Transarterial Embolization for Spontaneous Soft-Tissue Hematomas: Predictive Factors for Early Death
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Patient Characteristics
2.3. Imaging and Procedure Data
2.4. Outcomes
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Patient Characteristics
3.2. Imaging and Procedure Data
3.3. Outcomes and Prognostic Factors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| INR | International Normalized Ratio |
| MDCT | Multidetector row computed tomography |
| NBCA | N-butyl-2-cyanoacrylate |
| PC | Platelet count |
| PT | Prothrombin Time |
| RBC | Red Blood Cell |
| SSTH | Spontaneous soft-tissue hematoma |
| TAE | Transarterial Embolization |
References
- Decker, J.A.; Brill, L.M.; Orlowski, U.; Varga-Szemes, A.; Emrich, T.; Schoepf, U.J.; Schwarz, F.; Kröncke, T.J.; Scheurig-Münkler, C. Spontaneous Iliopsoas Muscle Hemorrhage–Predictors of Associated Mortality. Acad. Radiol. 2022, 29, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Neumayer, B.; Hassler, E.; Petrovic, A.; Widek, T.; Ogris, K.; Scheurer, E. Age determination of soft tissue hematomas: Hematoma Age Determination. NMR Biomed. 2014, 27, 1397–1402. [Google Scholar] [CrossRef]
- Touma, L.; Cohen, S.; Cassinotto, C.; Reinhold, C.; Barkun, A.; Tran, V.T.; Banon, O.; Valenti, D.; Gallix, B.; Dohan, A. Transcatheter Arterial Embolization of Spontaneous Soft Tissue Hematomas: A Systematic Review. Cardiovasc. Interv. Radiol. 2019, 42, 335–343. [Google Scholar] [CrossRef]
- Llitjos, J.F.; Daviaud, F.; Grimaldi, D.; Legriel, S.; Georges, J.L.; Guerot, E.; Bedos, J.P.; Fagon, J.Y.; Charpentier, J.; Mira, J.P. Ilio-psoas hematoma in the intensive care unit: A multicentric study. Ann. Intensive Care 2016, 6, 8. [Google Scholar] [CrossRef]
- Nagraj, S.K.; Prashanti, E.; Aggarwal, H.; Lingappa, A.; Muthu, M.S.; Krishanappa, S.K.K.; Hassan, H. Interventions for treating post-extraction bleeding. Cochrane Database Syst. Rev. 2018, 3, CD011930. [Google Scholar]
- Menditto, V.G.; Fulgenzi, F.; Lombardi, S.; Dimitriadou, A.; Mincarelli, C.; Rosati, M.; Candelari, R.; Pomponio, G.; Salvi, A.; Gabrielli, A. Management of spontaneous soft-tissue hemorrhage secondary to anticoagulant therapy: A cohort study. Am. J. Emerg. Med. 2018, 36, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.; Pellerin, O.; Tran, V.T.; Gallix, B.; Boucher, L.M.; Valenti, D.; Sapoval, M.; Soyer, P.; Dohan, A. Predictors of Mortality from Spontaneous Soft-Tissue Hematomas in a Large Multicenter Cohort Who Underwent Percutaneous Transarterial Embolization. Radiology 2019, 291, 250–258. [Google Scholar] [CrossRef]
- Luk, L.; Steinman, J.; Newhouse, J.H. Intravenous Contrast-Induced Nephropathy—The Rise and Fall of a Threatening Idea. Adv. Chronic Kidney Dis. 2017, 24, 169–175. [Google Scholar] [CrossRef]
- van der Molen, A.J.; Reimer, P.; Dekkers, I.A.; Bongartz, G.; Bellin, M.F.; Bertolotto, M.; Clement, O.; Heinz-Peer, G.; Stacul, F.; Webb, J.A.; et al. Post-contrast acute kidney injury—Part 1: Definition, clinical features, incidence, role of contrast medium and risk factors: Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur. Radiol. 2018, 28, 2845–2855. [Google Scholar] [CrossRef]
- Sharafuddin, M.J.; Andresen, K.J.; Sun, S.; Lang, E.; Stecker, M.S.; Wibbenmeyer, L.A. Spontaneous extraperitoneal hemorrhage with hemodynamic collapse in patients undergoing anticoagulation: Management with selective arterial embolization. J. Vasc. Interv. Radiol. 2001, 12, 1231–1234. [Google Scholar] [CrossRef]
- Klausenitz, C.; Kuehn, J.P.; Noeckler, K.; Radosa, C.G.; Hoffmann, R.T.; Teichgraeber, U.; Mensel, B. Efficacy of transarterial embolisation in patients with life-threatening spontaneous retroperitoneal haematoma. Clin. Radiol. 2021, 76, 157.e11–157.e18. [Google Scholar] [CrossRef] [PubMed]
- Popov, M.; Sotiriadis, C.; Gay, F.; Jouannic, A.-M.; Lachenal, Y.; Hajdu, S.D.; Doenz, F.; Qanadli, S.D. Spontaneous Intramuscular Hematomas of the Abdomen and Pelvis: A New Multilevel Algorithm to Direct Transarterial Embolization and Patient Management. Cardiovasc. Interv. Radiol. 2017, 40, 537–545. [Google Scholar] [CrossRef]
- Artzner, T.; Clere-Jehl, R.; Schenck, M.; Greget, M.; Merdji, H.; De Marini, P.; Tuzin, N.; Helms, J.; Meziani, F. Spontaneous ilio-psoas hematomas complicating intensive care unit hospitalizations. PLoS ONE 2019, 14, e0211680. [Google Scholar] [CrossRef]
- Basile, A.; Medina, J.G.; Mundo, E.; Medina, V.G.; Leal, R. Transcatheter arterial embolization of concurrent spontaneous hematomas of the rectus sheath and psoas muscle in patients undergoing anticoagulation. Cardiovasc. Interv. Radiol. 2004, 27, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Teta, M.; Drabkin, M.J. Fatal retroperitoneal hematoma associated with Covid-19 prophylactic anticoagulation protocol. Radiol. Case Rep. 2021, 16, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Qanadli, S.D.; El Hajjam, M.; Mignon, F.; Bruckert, F.; Chagnon, S.; Lacombe, P. Life-threatening spontaneous psoas haematoma treated by transcatheter arterial embolization. Eur. Radiol. 1999, 9, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Patidar, Y.; Srinivasan, S.V.; Singh, J.; Patel, R.K.; Chandel, K.; Mukund, A.; Sharma, M.K.; Sarin, S.K. Clinical Outcomes of Transcatheter Arterial Embolization Using N-butyl-2-cyanoacrylate (NBCA) in Cirrhotic Patients. J. Clin. Exp. Hepatol. 2022, 12, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.H.; Jae, H.J.; Kim, H.-C.; Chung, J.W.; Park, J.H. Transcatheter arterial embolization of intramuscular active hemorrhage with N-butyl cyanoacrylate. Cardiovasc. Interv. Radiol. 2012, 35, 292–298. [Google Scholar] [CrossRef]
- Khalilzadeh, O.; Baerlocher, M.O.; Shyn, P.B.; Connolly, B.L.; Devane, A.M.; Morris, C.S.; Cohen, A.M.; Midia, M.; Thornton, R.H.; Gross, K.; et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 2017, 28, 1432–1437.e3. [Google Scholar] [CrossRef]
- Nakayama, M.; Kato, K.; Yoshioka, K.; Sato, H. Coagulopathy-related soft tissue hematoma: A comparison between computed tomography findings and clinical severity. Acta Radiol. Open 2020, 9, 2058460120923266. [Google Scholar] [CrossRef]
- Dohan, A.; Sapoval, M.; Chousterman, B.G.; di Primio, M.; Guerot, E.; Pellerin, O. Spontaneous Soft-Tissue Hemorrhage in Anticoagulated Patients: Safety and Efficacy of Embolization. Am. J. Roentgenol. 2015, 204, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, Y.; Rodallec, M.H.; Boulay-Coletta, I.; Jullès, M.-C.; Ridereau-Zins, C.; Zins, M. Multidetector CT angiography in acute gastrointestinal bleeding: Why, when, and how. Radiographics 2011, 31, E35–E46. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.E.; Ridley, L.J. Diagnostic accuracy of CT angiography in acute gastrointestinal bleeding. J. Med. Imaging Radiat. Oncol. 2008, 52, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Djaber, S.; Bohelay, G.; Moussa, N.; Déan, C.; del Giudicce, C.; Sapoval, M.; Dohan, A.; Pellerin, O. Cutaneous necrosis after embolization of spontaneous soft-tissue hematoma of the abdominal wall. Diagn. Interv. Imaging 2018, 99, 831–833. [Google Scholar] [CrossRef]
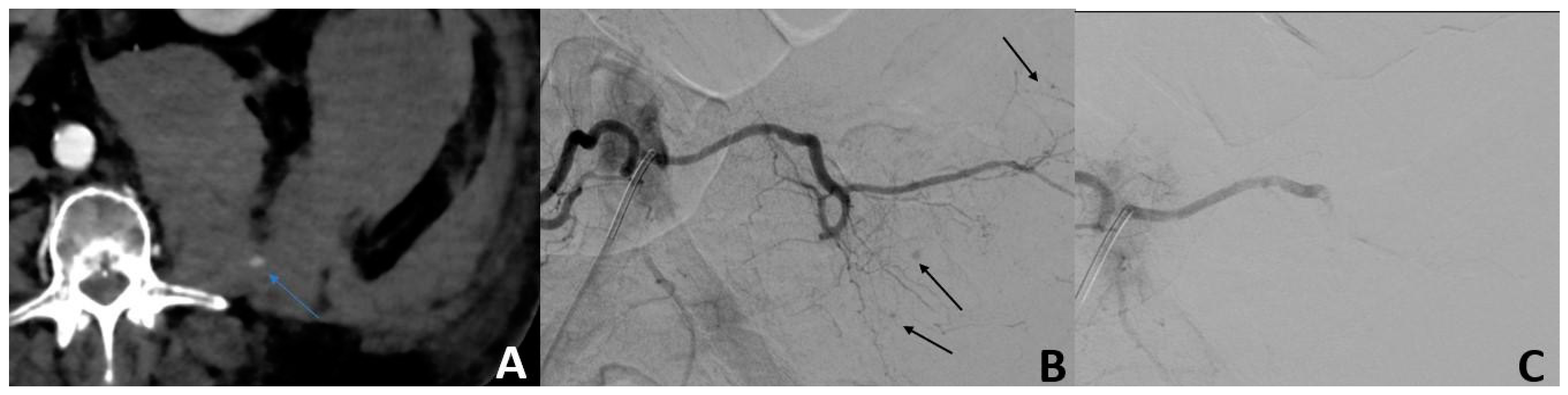
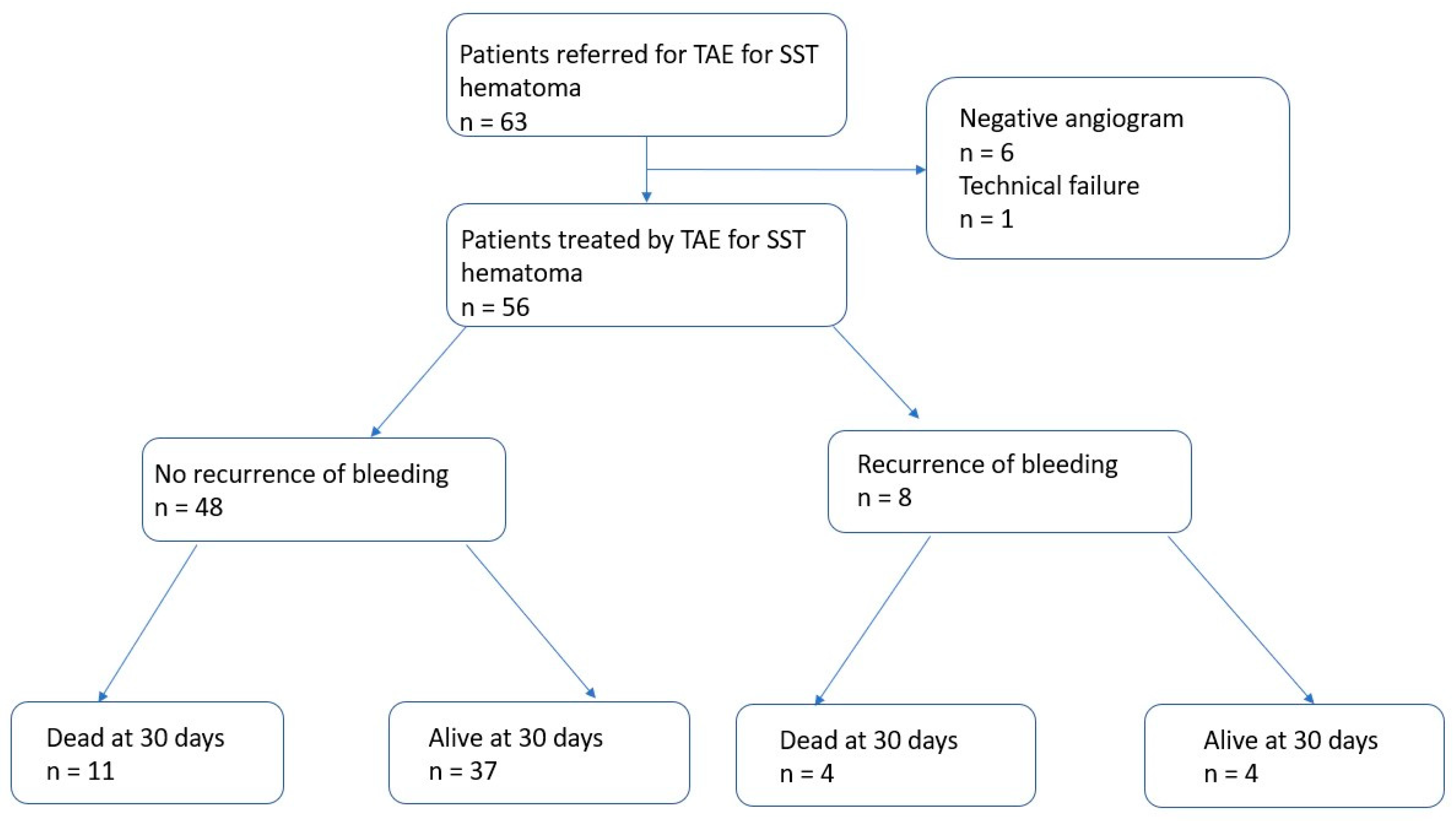

| Variables | 56 |
|---|---|
| Age, years median [Q25–75] | 75.5 [39–83] |
| Male n. (%) | 23 (41.1) |
| Performance Status median [Q25–75] | 1 [0–2] |
| Comorbidities n. (%) | |
| Diabete | 16 (33.9) |
| HBP | 27 (48.2) |
| Chronic renal failure | 9 (16.1) |
| History of cancer | 12 (21.4) |
| Cirrhosis | 4 (7.1) |
| Antithrombotic therapy, n. (%) | 51 (91.1) |
| Indication for antithrombotic therapy, n. (%) | |
| Atrial fibrillation | 27 (53.0) |
| Deep venous thrombosis | 10 (19.6) |
| Ischemic stroke | 5 (9.8) |
| Cardiopathy | 3 (5.9) |
| Mechanical valve prosthesis | 2 (3.9) |
| Prophylaxy | 2 (3.9) |
| Others | 2 (3.9) |
| Hospitalized at the time of diagnosis n. (%) | 24 (42.9) |
| Causes of hospitalization n. (%) | |
| Infection | 7 (12.5) |
| Surgery | 8 (14.3) |
| Cardiovascular disease | 7 (12.5) |
| Infection and surgery | 2 (3.6) |
| Hemodynamic instability n. (%) | 30 (53.6) |
| Main symptom n. (%) | |
| Abdominal pain | 26 (46.4) |
| Tumefaction | 4 (7.2) |
| No clinical symptom | 26 (46.4) |
| Variables | 56 |
|---|---|
| Antithrombotic therapy n. (%) | 51 |
| Antiplatelet | 5 (8.9) |
| Clopidogrel | 2 |
| Aspirin | 3 |
| Anticoagulant | 39 (69.6) |
| LMWH | 7 |
| UFH | 14 |
| VKA | 12 |
| Apixaban | 3 |
| Rivaroxaban | 2 |
| Antiplatelet and Anticoagulation | 7 (12.5) |
| VKA + Aspirin | 1 |
| UFH + Aspirin | 6 |
| No antithrombotic therapy | 5 (8.9) |
| Discontinuation of anticoagulant therapy n. (%) | 51 (100) |
| Overdosage n. (%) | 13 (25.5) |
| Reversion of anticoagulant therapy n. (%) | 13 (25.5) |
| Prothrombin complex concentrates | 10 (19.6) |
| K Vitamin | 9 (17.6) |
| Tranexamic Acid | 5 (9.8) |
| Biology, median [Q25–75] | |
| INR | 1.45 [1–2.7] |
| PT(%) | 65 [8–75] |
| Platelet (G/L) | 180 [38–255] |
| Hemoglobin (g/dL) | 7.6 [4.4–8.2] |
| Drop of Hemoblogin (g/dL) | 3.45 [0.9–4.9] |
| RBC Transfusion n. (%) | 41 (71.9) |
| Number of RBC, median [Q25–75] | 4 [1–7.5] |
| Fresh frozen plasma transfusion n. (%) | 25 (44.6) |
| Number of fresh frozen plasma units, median [Q25–75] | 3 [1–6] |
| Platelet transfusion n. (%) | 7 (12.5) |
| Number of platelet units, median [Q25–75] | 2 [2,3] |
| Variables | 56 |
|---|---|
| Preoperative CT n. (%) | |
| Active bleeding | 56 (100) |
| Volume of hematoma median [Q25–75)] | 1336 [135–1664] |
| Fluid level | 32 (57.14) |
| ≥2 locations of hematoma | 3 (5.4) |
| Location of hematoma n. (%) | |
| Retroperitoneum | 37 (66.0) |
| Rectus sheath | 13 (23.2) |
| Thigh | 3 (5.4) |
| Psoas + Thigh | 1 (1.8) |
| Rectus sheath + thigh | 1 (1.8) |
| Rectus sheath + retroperitoneum | 1 (1.8) |
| Angiographic data n. (%) | |
| Active bleeding | 50 (89.3) |
| Empirical Embolization | 6 (10.7) |
| Arteries Embolized n. (%) | |
| Lumbar | 30 (53.6) |
| Ilio-lumbar | 14 (25.0) |
| Epigastric inferior | 15 (26.8) |
| Deep femoral | 4 (7.1) |
| Number of embolized arteries n. (%) | |
| 1 | 48 (85.7) |
| ≥2 | 8 (14.3) |
| Embolic Agents n. (%) | |
| Gelatine sponge | 32 (57.2) |
| Microparticles | 11 (19.6) |
| Coils | 5 (8.9) |
| NBCA | 4 (7.1) |
| NBCA + gelatine sponge | 1 (1.8) |
| Microparticle + gelatine sponge | 2 (3.6) |
| Microparticle + gelatine sponge + coils | 1 (1.8) |
| Time of procedure (min) median [Q25–75] | 43 [16–60] |
| Variables | 56 |
|---|---|
| Clinical Success n. (%) | 48 (85.7) |
| Mortality during follow-up n. (%) | 20 (35.7) |
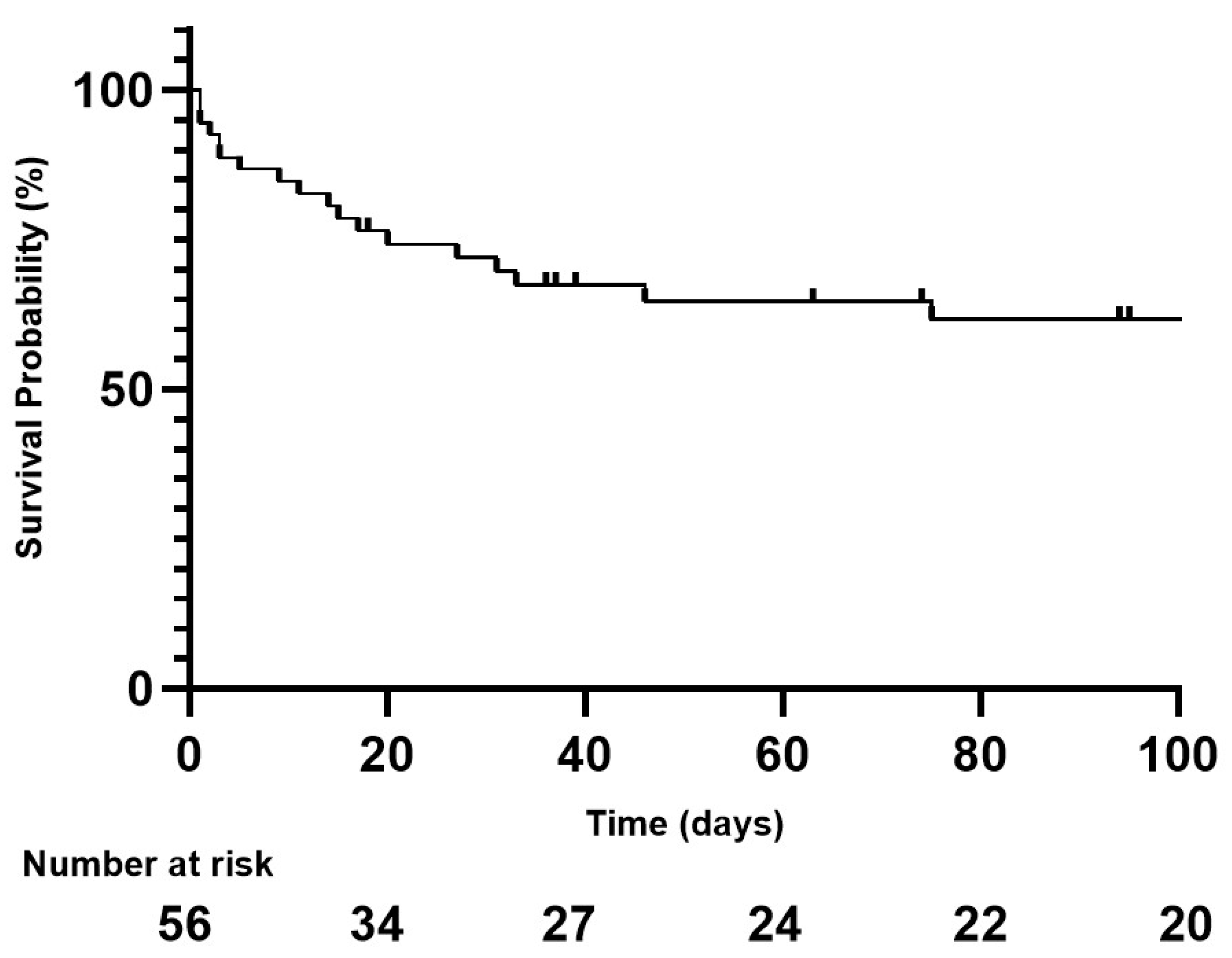
| Day-30 mortality n. (%) | 15 (26.8) |
| Day-3 mortality n. (%) | 5 (8.9) |
| Per-operative Complications n. (%) | 2 (3.6) |
| Post-Operative Complications n. (%) | 0 |
| Recurrence of Bleeding n. (%) | 8 (14.3) |
| Early ≤ 30 days | 8 (14.3) |
| Delayed > 30 days | 0 |
| Management of Early Rebleeding n. (%) | |
| Repeat TAE | 8/8 (100) |
| Duration of follow-up (days) median [Q25–75] | 31 [0–210] |
| Early Death ≤ 30 Days | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||
| Characteristics | OR | p Value | OR | p Value |
| Demographics data | ||||
| Age (years) | 1.03 (0.98–1.10) | 0.23 | - | - |
| Male | 1.36 (0.41–4.50) | 0.61 | - | - |
| HBP | 1.40 (0.41–4.80) | 0.59 | - | - |
| Diabetes | 2.22 (0.61–7.81) | 0.23 | - | - |
| Chronic renal failure | 0.76 (0.13–4.19) | 0.75 | - | - |
| Active cancer | 0.91 (0.16–5.17) | 0.92 | - | - |
| Antiplatelet therapy | 0.66 (0.06–6.46) | 0.72 | - | - |
| Anticoagulant therapy | 0.82 (0.23–2.93) | 0.77 | - | - |
| Hospitalization at diagnosis | 1.23 (0.37–4.05) | 0.72 | - | - |
| Coagulation disorder | ||||
| INR | 0.06 (0.76–1.46) | 0.72 | - | - |
| Hb < 7 | 1.31 (0.38–4.69) | 0.67 | - | - |
| MDCT Imaging data | ||||
| Retroperitoneum | 4.65 (1.32–16.31) | 0.016 | 4.08 (1.01–61.4) | 0.047 |
| Volume of hematoma (ml) | 7.7 (1.86–31.92) | 0.004 | 1.01 (1.00–1.02) | 0.023 |
| Fluid level on CT scan | 0.81 (0.24–2.66) | 0.727 | ||
| TAE data | ||||
| Blush at angiography | 1.82 (0–∞) | 0.99 | - | - |
| Use of gelatine sponge | 0.53 (0.61–1.78) | 0.3 | - | - |
| Lumbar artery | 2.1 (0.61–7.22) | 0.24 | - | - |
| Number of embolized arteries | 1.08 (0.23–5.04) | 0.92 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grange, R.; Grange, L.; Chevalier, C.; Mayaud, A.; Villeneuve, L.; Boutet, C.; Grange, S. Transarterial Embolization for Spontaneous Soft-Tissue Hematomas: Predictive Factors for Early Death. J. Pers. Med. 2023, 13, 15. https://doi.org/10.3390/jpm13010015
Grange R, Grange L, Chevalier C, Mayaud A, Villeneuve L, Boutet C, Grange S. Transarterial Embolization for Spontaneous Soft-Tissue Hematomas: Predictive Factors for Early Death. Journal of Personalized Medicine. 2023; 13(1):15. https://doi.org/10.3390/jpm13010015
Chicago/Turabian StyleGrange, Rémi, Lucile Grange, Clément Chevalier, Alexandre Mayaud, Loïc Villeneuve, Claire Boutet, and Sylvain Grange. 2023. "Transarterial Embolization for Spontaneous Soft-Tissue Hematomas: Predictive Factors for Early Death" Journal of Personalized Medicine 13, no. 1: 15. https://doi.org/10.3390/jpm13010015
APA StyleGrange, R., Grange, L., Chevalier, C., Mayaud, A., Villeneuve, L., Boutet, C., & Grange, S. (2023). Transarterial Embolization for Spontaneous Soft-Tissue Hematomas: Predictive Factors for Early Death. Journal of Personalized Medicine, 13(1), 15. https://doi.org/10.3390/jpm13010015







