A Quantitative and Qualitative Clinical Validation of Soft Tissue Simulation for Orthognathic Surgery Planning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Quantitative Validation
- Are the results of the fine mesh more accurate than those of the coarse mesh? It was studied if there were significant differences between the distance in the fine mesh and that of the coarse mesh, in order to know if they are equivalent or not.
- Are the results valid for clinical use? It was studied if the distances were significantly smaller than 2 and 3 mm (the most common values in the literature for the clinically accepted error in orthognathic surgery), and therefore if the results are clinically valid.
- Is the difference of mean distances between fine and coarse meshes correlated to simulation time? The reduction percentage of simulation time from fine meshes to coarse meshes was compared with the difference of their mean distances, to understand if there is a correlation between these variables.
- Is the mean distance of the anatomical landmarks equivalent to the one from mesh vertexes? The mean distance of anatomical landmarks for each type of mesh was compared with its corresponding percentage of mesh vertexes with simulation error below 3 mm, to see if these measures are equivalent or not.
2.2. Qualitative Validation
- For evaluation questions, differences between the surgeons and differences for each surgeon before and after seeing the actual surgical result were studied, to see if they agreed with each other and if they changed their opinion.
- For binary questions, percentage of answer coincidence between the surgeons was calculated, to see their level of agreement.
2.3. Quantitative vs. Qualitative Comparison
2.4. Statistical Methodology
- The normality of the quantitative variables was studied using the Kolmogorov-Smirnov test. Qualitative evaluation questions were described with first quartile, median and third quartile.
- For paired data, the Student’s t-test (parametric variables) and the Wilcoxon signed rank test (non-parametric variables) were used. For unpaired data, Pearson’s correlation (parametric variables) and Spearman’s correlation (non-parametric variables) were used.
3. Results
3.1. Quantitative Validation
3.2. Qualitative Validation
3.3. Quantitative vs. Qualitative Comparison
4. Discussion
4.1. Quantitative Validation
4.2. Qualitative Validation
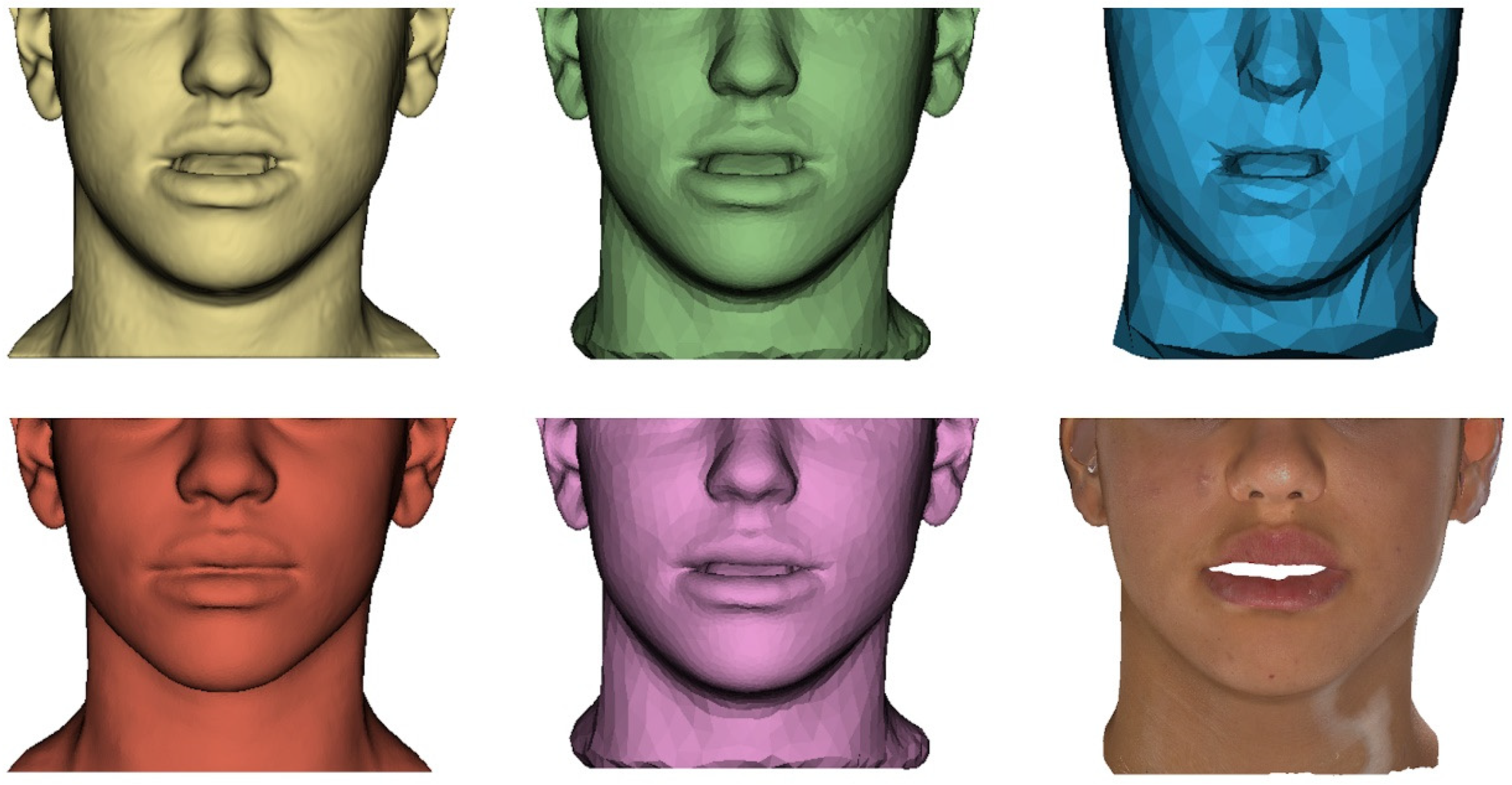
- Anatomical detail of the meshes: both surgeons consider that the fine mesh resembles the patient real anatomy (A = 4.5 and B = 4, due to lack of detail in the lips), although there is a significant difference between them (p = 0.047). However, both consider that the coarse mesh does not resemble the real anatomy (A = 2 and B = 1.5, since the characteristic features of the patient are not perceptible), with no significant differences. This means that, although the error is preserved in the coarse mesh and therefore it passes the quantitative validation, as mentioned previously, the surgeons do not consider this mesh realistic enough, so it does not pass the qualitative validation. In this regard, in the generic binary questions, surgeons comment that they could wait 1 min on average to obtain the final simulation result with the higher resolution mesh, but they do not agree on the use of the lower resolution mesh to carry out intermediate surgical planning steps in a few seconds (one surgeon would use it, the other would not).
- General accuracy: both surgeons consider that the simulation is a correct prediction of the possible result of the surgery (A = 4 and B = 4.5) and of the actual result (A = 4.25 and B = 4.75), even though there are significant differences between them in both cases (p = 0.047). The surgeons show a general positive opinion of the simulation results, although at different levels. In addition, both surgeons tend to increase the grade of the simulation accuracy when seeing the real result, as already stated in the literature [11]: for surgeon A (before = 4 and after = 4.25) the difference is not significant (p = 0.260), while for surgeon B (before = 4.5 and after = 4.75) it is (p = 0.014). Finally, the surgeons final scores to the simulations (A = 4 and B = 4.5, from the final evaluation question) coincide with these results, indicating that the overall experience matches the median of the evaluations.
- Regions accuracy: surgeons consider, both before and after seeing the actual result, that the simulation is correct, specifically, in the regions of the nose (A = 4.75 and B = 4.5 before, and A = B = 4.75 after) and chin (A = B = 4.75 before, and A = B = 5 after), without significant differences. However, both before and after seeing the actual result, there are significant differences between them (p = 0.017) in the evaluation of lips accuracy (A = 3.75 and B = 4.5 before, and A = 4 and B = 4.75 after), which is still high but lower than that of the other regions. This coincides with the quantitative results (where LL was one of the least accurate anatomical landmarks), but the difference between the two surgeons is appreciable, which shows different levels of satisfaction in this region. As in the general accuracy evaluation, the tendency of both surgeons when seeing the actual result is to maintain or increase the grade for the region accuracy of the nose (before = after = 4.75 for surgeon A, before = 4.5 and after = 4.75 for surgeon B), the lips (before = 3.75 and after = 4 for surgeon A, before = 4.5 and after = 4.75 for surgeon B) and the chin (before = 4.75 and after = 5 for both surgeons).
- Clinical use: before seeing the actual result, the simulation appears to be clinically acceptable in about half of the cases (surgeon A = 50% and surgeon B = 40%), and, after seeing it, in more than half of the cases (surgeon A = 60% and surgeon B = 80%). Even if these results are acceptable, they are way poorer than the quantitative results, where only two patients (patients 5 and 6) show a mean distance above 1 mm (Figure 4).
- Texture: both surgeons consider the textured simulation to be very realistic (surgeon A = 4.5 and surgeon B = 5), with no significant differences. In addition, they indicate that the simulation appearance improves by adding the texture in most cases (60% agreement) and therefore they would show the simulation to the patient (100% agreement), although they would need to manually modify some areas of the texture in most cases (100% agreement).
- Comparison with commercial software: the simulation seems much more reliable than the possible result from Dolphin Imaging (Dolphin Imaging & Management Solutions, Chatsworth, CA, USA), which is the commercial software used at Hospital Universitario La Paz for orthognathic surgery planning (surgeon A = 90% and surgeon B = 100%).
- Influence on the surgical planning process: both surgeons consider that in general the simulation would have influenced them considerably in the surgical planning process (A = 4.25 and B = 5, without significant differences), both to modify the bone planning if they were not satisfied with it and to maintain it if they were satisfied (90% agreement). This indicates the important role that surgical planning programs play for the surgeons, which show a great capacity to influence them.
4.3. Quantitative vs. Qualitative Comparison
- In one of the first validation studies of soft tissue simulation results [10], the authors simulated 3 patient cases using FEM. Quantitatively, they obtained a mean error of the whole face between 1 and 1.5 mm, while for qualitative validation they made a visual comparison, first superimposing the meshes and then placing them side by side. They neither indicate the computation time nor texture the results.
- The study from Mollemans and colleagues [11] represents one of the most comprehensive simulation and validation works to date. The authors compared FEM, mass spring model (MSM) and mass tensor model (MTM) simulation methods in 10 patients, obtaining a 90th percentile error of 1.51 mm for FEM, with their own system to measure distances between the two meshes. An average time of 25.7 s is reported for the FEM simulation, and texture was applied to results for greater realism. Qualitative validation was carried out with 8 surgeons through 2 experiments, corresponding to questions before seeing the actual surgical outcome and after seeing it, as in our study. The means of the surgeons’ answers to 3 questions for each patient and another 3 generic ones are mostly positive. Finally, the authors briefly comment, without analysing them in detail, some inconsistencies between the qualitative and quantitative results, which justify the need to use both validations.
- In a recent publication [13], the authors performed an automatic segmentation, MSM simulation and surgical navigation study in a single patient. On the one hand, the quantitative validation was based on the computation of the distance with three different methods, obtaining a 91% of mesh error below 2 mm and a mean error below 1 mm. The qualitative validation, on the other hand, was based on 17 evaluation questions on a 4-point Likert scale, with 12 surgeons, but the questions and answers are not indicated. Moreover, the simulation result has no texture, and the computation time is not indicated either.
- Lastly, Kim D. et al. have recently published many studies on soft tissue simulation in orthognathic surgery, focusing mainly on the lip region. In 2017 they published a simulation and validation study [12] with a cohort of 40 patients, with both types of validation. For the quantitative part, they divided the face into 8 regions, and found that all of them, except the one corresponding to the lower lip, had a mean error below 1.5 mm and a maximum error below 3 mm. For the qualitative validation, they asked 2 surgeons if the simulation results were clinically acceptable, obtaining 32 out of 40 positive answers, but they did not relate these results to the quantitative ones. In their latest study [14], the authors simulated and validated the lip region in 35 patient cases. For the quantitative validation, they divided the face into 6 regions, obtaining a mean error of 1 mm. For the qualitative part, in this case they did not consult the surgeons but mathematically analysed the shape of the lips instead, assuming that it represents the opinion of a surgeon. They analysed only the lips, obtaining 26 out of 35 clinically acceptable results. In both publications, they textured the simulation result and highlighted that the main limitation of their study was the computation time, which was less than 10 min in their first study and approximately 30 min (due to the greater complexity of the algorithm) in their second study, which precludes its use in clinical practice.
4.4. Study Limitations and Sources of Error
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Anatomical detail of the meshes: the soft tissue fine and coarse meshes before simulation were shown, together with the segmentation mesh from the preoperative CBCT image, for the validation of the simulation mesh preparation.
- Potential accuracy of the simulation: the preoperative CBCT image, bone surgical planning (osteotomies and displacements) and simulated soft tissue mesh were shown without the actual result, for a first evaluation of the simulation, as in a clinical use scenario.
- Real accuracy of the simulation: the simulated soft tissue mesh was shown again, but this time accompanied by the actual surgical outcome, for the final validation of the prediction accuracy.
- Texture: for patients with preoperative texture data (5 out of 10), the textured simulation result was shown, for the evaluation of the simulation texturing process.
- Influence on the surgical planning process: all the mentioned files were shown together for an evaluation of the influence of the simulation results on the surgical planning process.
References
- Grauer, D.; Cevidanes, L.S.H.; Proffit, W.R. Working with DICOM craniofacial images. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Cevidanes, L.H.C.; Oliveira, A.E.F.; Grauer, D.; Styner, M.; Proffit, W.R. Clinical Application of 3D Imaging for Assessment of Treatment Outcomes. Semin. Orthod. 2011, 17, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Hertanto, M.; Ayoub, A.F.; Benington, P.C.M.; Naudi, K.B.; McKenzie, P.S. Orthognathic patient perception of 3D facial soft tissue prediction planning. J. Craniomaxillofac. Surg. 2021, 49, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Rasteau, S.; Sigaux, N.; Louvrier, A.; Bouletreau, P. Three-dimensional acquisition technologies for facial soft tissues—Applications and prospects in orthognathic surgery. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 721–728. [Google Scholar] [CrossRef]
- Olivetti, E.C.; Nicotera, S.; Marcolin, F.; Vezzetti, E.; Sotong, J.P.A.; Zavattero, E.; Ramieri, G. 3D Soft-tissue prediction methodologies for orthognathic surgery—A literature review. Appl. Sci. 2019, 9, 4550. [Google Scholar] [CrossRef]
- Lee, K.J.C.; Tan, S.L.; Low, H.Y.A.; Chen, L.J.; Yong, C.W.; Chew, M.T. Accuracy of 3-dimensional soft tissue prediction for orthognathic surgery in a Chinese population. J. Stomatol. Oral Maxillofac. Surg. 2021, in press. [Google Scholar] [CrossRef]
- Khambay, B.; Ullah, R. Current methods of assessing the accuracy of three-dimensional soft tissue facial predictions: Technical and clinical considerations. Int. J. Oral Maxillofac. Surg. 2015, 44, 132–138. [Google Scholar] [CrossRef]
- Liebregts, J.; Xi, T.; Timmermans, M.; De Koning, M.; Bergé, S.; Hoppenreijs, T.; Maal, T. Accuracy of three-dimensional soft tissue simulation in bimaxillary osteotomies. J. Craniomaxillofac. Surg. 2015, 43, 329–335. [Google Scholar] [CrossRef]
- Chabanas, M.; Luboz, V.; Payan, Y. Patient specific finite element model of the face soft tissues for computer-assisted maxillofacial surgery. Med. Image Anal. 2003, 7, 131–151. [Google Scholar] [CrossRef]
- Chabanas, M.; Marecaux, C.; Chouly, F.; Boutault, F.; Payan, Y. Evaluating soft tissue simulation in maxillofacial surgery using preoperative and postoperative CT scans. Int. Congr. Ser. 2004, 1268, 419–424. [Google Scholar] [CrossRef]
- Mollemans, W.; Schutyser, F.; Nadjmi, N.; Maes, F.; Suetens, P. Predicting soft tissue deformations for a maxillofacial surgery planning system: From computational strategies to a complete clinical validation. Med. Image Anal. 2007, 11, 282–301. [Google Scholar] [CrossRef]
- Kim, D.; Ho, D.C.Y.; Mai, H.; Zhang, X.; Shen, S.G.F.; Shunyao, S.; Yuan, P.; Liu, S.; Zhang, G.; Zhou, X.; et al. A clinically validated prediction method for facial soft-tissue changes following double-jaw surgery. Med. Phys. 2017, 44, 4252–4261. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.C.; Hostettler, A.; Agnus, V.; Nicolau, S.; George, D.; Soler, L.; Rémond, Y. A New Software Suite in Orthognathic Surgery: Patient Specific Modeling, Simulation and Navigation. Surg. Innov. 2019, 26, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kuang, T.; Rodrigues, Y.L.; Gateno, J.; Shen, S.G.F.; Wang, X.; Stein, K.; Deng, H.H.; Liebschner, M.A.K.; Xia, J.J. A novel incremental simulation of facial changes following orthognathic surgery using FEM with realistic lip sliding effect. Med. Image Anal. 2021, 72, 102095. [Google Scholar] [CrossRef] [PubMed]
- Alcañiz, P.; Pérez, J.; Gutiérrez, A.; Barreiro, H.; Miraut, D.; Illana, C.; Guiñales, J.; Otaduy, M.A. Soft-Tissue Simulation for Computational Planning of Orthognathic Surgery. J. Pers. Med. 2021, 11, 982. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Ahrens, J.; Geveci, B.; Law, C. 36—ParaView: An End-User Tool for Large-Data Visualization. In The Visualization Handbook; Hansen, C.D., Johnson, C.R., Eds.; Butterworth-Heinemann: Oxford, UK, 2005; pp. 717–731. [Google Scholar] [CrossRef]
- Kolokitha, O.E.; Chatzistavrou, E. Factors Influencing the Accuracy of Cephalometric Prediction of Soft Tissue Profile Changes Following Orthognathic Surgery. J. Maxillofac. Oral Surg. 2012, 11, 82–90. [Google Scholar] [CrossRef]
- Schendel, S.A.; Jacobson, R.; Khalessi, S. 3-dimensional facial simulation in orthognathic surgery: Is it accurate? J. Oral Maxillofac. Surg. 2013, 71, 1406–1414. [Google Scholar] [CrossRef]
- Nadjmi, N.; Defrancq, E.; Mollemans, W.; Hemelen, G.V.; Bergé, S. Quantitative validation of a computer-aided maxillofacial planning system, focusing on soft tissue deformations. Ann. Maxillofac. Surg. 2014, 4, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Nam, K.U.; Hong, J. Is three-dimensional soft tissue prediction by software accurate? J. Craniofac. Surg. 2015, 26, e729–e733. [Google Scholar] [CrossRef]
- Holzinger, D.; Juergens, P.; Shahim, K.; Reyes, M.; Schicho, K.; Millesi, G.; Perisanidis, C.; Zeilhofer, H.-F.; Seemann, R. Accuracy of soft tissue prediction in surgery-first treatment concept in orthognathic surgery: A prospective study. J. Craniomaxillofac. Surg. 2018, 46, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kuang, T.; Rodrigues, Y.L.; Gateno, J.; Shen, S.G.F.; Wang, X.; Deng, H.; Yuan, P.; Alfi, D.M.; Liebschner, M.A.K.; et al. A New Approach of Predicting Facial Changes Following Orthognathic Surgery Using Realistic Lip Sliding Effect. Med. Image Comput. Comput. Assist. Interv. 2019, 11768, 336–344. [Google Scholar] [CrossRef]
- Thomas, P.M. Three-Dimensional Soft Tissue Simulation in Orthognathic Surgery. Atlas Oral Maxillofac. Surg. Clin. 2020, 28, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, R.; van Weert, H.; Loonen, T.; Bergé, S.; Vinayahalingam, S.; Baan, F.; Maal, T.; de Jong, G.; Xi, T. Three-dimensional virtual planning in mandibular advancement surgery: Soft tissue prediction based on deep learning. J. Craniomaxillofac. Surg. 2021, 49, 775–782. [Google Scholar] [CrossRef]
- Resnick, C.M.; Dang, R.R.; Glick, S.J.; Padwa, B.L. Accuracy of three-dimensional soft tissue prediction for Le Fort I osteotomy using Dolphin 3D software: A pilot study. Int. J. Oral Maxillofac. Surg. 2017, 46, 289–295. [Google Scholar] [CrossRef]
- Knoops, P.G.M.; Borghi, A.; Ruggiero, F.; Badiali, G.; Bianchi, A.; Marchetti, C.; Rodriguez-Florez, N.; Breakey, R.W.F.; Jeelani, O.; Dunaway, D.J.; et al. A novel soft tissue prediction methodology for orthognathic surgery based on probabilistic finite element modelling. PLoS ONE 2018, 13, e0197209. [Google Scholar] [CrossRef] [PubMed]
- Cunha, H.S.; da Costa Moraes, C.A.; de Faria Valle Dornelles, R.; da Rosa, E.L.S. Accuracy of three-dimensional virtual simulation of the soft tissues of the face in OrtogOnBlender for correction of class II dentofacial deformities: An uncontrolled experimental case-series study. Oral Maxillofac. Surg. 2020, 25, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Shafi, M.I.; Ayoub, A.; Ju, X.; Khambay, B. The accuracy of three- dimensional prediction planning for the surgical correction of facial deformities using Maxilim. Int. J. Oral Maxillofac. Surg. 2013, 42, 801–806. [Google Scholar] [CrossRef]
- Ullah, R.; Turner, P.J.; Khambay, B.S. Accuracy of three-dimensional soft tissue predictions in orthognathic surgery after Le Fort I advancement osteotomies. Br. J. Oral Maxillofac. Surg. 2015, 53, 153–157. [Google Scholar] [CrossRef]
- Mundluru, T.; Almukhtar, A.; Ju, X.; Ayoub, A. The accuracy of three-dimensional prediction of soft tissue changes following the surgical correction of facial asymmetry: An innovative concept. Int. J. Oral Maxillofac. Surg. 2017, 46, 1517–1524. [Google Scholar] [CrossRef]
- Knoops, P.G.M.; Borghi, A.; Breakey, R.W.F.; Ong, J.; Jeelani, N.U.O.; Bruun, R.; Schievano, S.; Dunaway, D.J.; Padwa, B.L. Three-dimensional soft tissue prediction in orthognathic surgery: A clinical comparison of Dolphin, ProPlan CMF, and probabilistic finite element modelling. Int. J. Oral Maxillofac. Surg. 2018, 48, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Elshebiny, T.; Morcos, S.; Mohammad, A.; Quereshy, F.; Valiathan, M. Accuracy of three-dimensional soft tissue prediction in orthognathic cases using Dolphin three-dimensional software. J. Craniofac. Surg. 2019, 30, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Bianchi, A.; Bassi, M.; Gori, R.; Lamberti, C.; Sarti, A. Mathematical modeling and numerical simulation in maxillofacial virtual surgery. J. Craniofac. Surg. 2007, 18, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Juergens, P.; Weber, S.; Nolte, L.P.; Reyes, M. A New Soft-Tissue Simulation Strategy for Cranio-Maxillofacial Surgery Using Facial Muscle Template Model. Prog. Biophys. Mol. Biol. 2010, 103, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.; Zachow, S.; Deuflhard, P. Craniofacial Surgery Planning Based on Virtual Patient Models. IT Inf. Technol. 2010, 52, 258–264. [Google Scholar] [CrossRef]
- Marchetti, C.; Bianchi, A.; Muyldermans, L.; Di Martino, M.; Lancellotti, L.; Sarti, A. Validation of new soft tissue software in orthognathic surgery planning. Int. J. Oral Maxillofac. Surg. 2011, 40, 26–32. [Google Scholar] [CrossRef]
- Terzic, A.; Combescure, C.; Scolozzi, P. Accuracy of Computational Soft Tissue Predictions in Orthognathic Surgery from Three-Dimensional Photographs 6 Months after Completion of Surgery: A Preliminary Study of 13 Patients. Aesthetic Plast. Surg. 2014, 38, 184–191. [Google Scholar] [CrossRef]
- El-Molla, M.M.; El-Beialy, A.R.; Kandil, A.H.; El-Bialy, A.M.; Mostafa, Y.A. Three Dimensional approach for realistic simulation of facial soft tissue response: A pilot study. Prog. Orthod. 2011, 12, 59–65. [Google Scholar] [CrossRef]
- Olate, S.; Zaror, C.; Mommaerts, M.Y. A systematic review of soft-to-hard tissue ratios in orthognathic surgery. Part IV: 3D análisis—Is there evidence? J. Craniomaxillofac. Surg. 2017, 45, 1278–1286. [Google Scholar] [CrossRef]



| Experiment | Shown Files | Question | Type |
|---|---|---|---|
| Anatomical detail of the meshes | Segmentation mesh from the preoperative CBCT image and soft tissue fine and coarse meshes | Anatomical detail of fine mesh | Evaluation |
| Anatomical detail of coarse mesh | Evaluation | ||
| Potential accuracy of the simulation | Preoperative CBCT image, bone planning and soft tissue simulation result | General accuracy (before) | Evaluation |
| Nose accuracy (before) | Evaluation | ||
| Lips accuracy (before) | Evaluation | ||
| Chin accuracy (before) | Evaluation | ||
| Valid for clinical use (before) | Binary | ||
| Real accuracy of the simulation | Postoperative CBCT image with its segmentation mesh and soft tissue simulation result | General accuracy (after) | Evaluation |
| Nose accuracy (after) | Evaluation | ||
| Lips accuracy (after) | Evaluation | ||
| Chin accuracy (after) | Evaluation | ||
| Valid for clinical use (after) | Binary | ||
| Better than commercial software | Binary | ||
| Texture | Textured simulation result | Texture realism | Evaluation |
| Improvement using texture | Binary | ||
| Perfect texture | Binary | ||
| Communication with patient | Binary | ||
| Influence on the surgical planning process | All the above | General influence | Evaluation |
| Influence on the bone planning | Binary |
| Variable | 25th (mm) | Median (mm) | 75th (mm) |
|---|---|---|---|
| Sn fine | 0.26 | 0.99 | 1.35 |
| Sn coarse | 0.42 | 1.20 | 1.52 |
| A fine | 0.12 | 0.15 | 0.77 |
| A coarse | 0.31 | 0.50 | 0.97 |
| UL fine | 0.26 | 0.70 | 1.08 |
| UL coarse | 0.34 | 0.88 | 1.58 |
| LL fine | 0.31 | 0.92 | 2.03 |
| LL coarse | 0.35 | 0.73 | 2.27 |
| B fine | 0.15 | 0.45 | 0.75 |
| B coarse | 0.21 | 0.25 | 0.53 |
| Pog fine | 0.25 | 0.29 | 0.41 |
| Pog coarse | 0.20 | 0.27 | 0.51 |
| Me fine | 0.26 | 0.43 | 0.84 |
| Me coarse | 0.40 | 0.79 | 1.10 |
| RGo fine | 0.21 | 1.04 | 2.50 |
| RGo coarse | 0.36 | 1.57 | 2.13 |
| LGo fine | 0.38 | 0.63 | 2.70 |
| LGo coarse | 0.51 | 0.91 | 2.51 |
| Mean fine | 0.61 | 0.69 | 1.04 |
| Mean coarse | 0.69 | 0.84 | 1.30 |
| Difference of mean distances | 0.10 | 0.11 | 0.20 |
| Variable | Comparison Value (mm) | Fine Mesh | Coarse Mesh |
|---|---|---|---|
| Sn | 2 | <0.001 | 0.001 |
| A | 2 | 0.005 | 0.001 |
| UL | 2 | <0.001 | <0.001 |
| LL | 2 | 0.203 | 0.381 |
| 3 | 0.059 | 0.023 | |
| B | 2 | <0.001 | <0.001 |
| Pog | 2 | 0.005 | <0.001 |
| Me | 2 | 0.007 | 0.017 |
| RGo | 2 | 0.073 | 0.074 |
| 3 | 0.001 | <0.001 | |
| LGo | 2 | 0.074 | 0.162 |
| 3 | 0.013 | 0.002 | |
| Mean | 2 | 0.005 | <0.001 |
| Question | A | B | ||||
|---|---|---|---|---|---|---|
| 25th | Median | 75th | 25th | Median | 75th | |
| Anatomical detail of fine mesh | 4.375 | 4.500 | 5.000 | 3.875 | 4.000 | 4.500 |
| Anatomical detail of coarse mesh | 2.000 | 2.000 | 3.000 | 1.375 | 1.500 | 2.125 |
| General accuracy (before) | 3.500 | 4.000 | 4.125 | 4.000 | 4.500 | 4.625 |
| Nose accuracy (before) | 3.750 | 4.750 | 5.000 | 4.000 | 4.500 | 5.000 |
| Lips accuracy (before) | 3.375 | 3.750 | 4.000 | 3.875 | 4.500 | 4.625 |
| Chin accuracy (before) | 4.000 | 4.750 | 5.000 | 4.500 | 4.750 | 5.000 |
| General accuracy (after) | 3.750 | 4.250 | 5.000 | 4.500 | 4.750 | 5.000 |
| Nose accuracy (after) | 4.000 | 4.750 | 5.000 | 4.375 | 4.750 | 5.000 |
| Lips accuracy (after) | 3.000 | 4.000 | 4.625 | 4.500 | 4.750 | 5.000 |
| Chin accuracy (after) | 4.000 | 5.000 | 5.000 | 5.000 | 5.000 | 5.000 |
| Texture realism | 4.250 | 4.500 | 5.000 | 4.500 | 5.000 | 5.000 |
| General influence | 3.750 | 4.250 | 5.000 | 4.375 | 5.000 | 5.000 |
| Question | A | B | Agreement (%) | ||
|---|---|---|---|---|---|
| Yes | No | Yes | No | ||
| Valid for clinical use (before) | 5 | 5 | 4 | 6 | 70 |
| Valid for clinical use (after) | 6 | 4 | 8 | 2 | 60 |
| Better than commercial software | 9 | 1 | 10 | 0 | 90 |
| Improvement using texture | 3 | 2 | 5 | 0 | 60 |
| Perfect texture | 1 | 4 | 1 | 4 | 100 |
| Communication with patient | 5 | 0 | 5 | 0 | 100 |
| Influence on the bone planning | 9 | 1 | 10 | 0 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez Venturini, A.; Guiñales Díaz de Cevallos, J.; del Castillo Pardo de Vera, J.L.; Alcañiz Aladrén, P.; Illana Alejandro, C.; Cebrián Carretero, J.L. A Quantitative and Qualitative Clinical Validation of Soft Tissue Simulation for Orthognathic Surgery Planning. J. Pers. Med. 2022, 12, 1460. https://doi.org/10.3390/jpm12091460
Gutiérrez Venturini A, Guiñales Díaz de Cevallos J, del Castillo Pardo de Vera JL, Alcañiz Aladrén P, Illana Alejandro C, Cebrián Carretero JL. A Quantitative and Qualitative Clinical Validation of Soft Tissue Simulation for Orthognathic Surgery Planning. Journal of Personalized Medicine. 2022; 12(9):1460. https://doi.org/10.3390/jpm12091460
Chicago/Turabian StyleGutiérrez Venturini, Alessandro, Jorge Guiñales Díaz de Cevallos, José Luis del Castillo Pardo de Vera, Patricia Alcañiz Aladrén, Carlos Illana Alejandro, and José Luis Cebrián Carretero. 2022. "A Quantitative and Qualitative Clinical Validation of Soft Tissue Simulation for Orthognathic Surgery Planning" Journal of Personalized Medicine 12, no. 9: 1460. https://doi.org/10.3390/jpm12091460






