 (4.8) | 11 (1.3) | 5 (1.5) | 14 (1.8) |
| thorax wall | 23 (0.8) | 1 (0.1) | 2 (1.4) | 4 (0.5) | 8 (2.3) | 8 (1.0) |
| brain | 8 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (2.0) | 1 (0.1) |
| missing | 1 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) |
| SBRT (%) | 1096 (38.6) | 643 (90.1) | 61 (42.1) | 29 (3.3) | 39 (11.4) | 324 (42.2) |
| deceased (%) | 1975 (69.5) | 364 (51.0) | 96 (66.2) | 674 (77.4) | 284 (82.8) | 557 (72.6) |
| survival (median) | 1.71 | 3.32 | 2.15 | 1.41 | 0.53 | 1.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Amsterdam, W.A.C.; Harlianto, N.I.; Verhoeff, J.J.C.; Moeskops, P.; de Jong, P.A.; Leiner, T. The Association between Muscle Quantity and Overall Survival Depends on Muscle Radiodensity: A Cohort Study in Non-Small-Cell Lung Cancer Patients. J. Pers. Med. 2022, 12, 1191. https://doi.org/10.3390/jpm12071191
van Amsterdam WAC, Harlianto NI, Verhoeff JJC, Moeskops P, de Jong PA, Leiner T. The Association between Muscle Quantity and Overall Survival Depends on Muscle Radiodensity: A Cohort Study in Non-Small-Cell Lung Cancer Patients. Journal of Personalized Medicine. 2022; 12(7):1191. https://doi.org/10.3390/jpm12071191
Chicago/Turabian Stylevan Amsterdam, Wouter A. C., Netanja I. Harlianto, Joost J. C. Verhoeff, Pim Moeskops, Pim A. de Jong, and Tim Leiner. 2022. "The Association between Muscle Quantity and Overall Survival Depends on Muscle Radiodensity: A Cohort Study in Non-Small-Cell Lung Cancer Patients" Journal of Personalized Medicine 12, no. 7: 1191. https://doi.org/10.3390/jpm12071191
APA Stylevan Amsterdam, W. A. C., Harlianto, N. I., Verhoeff, J. J. C., Moeskops, P., de Jong, P. A., & Leiner, T. (2022). The Association between Muscle Quantity and Overall Survival Depends on Muscle Radiodensity: A Cohort Study in Non-Small-Cell Lung Cancer Patients. Journal of Personalized Medicine, 12(7), 1191. https://doi.org/10.3390/jpm12071191







