Novel Machine Learning Approach to Predict and Personalize Length of Stay for Patients Admitted with Syncope from the Emergency Department
Abstract
:1. Introduction
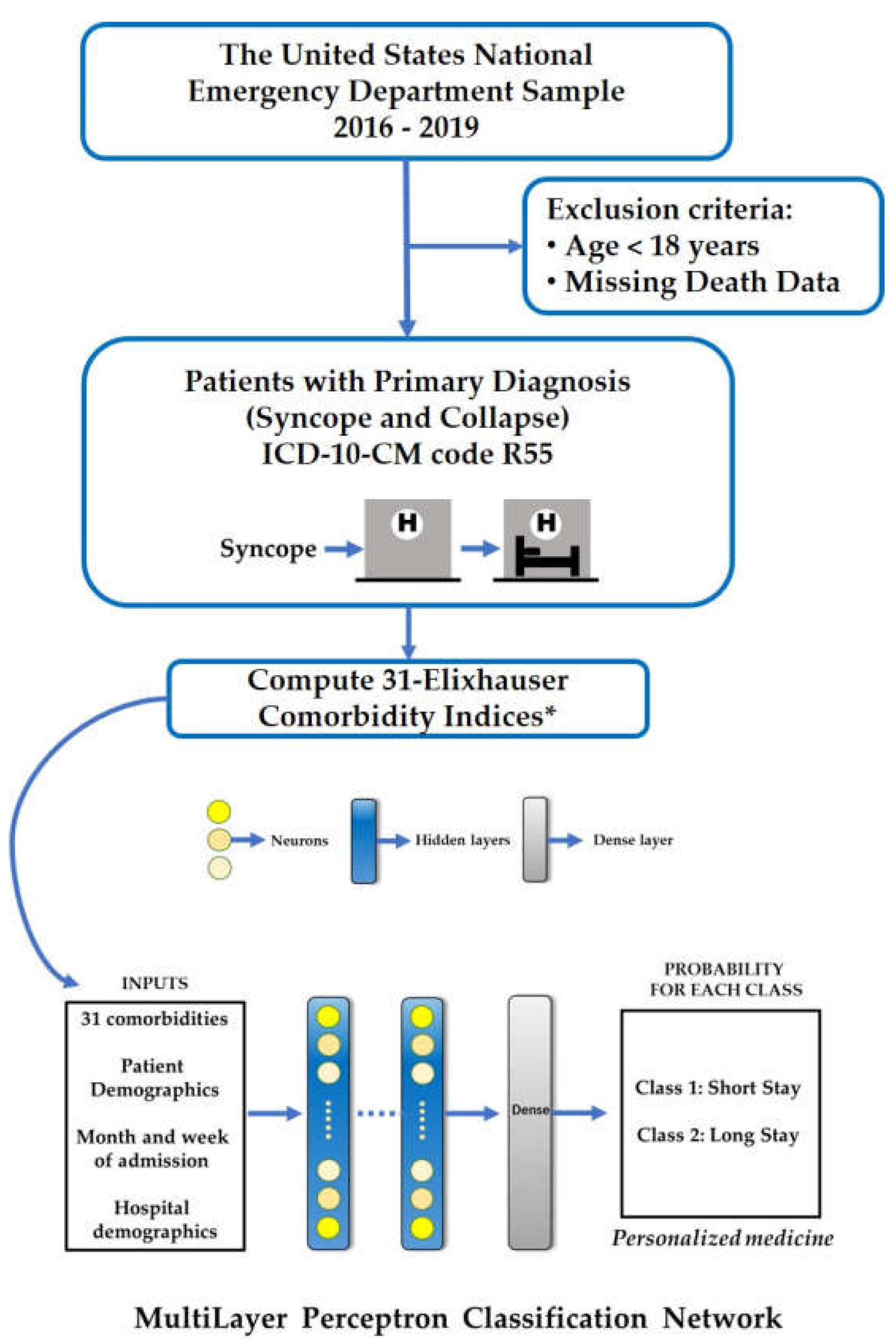
2. Materials and Methods
2.1. Data Source
2.2. Input Variables
2.3. Target Variables (Study Outcomes)
2.4. Statistical Analysis
2.5. Data Processing and Machine Learning
3. Results
3.1. Participant Characteristics
3.2. Prediction Model Performance for Short and Long LoS after Syncope
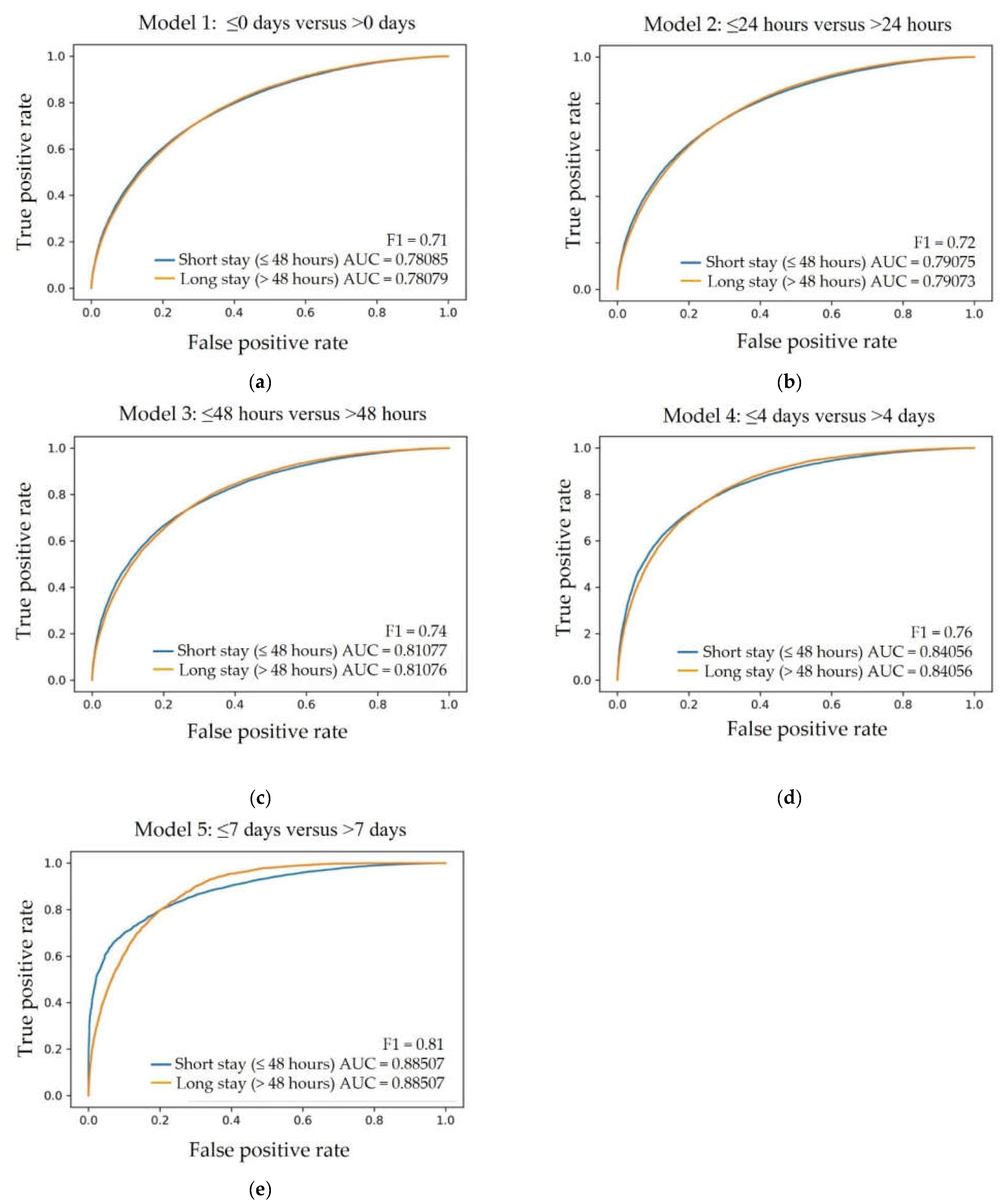
3.3. The Receiver Operator Characteristics for Short versus Long LoS
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.S.; Morillo, C.A.; Krahn, A.D.; O'Neill, B.; Thiruganasambandamoorthy, V.; Parkash, R.; Talajic, M.; Tu, J.V.; Seifer, C.; Johnstone, D.; et al. Standardized approaches to the investigation of syncope: Canadian Cardiovascular Society position paper. Can. J. Cardiol. 2011, 27, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Dipaola, F.; Shiffer, D.; Gatti, M.; Mene, R.; Solbiati, M.; Furlan, R. Machine Learning and Syncope Management in the ED: The Future Is Coming. Medicina 2021, 571. [Google Scholar] [CrossRef] [PubMed]
- Dipaola, F.; Gatti, M.; Pacetti, V.; Bottaccioli, A.G.; Shiffer, D.; Minonzio, M.; Mene, R.; Giaj Levra, A.; Solbiati, M.; Costantino, G.; et al. Artificial Intelligence Algorithms and Natural Language Processing for the Recognition of Syncope Patients on Emergency Department Medical Records. J. Clin. Med. 2019, 8, 1677. [Google Scholar] [CrossRef]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martin, A.; et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee, M.; Shen, W.K.; Sheldon, R.S.; Benditt, D.G.; Cohen, M.I.; Forman, D.E.; Goldberger, Z.D.; Grubb, B.P.; Hamdan, M.H.; Krahn, A.D.; et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2017, 14, e155–e217. [Google Scholar] [CrossRef]
- Angus, S. The Cost-Effective Evaluation of Syncope. Med. Clin. N. Am. 2016, 100, 1019–1032. [Google Scholar] [CrossRef]
- Kadri, A.N.; Abuamsha, H.; Nusairat, L.; Kadri, N.; Abuissa, H.; Masri, A.; Hernandez, A.V. Causes and Predictors of 30-Day Readmission in Patients With Syncope/Collapse: A Nationwide Cohort Study. J. Am. Heart Assoc. 2018, 7, e009746. [Google Scholar] [CrossRef]
- Stone, K.; Zwiggelaar, R.; Jones, P.; Mac, P. A systematic review of the prediction of hospital length of stay: Towards a unified framework. PLoS Digit. Health 2022, 1, e0000017. [Google Scholar] [CrossRef]
- Anand, V.; Benditt, D.G.; Adkisson, W.O.; Garg, S.; George, S.A.; Adabag, S. Trends of hospitalizations for syncope/collapse in the United States from 2004 to 2013-An analysis of national inpatient sample. J. Cardiovasc. Electrophysiol. 2018, 29, 916–922. [Google Scholar] [CrossRef]
- Shea, S.; Sideli, R.V.; DuMouchel, W.; Pulver, G.; Arons, R.R.; Clayton, P.D. Computer-generated informational messages directed to physicians: Effect on length of hospital stay. J. Am. Med. Inform. Assoc. 1995, 2, 58–64. [Google Scholar] [CrossRef]
- Costantino, G.; Falavigna, G.; Solbiati, M.; Casagranda, I.; Sun, B.C.; Grossman, S.A.; Quinn, J.V.; Reed, M.J.; Ungar, A.; Montano, N.; et al. Neural networks as a tool to predict syncope risk in the Emergency Department. Europace 2017, 19, 1891–1895. [Google Scholar] [CrossRef]
- Tu, J.V.; Guerriere, M.R. Use of a neural network as a predictive instrument for length of stay in the intensive care unit following cardiac surgery. Comput. Biomed. Res. 1993, 26, 220–229. [Google Scholar] [CrossRef]
- Muhlestein, W.E.; Akagi, D.S.; Kallos, J.A.; Morone, P.J.; Weaver, K.D.; Thompson, R.C.; Chambless, L.B. Using a Guided Machine Learning Ensemble Model to Predict Discharge Disposition following Meningioma Resection. J. Neurol. Surg. B Skull. Base 2018, 79, 123–130. [Google Scholar] [CrossRef]
- Pasupula, D.K.; Bhat, A.; Siddappa Malleshappa, S.K.; Munir, M.B.; Barakat, A.; Jain, S.; Wang, N.C.; Saba, S.; Bhonsale, A. Impact of Change in 2010 American Heart Association Cardiopulmonary Resuscitation Guidelines on Survival After Out-of-Hospital Cardiac Arrest in the United States: An Analysis From 2006 to 2015. Circ. Arrhythm. Electrophysiol. 2020, 13, e007843. [Google Scholar] [CrossRef]
- Pasupula, D.K.; Bhat, A.G.; Meera, S.J.; Siddappa Malleshappa, S.K. Influence of comorbidity on survival after out-of-hospital cardiac arrest in the United States. Resuscitation 2019, 145, 21–25. [Google Scholar] [CrossRef]
- Hirshon, J.M.; Warner, M.; Irvin, C.B.; Niska, R.W.; Andersen, D.A.; Smith, G.S.; McCaig, L.F. Research using emergency department-related data sets: Current status and future directions. Acad. Emerg. Med. 2009, 16, 1103–1109. [Google Scholar] [CrossRef]
- Ruwald, M.H.; Hansen, M.L.; Lamberts, M.; Kristensen, S.L.; Wissenberg, M.; Olsen, A.M.; Christensen, S.B.; Vinther, M.; Kober, L.; Torp-Pedersen, C.; et al. Accuracy of the ICD-10 discharge diagnosis for syncope. Europace 2013, 15, 595–600. [Google Scholar] [CrossRef]
- Elixhauser Comorbidity Software Refined for ICD-10-CM. Available online: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp (accessed on 18 December 2022).
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Patel, S.; Gabunilas, J.; Qian, A.S.; Cecil, A.; Jairath, V.; Sandborn, W.J.; Ohno-Machado, L.; Chen, P.L.; Singh, S. Simplified Machine Learning Models Can Accurately Identify High-Need High-Cost Patients With Inflammatory Bowel Disease. Clin. Transl. Gastroenterol. 2022, 13, e00507. [Google Scholar] [CrossRef]
- Iwase, S.; Nakada, T.A.; Shimada, T.; Oami, T.; Shimazui, T.; Takahashi, N.; Yamabe, J.; Yamao, Y.; Kawakami, E. Prediction algorithm for ICU mortality and length of stay using machine learning. Sci. Rep. 2022, 12, 12912. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. The current role of the virtual elements of artificial intelligence in total knee arthroplasty. EFORT Open Rev. 2022, 7, 491–497. [Google Scholar] [CrossRef]
- Saravi, B.; Zink, A.; Ulkumen, S.; Couillard-Despres, S.; Hassel, F.; Lang, G. Performance of Artificial Intelligence-Based Algorithms to Predict Prolonged Length of Stay after Lumbar Decompression Surgery. J. Clin. Med. 2022, 11, 4050. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xie, Y.; Zhang, X.; Li, W. Cerebral Angiography under Artificial Intelligence Algorithm in the Design of Nursing Cooperation Plan for Intracranial Aneurysm Patients in Craniotomy Clipping. Comput. Math. Methods Med. 2022, 2022, 2182931. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 4,645,483) | 2016 (n = 1,135,359) | 2017 (n = 1,174,452) | 2018 (n = 1,137,276) | 2019 (n = 1,188,396) | P trend | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (clustered) | |||||||||||
| 18–54 years | 2,108,750 | (45.4%) | 533,875 | (46.6%) | 528,642 | (45.0%) | 516,383 | (45.4%) | 529,850 | (44.6%) | <0.001 |
| 55–64 years | 693,974 | (14.9%) | 167,632 | (14.6%) | 175,699 | (15.0%) | 171,566 | (15.1%) | 179,077 | (15.1%) | |
| 65–74 years | 727,565 | (15.7%) | 171,271 | (15.0%) | 184,783 | (15.7%) | 178,412 | (15.7%) | 193,099 | (16.2%) | |
| 75–84 years | 668,526 | (14.4%) | 160,514 | (14.0%) | 169,815 | (14.5%) | 163,382 | (14.4%) | 174,814 | (14.7%) | |
| ≥85 years | 446,668 | (9.6%) | 112,067 | (9.8%) | 115,513 | (9.8%) | 107,532 | (9.5%) | 111,556 | (9.4%) | |
| Gender | |||||||||||
| Males | 2,042,422 | (44.0%) | 498,284 | (43.5%) | 514,575 | (43.8%) | 499,703 | (43.9%) | 529,860 | (44.6%) | <0.001 |
| Females | 2,602,663 | (56.0%) | 646,853 | (56.5%) | 659,848 | (56.2%) | 637,502 | (56.1%) | 658,460 | (55.4%) | |
| ECI Cluster | |||||||||||
| ECI = 0 | 1,670,288 | (36.0%) | 426,332 | (37.2%) | 421,696 | (35.9%) | 403,301 | (35.5%) | 418,959 | (35.3%) | 0.0043 |
| ECI = 1–2 | 1,905,336 | (41.0%) | 470,893 | (41.1%) | 482,367 | (41.1%) | 465,062 | (40.9%) | 487,014 | (41.0%) | |
| ECI ≥ 3 | 1,069,859 | (23.0%) | 248,135 | (21.7%) | 270,389 | (23.0%) | 268,913 | (23.6%) | 282,423 | (23.8%) | |
| Primary expected payer | |||||||||||
| Medicare | 1,877,544 | (40.5%) | 458,255 | (40.0%) | 479,524 | (40.9%) | 457,512 | (40.3%) | 482,252 | (40.6%) | 0.1328 |
| Medicaid | 679,442 | (14.6%) | 169,790 | (14.8%) | 170,883 | (14.6%) | 171,253 | (15.1%) | 167,515 | (14.1%) | |
| Private insurance | 1,514,372 | (32.6%) | 376,475 | (32.9%) | 377,400 | (32.2%) | 370,989 | (32.7%) | 389,507 | (32.8%) | |
| Self-pay | 393,866 | (8.5%) | 94,992 | (8.3%) | 99,525 | (8.5%) | 95,935 | (8.4%) | 103,413 | (8.7%) | |
| No charge | 14,042 | (0.3%) | 3299 | (0.3%) | 3,948 | (0.3%) | 2864 | (0.3%) | 3931 | (0.3%) | |
| Other | 159,652 | (3.4%) | 41,594 | (3.6%) | 40,110 | (3.4%) | 37,654 | (3.3%) | 40,293 | (3.4%) | |
| Death/Alive | |||||||||||
| Alive | 4,643,245 | (100.0%) | 1,144,608 | (99.9%) | 1,173,888 | (100.0%) | 1,136,846 | (100.0%) | 1,187,904 | (100.0%) | 0.0027 |
| Died in ED | 1309 | (<0.01%) | 464 | (<0.01%) | 330 | (<0.01%) | 227 | (<0.01%) | 288 | (<0.01%) | |
| Died in the Hospital | 929 | (<0.01%) | 287 | (<0.01%) | 235 | (<0.01%) | 203 | (<0.01%) | 204 | (<0.01%) | |
| Length of Stay | AUC * | Precision | Recall | F1 | Average Accuracy |
|---|---|---|---|---|---|
| ≤0 days # | 0.78 | 0.70 | 0.72 | 0.71 | 0.71 |
| ≤24 h | 0.79 | 0.72 | 0.72 | 0.72 | 0.72 |
| ≤48 h | 0.81 | 0.72 | 0.76 | 0.74 | 0.73 |
| ≤4 days | 0.84 | 0.76 | 0.75 | 0.76 | 0.76 |
| ≤7 days | 0.88 | 0.78 | 0.83 | 0.81 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Reddy Mudireddy, A.; Kumar Pasupula, D.; Adhaduk, M.; Barsotti, E.J.; Sonka, M.; Statz, G.M.; Bullis, T.; Johnston, S.L.; Evans, A.Z.; et al. Novel Machine Learning Approach to Predict and Personalize Length of Stay for Patients Admitted with Syncope from the Emergency Department. J. Pers. Med. 2023, 13, 7. https://doi.org/10.3390/jpm13010007
Lee S, Reddy Mudireddy A, Kumar Pasupula D, Adhaduk M, Barsotti EJ, Sonka M, Statz GM, Bullis T, Johnston SL, Evans AZ, et al. Novel Machine Learning Approach to Predict and Personalize Length of Stay for Patients Admitted with Syncope from the Emergency Department. Journal of Personalized Medicine. 2023; 13(1):7. https://doi.org/10.3390/jpm13010007
Chicago/Turabian StyleLee, Sangil, Avinash Reddy Mudireddy, Deepak Kumar Pasupula, Mehul Adhaduk, E. John Barsotti, Milan Sonka, Giselle M. Statz, Tyler Bullis, Samuel L. Johnston, Aron Z. Evans, and et al. 2023. "Novel Machine Learning Approach to Predict and Personalize Length of Stay for Patients Admitted with Syncope from the Emergency Department" Journal of Personalized Medicine 13, no. 1: 7. https://doi.org/10.3390/jpm13010007
APA StyleLee, S., Reddy Mudireddy, A., Kumar Pasupula, D., Adhaduk, M., Barsotti, E. J., Sonka, M., Statz, G. M., Bullis, T., Johnston, S. L., Evans, A. Z., Olshansky, B., & Gebska, M. A. (2023). Novel Machine Learning Approach to Predict and Personalize Length of Stay for Patients Admitted with Syncope from the Emergency Department. Journal of Personalized Medicine, 13(1), 7. https://doi.org/10.3390/jpm13010007







